Cabazitaxel (Jevtana) for metastatic castration-resistant prostate cancer
Keywords: cabazitaxel, castration-resistant prostate cancer, docetaxel resistance, XRP6258
Abstract
Objective:
This article presents current clinical evidence supporting the use of cabazitaxel (Jevtana) in men with metastatic castration-resistant prostate cancer (mCRPC).
Data Sources:
We conducted a literature search using abstracts from MEDLINE and PubMed (from January 1966 to December 2011) and the American Society of Clinical Oncology (from January 2000 to December 2011). The search included clinical studies and abstracts in the English language that described the pharmacology, pharmacokinetics, clinical activity, and safety of cabazitaxel in mCRPC.
Results:
Cabazitaxel, a semisynthetic microtubule inhibitor that induces cell death by microtubule stabilization, was approved in combination with prednisone for the treatment of mCRPC in patients who had been treated with a docetaxel-(Taxotere)-containing regimen. The approval of this taxane derivative was based primarily on the results of a randomized, open-label trial in patients with mCRPC who were treated with either cabazitaxel 25 mg/m2 or mitoxantrone (Novantrone) 12 mg/m2 intravenously every 3 weeks, both in combination with prednisone 10 mg/day. The median survival period was 15.1 months with cabazitaxel and 12.7 months with mitoxantrone. Neither group experienced complete responses. Cabazitaxel has also shown activity in breast cancer and other malignancies. In clinical trials, common grade 3 or grade 4 adverse reactions were myelosuppression, febrile neutropenia, diarrhea, fatigue, and asthenia. Other adverse effects included abdominal pain, back pain, arthralgia, and peripheral neuropathy.
Conclusion:
Cabazitaxel appeared to be an effective second-line agent in patients with mCRPC refractory to a docetaxel-containing regimen. Studies comparing cabazitaxel with existing first-line regimens for mCRPC are under way. Until the results of these head-to-head trials are published, it remains uncertain whether cabazitaxel is more effective or more tolerable than the currently available first-line regimens.
INTRODUCTION
Prostate cancer is the most common malignancy in American men and represents the second leading cause of cancer-related deaths in men in the U.S. An estimated 217,730 new cases and 32,050 deaths were reported in 2010.1 Earlystage, localized prostate cancer has an excellent prognosis and can be cured by radical prostatectomy, radiation, or both.
From 20% to 40% of patients who undergo primary therapy for early-stage prostate cancer experience relapse, and 30% to 70% of those with biochemical recurrence (i.e., prostate-specific antigen [PSA] levels above 0.2 ng/mL) develop metastatic disease within 10 years after local therapy.2–5 In patients with advanced or metastatic prostate cancer, symptomatic disease responds to surgical intervention (orchiectomy) or medical castration with androgen-deprivation therapy, namely, a luteinizing hormone–releasing hormone (LHRH) agonist with or without an antiandrogen. Medical castration with hormone-deprivation treatments reduces testosterone levels to below 50 ng/dL and achieves response rates as high as 80%.6,7 Although such therapies improve symptoms, tumors invariably become hormone-refractory—castration-resistant prostate cancer (CRPC)—and patients experience disease progression within a median period of 18 to 24 months.8
The Prostate Cancer Working Group describes CRPC as a “continuum,” because the disease can progress despite androgen-deprivation therapy and may be manifested as an increase in serum PSA levels, as the progression of pre-existing disease, or as the appearance of new metastases.9 The current standard of care for patients with CRPC and detectable macroscopic, metastatic disease is systemic chemotherapy with a regimen containing docetaxel (Taxotere, Sanofi) every 3 weeks, combined with oral prednisone 5 mg twice daily. With reduced production of adrenal androgen and secretion of adrenocorticotropic hormone (ACTH), low-dose prednisone has an antineoplastic effect on prostate cancer cells.
First-line therapy with docetaxel and prednisone is based on two large randomized trials, TAX 327 and Southwest Oncology Group (SWOG) 9916, both published in 2004.10,11
The TAX 327 trial compared docetaxel 75 mg/m2 every 3 weeks plus an oral corticosteroid with the established standard of mitoxantrone (Novantrone, EMD Serono) 12 mg/m2 every 3 weeks. Mitoxantrone is a type-2 topoisomerase inhibitor that disrupts DNA synthesis and repair. It was previously the FDA’s standard of care for prostate cancer because of its palliative effects. In TAX 327, a significant survival benefit was observed with docetaxel (18.9 months) compared with mitoxantrone (16.5 months) (P = 0.009).10
SWOG 9916 also demonstrated a significant survival benefit with a combination of docetaxel and estramustine (Emcyt, Pfizer) (17.5 months) compared with mitoxantrone (15.6 months) (P = 0.02).11
Although mitoxantrone-based treatment provides a palliative benefit for patients with disease progression after failure with docetaxel, no second-line regimens have shown a survival benefit or improved quality of life in patients with mCRPC, underscoring the need for novel salvage therapies in this population.
Cabazitaxel (Jevtana, Sanofi), abiraterone (Zytiga, Janssen Biotech), and sipuleucel-T (Provenge, Dendreon) represent the newest agents in the expanding realm of treating mCRPC. Sipuleucel-T was the first vaccine-based immunotherapy approved by the FDA, based on a trial by Kantoff and colleagues, which demonstrated a survival benefit in men with asymptomatic to minimally symptomatic mCRPC.12 Sipuleucel-T is administered as an infusion of three doses over a period of 60 minutes every 2 weeks.
Abiraterone specifically targets cytochrome P450 (CYP) 17A1, an enzyme necessary for the synthesis of androgen and estrogen. It is a secondary therapeutic hormonal option that works in a fashion similar to that of ketoconazole (Nizoral, Janssen) in prostate cancer. The approval of abiraterone was based on the results of a trial by de Bono et al. that demonstrated an overall survival benefit compared with placebo.13
In June 2010, the FDA approved cabazitaxel in combination with prednisone for men with mCRPC who had already received a docetaxel-containing regimen.14 Cabazitaxel is available in the U.S. and is being considered by the European Medicines Agency and other regulatory bodies.
MECHANISM OF ACTION
Cabazitaxel is a novel second-generation, semisynthetic microtubule inhibitor (specifically, a taxane derivative) that induces cell death by microtubule stabilization. Its mechanism of action is similar to that of paclitaxel and docetaxel.15 Microtubules are cytoskeletal polymers composed of alpha-tubulin and beta-tubulin heterodimers, which have a key role in the maintenance of cell shape, vesicle transport, cell signaling, and cell division. Cabazitaxel binds the N-terminal amino acids of the beta-tubulin subunit and promotes microtubule polymerization (tubulin dimer elongation).16 During mitosis, microtubules extend toward the mitotic spindle, which is responsible for the separation and distribution of chromosomes and for cell division into daughter cells. Cabazitaxel stimulates microtubule polymerization and inhibits microtubule cell division, thereby arresting the tumor cell cycle and tumor proliferation.
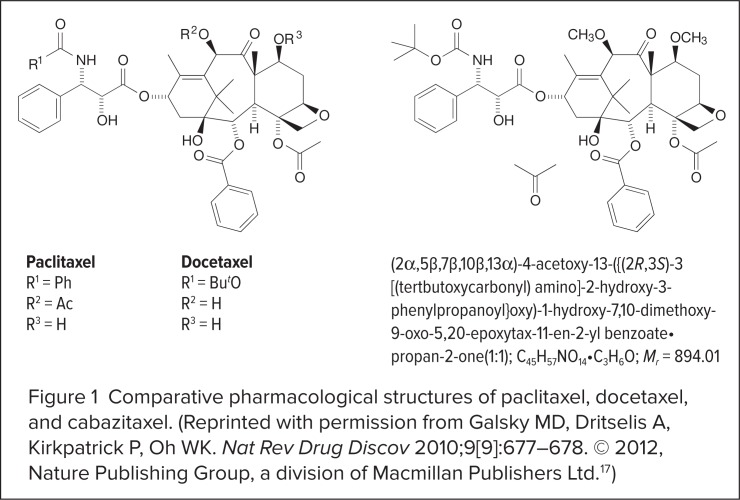
Although cabazitaxel shares similarities with its taxane predecessors, its structure and pharmacology are distinctly different. The molecular structure of cabazitaxel differs from that of docetaxel at the side chain, where the hydroxyl groups are replaced with methoxy side chains (Figure 1).17 Extra methyl groups counteract constitutive and acquired antitumor drug resistance by disabling the adenosine-5′-triphosphate (ATP)-dependent efflux pump, which is encoded by the multidrug-resistant gene ABCB.18
Figure 1.
Comparative pharmacological structures of paclitaxel, docetaxel, and cabazitaxel. (Reprinted with permission from Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Nat Rev Drug Discov 2010;9[9]:677–678. © 2012, Nature Publishing Group, a division of Macmillan Publishers Ltd.17)
Docetaxel and paclitaxel have a high affinity for the P-glycoprotein (P-gp) efflux pump; therefore, an effort was made to synthesize a taxane derivative that is unsusceptible to the effects of P-gp. In addition, as a consequence of its low affinity for P-gp, cabazitaxel can cross the blood–brain barrier, which may have implications for central nervous system (CNS) metastases.18
PHARMACODYNAMICS AND PHARMACOKINETICS
Mita et al. conducted a phase 1 study to determine the recommended phase 2 dose and the pharmacokinetic properties of cabazitaxel.19 Patients with refractory solid tumors received cabazitaxel at four dose levels (10, 15, 20, and 25 mg/m2) as an intravenous (IV) infusion every 3 weeks to identify the maximum tolerated dose and to characterize the drug’s toxicity profile.
The maximum plasma concentration (Cmax) and the area-under-the-concentration–time curve (AUC0–48 h) were determined in 23 evaluable patients. In these patients, an increase in the dose of cabazitaxel was proportionally related to an increase in the Cmax and AUC concentration. The 25-mg/m2 dose was correlated with a Cmax of 535 ± 305 mcg/L and an AUC concentration of 642 ± 320 mcg/L per hour. A triphasic model was used to describe the drug’s decreased plasma concentrations.
The plasma concentration pharmacokinetic activity was characterized by a rapid initial elimination phase (average terminal half-life [t1/2] = 2.6 ± 1.4 minutes), followed by an intermediate elimination phase (average t1/2 = 1.3 ± 0.6 hours) and a prolonged terminal elimination phase (average t1/2 = 77.3 ± 45.5 hours). Approximately 80% of the dose was eliminated within 2 weeks. The volume of distribution at steady state was large and highly variable (2,034 ± 1,495 L/m2).19
Cabazitaxel is metabolized in the liver primarily by CYP 3A4/5 isoenzymes and, to a lesser degree, by CYP2C8 enzymes. Clearance rates are high, averaging 53.5 ± 20.3 L/hour (27.3 ± 9.7 L/hour/m2), which represents 61% of hepatic blood flow (87 L/hour). Cabazitaxel binds mainly to human serum albumin (82%) and lipoproteins. The main route of elimination is via feces (76% of the dose, as metabolites); renal elimination accounts for 3.7% of the dose.20
Cabazitaxel should be used with caution in patients with severe renal impairment (i.e., a creatinine clearance of less than 30 mL/minute or end-stage renal disease); however, no dosage adjustments are necessary for patients with mild-tomoderate renal impairment.20
Because of its extensive metabolism in the liver, cabazitaxel should be used with caution in patients with hepatic impairment to avoid drug accumulation and subsequent toxicity. It should not be used in patients with hepatic impairment characterized by a total bilirubin level at or above the upper limit of normal (ULN) or by an aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) level at 1.5 times the ULN or higher, because available studies of cabazitaxel did not include populations with abnormal bilirubin or albumin levels.20
Cabazitaxel has not been evaluated in pediatric patients.20
LITERATURE SEARCH
We conducted a literature search of MEDLINE and PubMed abstracts (from January 1966 to December 2011), as well as American Society of Clinical Oncology (ASCO) abstracts (from January 2000 to December 2011), using the primary search terms cabazitaxel, castration-resistant prostate cancer, docetaxel resistance, and XRP6258 (i.e., cabazitaxel). Ongoing and published phase 1, phase 2, and phase 3 clinical trials that evaluated the safety and efficacy of cabazitaxel were reviewed. We included the manufacturer’s prescribing information to supplement the clinical data.
Clinical Trials
Mita et al.19
Mita et al. conducted a phase 1 study to determine the recommended phase 2 dose and pharmacokinetic properties of cabazitaxel in 25 patients with advanced solid tumors (see page 441). Cabazitaxel anticancer activity was seen in two patients with mCRPC. One patient receiving a 15-mg/m2 dose of cabazitaxel for four courses experienced a decline in PSA levels from 62 to 21 ng/mL and reduced disease-related bone pain. The size of a target lesion was also found to be smaller on radiographic imaging. The other patient received cabazitaxel 25 mg/m2 for eight courses and experienced a reduction in PSA levels from 415 to 44 ng/mL along with a partial response in measurable disease lesions.
The principal dose-limiting toxicity in this study was hematological bone-marrow suppression (i.e., neutropenia). One patient (4%) experienced prolonged grade 4 neutropenia, and a second patient (4%) experienced febrile neutropenia, both with the 25-mg/m2 dose. Nonhematological toxicities also occurred. Two patients (8%) experienced flushing, dizziness, and chest tightness, which were identified as grade 1 hypersensitivity reactions. Other nonhematological toxicities included diarrhea (14 patients; 56%), nausea (10 patients; 40%), fatigue (nine patients; 36%), neurotoxicity (nine patients; 36%), and vomiting (four patients; 16%).
Pivot et al.21
A phase 2 study of cabazitaxel in patients with advanced prostate cancer has not been conducted; however, in a phase 2 trial, 71 patients with metastatic breast cancer were treated with IV cabazitaxel 20 mg/m2 or 25 mg/m2 every 3 weeks, for a median of four cycles. After the first cycle, the dose for 20 patients was increased to 25 mg/m2 in the absence of significant toxicity. After a median follow-up period of 20 months, the median overall survival was 12.3 months, and the median time to progression was 2.7 months.
Notable hematological and nonhematological toxicities were observed. Eighteen patients (30%) achieved stable disease for at least 3 months. Adverse effects included neutropenia (73%), leukopenia (55%), fatigue (35%), diarrhea (30%), sensory neuropathy (17%), and hypersensitivity reactions (6%). Two patients died within 30 days after their last study treatment. One death that occurred as a result of shock and respiratory failure was related to cabazitaxel. The cause of the other patient’s death was unknown.
De Bono et al.22
Results from the large phase 3 TROPIC trial were published in 2010. This randomized, open-label study evaluated cabazitaxel in 755 mCRPC patients with documented disease progression during or after treatment with a docetaxel-containing regimen at a cumulative docetaxel dose greater than 225 mg/m2. Enrolled patients had to have either elevated PSA levels or measurable disease, as documented by the Response Evaluation Criteria in Solid Tumors (RECIST). All patients were receiving LHRH agonist therapy or had undergone surgical orchiectomy.
Patients received either cabazitaxel 25 mg/m2 IV (n = 378) or mitoxantrone 12 mg/m2 IV (n = 377) every 3 weeks, both administered with oral prednisone 10 mg daily, for a maximum of 10 cycles. Patients were categorized as having measurable or nonmeasurable disease and by Eastern Cooperative Oncology Group (ECOG) performance status. ECOG performance status provides an objective quantification of cancer patients’ general well-being and of their ability to perform activities of daily living. ECOG scores range from 0 to 5; 0 indicates a fully active person, and 5 indicates death.
Patients were eligible for enrollment if they were at least 18 years of age and had an ECOG performance status of 0 to 2. Patients who had previously received mitoxantrone, radiotherapy to 40% or more of the bone marrow, or cancer therapy (other than LHRH analogues) within 4 weeks before enrollment were excluded from the study. Eligible patients had adequate hematological, hepatic, renal, and cardiac function. Their median age was 68 years (cabazitaxel) and 67 years (mitoxantrone). Of the enrolled patients in both treatment arms,16% were non-Caucasian.
Overall survival was the primary endpoint. Secondary endpoints included progression-free survival, PSA response, response rates according to RECIST criteria, and pain response.
Cabazitaxel demonstrated an overall survival benefit, with a median survival of 15.1 months compared with 12.7 months for mitoxantrone, corresponding to a 30% reduction in the relative risk of death for cabazitaxel. The hazard ratio (HR) was 0.70, with a 95% confidence interval (CI) of 0.59 to 0.83 (P < 0.0001). The intent-to-treat analysis of overall survival consistently favored cabazitaxel in all subgroups; the total previous dose of docetaxel did not show a significant correlation with the response to cabazitaxel.
Secondary analyses demonstrated median progression-free survival of 2.8 months with cabazitaxel and 1.4 months with mitoxantrone (HR, 0.74; 95% CI, 0.64–0.86; P < 0.0001). PSA levels fell by at least 50% in 39% of the cabazitaxel group and in 18% of the mitoxantrone group (P = 0.0002). Tumor response rates were 14% for cabazitaxel and 4% for mitoxantrone (P = 0.0005). The median time to tumor progression was 8.8 months with cabazitaxel patients and 5.4 months with mitoxantrone (P < 0.0001). Overall pain reduction was similar between the two groups.
Disease progression was the primary reason for treatment discontinuation in both groups. Treatment delays occurred in 28% of patients in the cabazitaxel arm and in 15% of those in the mitoxantrone arm. Dose reductions were required in 12% of the cabazitaxel patients and in 4% of mitoxantrone patients.
Neutropenia (all grades) occurred in 94% and 88% of the cabazitaxel and mitoxantrone groups, respectively. The most common grade 3 and grade 4 hematological adverse events are listed in Table 1. Grade 3 or 4 febrile neutropenia occurred in 8% of the cabazitaxel patients and in 1% of the mitoxantrone patients.
Table 1.
Hematological Adverse Events Occurring at Grade 3 or Higher in the TROPIC Trial
| Cabazitaxel + Prednisone (n = 371) % | Mitoxantrone + Prednisone (n = 371) % | |
|---|---|---|
| Neutropenia | 82 | 58 |
| Leukopenia | 69 | 42 |
| Anemia | 11 | 5 |
| Febrile neutropenia | 7 | 1 |
| Thrombocytopenia | 4 | 2 |
From Jevtana (cabazitaxel) prescribing information.20
The most common nonhematological adverse events included diarrhea (47%, cabazitaxel vs. 11%, mitoxantrone), fatigue (37% vs. 27%), and asthenia (20% vs. 12%). Peripheral neuropathy (all grades) occurred in 14% of patients receiving cabazitaxel and in 3% of those receiving mitoxantrone. Grade 3 peripheral neuropathy was reported in 1% of patients in each group. Diarrhea (all grades) occurred in 47% of cabazitaxel patients and in 11% of mitoxantrone patients.
The cabazitaxel dose used in this study (25 mg/m2) was higher than the initial dose (20 mg/m2) administered to younger patients with advanced breast cancer in the phase 2 study by Pivot et al. Based on the hematological and nonhematological adverse events observed in this phase 3 trial, a randomized, openlabel study is under way to determine the non-inferiority of cabazitaxel 20 mg/m2 versus cabazitaxel 25 mg/m2 in combination with oral prednisone 10 mg daily in terms of overall survival in patients with mCRPC previously treated with a docetaxel-containing regimen.
As secondary objectives, the two cabazitaxel doses are also being investigated for efficacy and to see whether the lower dose is better tolerated.23
NCCN Practice Guidelines24
Treatment guidelines from the National Comprehensive Cancer Network (NCCN) have been updated to include cabazitaxel as a second-line option for patients with mCRPC who have not responded to a first-line docetaxel-containing regimen (a category 1 recommendation). The revised guidelines underscore the need to select appropriate patients for treatment with cabazitaxel. Patients should not have severe neuropathy and should have adequate liver, kidney and bone marrow function, given the drug’s safety profile. In addition, clinicians should consider using prophylactic granulocyte–colonystimulating factor (G–CSF) to reduce the risk of neutropenic complications.
Ongoing Studies
In view of the positive results achieved in patients with mCRPC, evaluation of cabazitaxel in locally advanced disease is warranted. A phase 1 trial of weekly cabazitaxel, given in combination with intensity-modulated radiation therapy and androgen-deprivation therapy, is being conducted to determine the efficacy of this treatment approach in patients with locally advanced, high-risk prostate cancer.25
Interest is also focused on cabazitaxel in combination with the monoclonal antibody bavituximab (Peregrine Pharmaceuticals), a first-in-class chimeric phosphatidylserine-targeted monoclonal antibody, in patients with CRPC.26 Cabazitaxel is also being evaluated in combination with the somatostatin analogue octreotide (Sandostatin, Novartis; Octreo, New MediconPharma), an antisecretory agent used to treat chemotherapy-induced diarrhea.27 Octreotide may have the potential to lessen the occurrence of cabazitaxel-associated diarrhea.
Sanofi is now recruiting patients for a study to compare the efficacy of first-line treatment with cabazitaxel 20 mg/m2, cabazitaxel 25 mg/m2, and docetaxel 25 mg/m2, each administered with oral prednisone 10 mg daily, in patients with mCRPC.28 Cabazitaxel is also being studied in head and neck cancer, advanced non–small-cell lung cancer, urothelial cancer, and gastroesophageal cancer.29–32
DOSAGE AND ADMINISTRATION
The approved dosage of cabazitaxel for mCRPC is 25 mg/m2 infused over 1 hour every 3 weeks, combined with oral prednisone 10 mg daily.20 Cabazitaxel is administered until disease progression or the occurrence of intolerable adverse events.
The 25-mg/m2 dose was approved on the basis of results from De Bono’s pivotal phase 3 study.22 The 25-mg/m2 dose had been selected instead of the 20-mg/m2 dose used in earlier studies19 based on the results of a phase 1 trial, which established two different maximum tolerated doses of cabazitaxel––20 mg/m2 and 25 mg/m2.33 The results of Pivot’s phase 2 study in patients with metastatic breast cancer demonstrated that cabazitaxel, given at 20 mg/m2 and increased to 25 mg/m2, was not associated with increased toxicity and resulted in low rates of febrile neutropenia and related infections.21
Cabazitaxel must be reconstituted and diluted with 250 mL of 0.9% sodium chloride or dextrose 5% water in a non–poly-vinyl chloride container before administration as an IV infusion. The final cabazitaxel concentration should be between 0.10 and 0.26 mg/mL. For total doses exceeding 65 mg, a larger infusion volume is required. Solutions for infusion are stable for 8 hours at room temperature or for 24 hours if they are refrigerated. Cabazitaxel should not be infused with other medications because information on IV compatibility is not available.20,34
Adjustments for Toxicity
Cabazitaxel therapy should be stopped or delayed if hematological or nonhematological toxicity occurs.20 The types and severity of toxicity that can be associated with cabazitaxel are listed in Table 2.35 The recommended dosage adjustments and treatment modifications for these adverse events are shown in Table 3.20 Primary prophylaxis for febrile neutropenia with G–CSF was not used in clinical studies of cabazitaxel; however, G–CSF was administered to patients who developed grade 4 neutropenia.
Table 2.
Hematological and Nonhematological Toxicity in Common Terminology Criteria for Adverse Events, Version 4.0
| Reaction | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Neutropenia | Neutrophils
|
Neutrophils
|
Neutrophils
|
Neutrophils
|
Death |
| Febrile neutropenia* | — | — | — |
|
Death |
| Hypersensitivity or allergic reaction |
|
|
|
|
Death |
| Diarrhea |
|
|
|
|
Death |
ADL = activities of daily living; ANC = absolute neutrophil count; LLN = lower limit of normal.
Fever of unknown origin without clinically or microbiologically documented infection; ANC < 1.0 × 109/L and fever ≥ 38.5°C.
Data compiled from National Institutes of Health.35
Table 3.
Suggested Cabazitaxel Dose Modifications for Hematological and Nonhematological Toxicities
| Hematological | Recommended Dose Adjustment for Toxicity |
| Neutropenia ≥ grade 3 for more than 1 week despite prophylaxis with G–CSF | Delay treatment until ANC > 1,500/mm3; then reduce dose to 20 mg/m2 with continued secondary WBC growth factor prophylaxis. |
| Febrile neutropenia | Delay treatment until improvement/resolution and ANC > 1,500/mm3; then reduce dose to 20 mg/m2 with continued secondary WBC growth factor prophylaxis. |
| Persistent hematological toxicity despite dose reduction | Discontinue treatment. |
| Nonhematological | Recommended Dose Adjustment for Toxicity |
| Severe hypersensitivity reaction | Discontinue treatment immediately. |
| Diarrhea ≥ grade 3 or persistent despite appropriate medications, fluids, and electrolyte replacement | Delay treatment until improvement or resolution; then reduce dose to 20 mg/m2 . |
| Persistent diarrhea despite dose reduction | Discontinue treatment. |
ANC = absolute neutrophil count; G–CSF = granulocyte–colony-stimulating factor; WBC = white blood cell.
From Jevtana (cabazitaxel) prescribing information.20
Cabazitaxel is formulated in polysorbate 80, an emulsifier, which has the potential to cause hypersensitivity reactions. Clinical manifestations of cabazitaxel-associated hypersensitivity reactions include hypotension, bronchospasm, rash, and erythema. These manifestations usually develop within minutes of the infusion, especially during the first and second infusions, and are reversible, resolving after the infusion is withheld. Appropriate management of cabazitaxel-related hypersensitivity reactions includes supportive-care measures, such as antihistamines, corticosteroids, IV fluids, oxygen, and bronchodilators.
Cabazitaxel-associated gastrointestinal (GI) toxicity, such as severe, persistent diarrhea, may occur and should be treated with IV fluids, electrolyte repletion, and an antidiarrheal medication, such as loperamide (e.g., Imodium, McNeil), diphenoxylate/atropine (Lomotil, Pfizer), or octreotide acetate (Sandostatin, Novartis), after GI infection has been ruled out.20
Premedication
To minimize the hypersensitivity reactions associated with cabazitaxel, the product labeling recommends the administration of an antihistamine (diphenhydramine 25 mg or equivalent), a corticosteroid (e.g., dexamethasone 8 mg or equivalent), or a histamine (H2)-receptor antagonist (e.g., ranitidine 50 mg or equivalent) at least 30 minutes before cabazitaxel is given.20
ASCO guidelines state that cabazitaxel poses a low risk for emesis and suggest that a single 8-mg dose of dexamethasone provides optimal prevention of nausea and vomiting.36 The need for additional antiemetic medications should be tailored for each patient according to risk factors for chemotherapy-induced nausea and vomiting and the patient’s previous treatment experience.
SAFETY PROFILE
The key safety data for cabazitaxel in patients with mCRPC were derived from the pivotal TROPIC trial.22 The most common serious adverse events in that study included myelosuppression (i.e., anemia, neutropenia, leukopenia, and thrombocytopenia), fatigue, diarrhea, peripheral neuropathy, back pain, and arthralgia.
DRUG INTERACTIONS
No formal drug-interaction studies have been conducted with cabazitaxel. Prednisone or prednisolone (10 mg daily) did not affect the pharmacokinetics of cabazitaxel.20
Because cabazitaxel is metabolized primarily via the CYP3A4 pathway, the concomitant administration of strong CYP3A4 inhibitors, such as ketoconazole, itraconazole (Sporanox, Janssen), and clarithromycin (e.g., Biaxin, Abbott), is expected to increase concentrations of cabazitaxel. Conversely, the concomitant administration of strong CYP3A inducers, such as phenytoin (Dilantin, Pfizer), carbamazepine (Carbatrol, Shire), and rifampin (Rifadin, Sanofi), is expected to decrease cabazitaxel concentrations. Therefore, the coadministration of cabazitaxel with strong CYP3A inhibitors and inducers should be avoided.20
WARNINGS AND PRECAUTIONS
The labeling for cabazitaxel includes a boxed warning regarding the potential for death from neutropenia. Cabazitaxel should not be administered to patients with neutrophil counts of 1,500/mm3 or below. Dose reductions are recommended following neutropenic fever or prolonged neutropenia. The administration of growth factor may reduce the risk of complications resulting from neutropenia, and primary prophylaxis with G–CSF should be considered in high-risk patients.20 According to the NCCN treatment guidelines, risk factors for complications from neutropenia include age older than 65 years, poor performance status, a history of neutropenic fever, extensive prior radiation, and poor nutrition status.37 Secondary prophylaxis with G–CSF should be considered if febrile neutropenia develops during treatment with cabazitaxel.20
The boxed warning also mentions the potential for severe hypersensitivity reactions, including generalized rash or erythema, hypotension, and bronchospasm. If such reactions occur, treatment should be discontinued immediately and appropriate therapy should be administered. Cabazitaxel is contraindicated in patients with a history of severe hypersensitivity reactions to this drug or to other medications that are formulated with polysorbate 80.20
Additional warnings and precautions include the potential for GI symptoms, such as nausea, vomiting, and diarrhea, during treatment with cabazitaxel. Diarrhea may be severe and may result in dehydration and electrolyte imbalance requiring rehydration and treatment with antiemetic and antidiarrheal medications. Treatment with cabazitaxel might need to be delayed, or the dosage may have to be reduced in patients with severe diarrhea (grade 3 or greater).20
Renal failure was reported in the pivotal clinical trial of cabazitaxel. Most cases occurred in association with dehydration, sepsis, or obstructive uropathy.20
PATIENT COUNSELING
Clinicians should inform patients about the risk of myelosuppression, GI symptoms, and hypersensitivity reactions during cabazitaxel therapy. Patients should also be aware of the potential risk for neurological symptoms (i.e., peripheral neuropathy) and should be counseled on the importance of reporting palpitations, difficulty in breathing, and the occurrence of rash or erythema during the infusion.20
P&T CONSIDERATIONS
Current clinical data indicate that cabazitaxel is a viable second-line option for patients with mCRPC who have received first-line treatment with a docetaxel-containing regimen. The FDA’s approval of cabazitaxel (in combination with prednisone) for this indication meets a definite clinical need in patients with mCRPC who experience disease progression after docetaxel-based therapy. Until recently, existing salvage regimens did not demonstrated a survival benefit in patients with advanced prostate cancer.
Cabazitaxel should be considered as an addition to the formulary (in combination with prednisone) for patients with mCRPC who previously received a docetaxel-containing regimen. The results of ongoing studies may support additional indications.
COST
The average wholesale cost of cabazitaxel per treatment cycle is $5,598, compared with $2,483 for docetaxel.38–40 No studies have evaluated the cost-effectiveness of cabazitaxel in patients with mCRPC.
CONCLUSION
Cabazitaxel induces cell death by microtubule stabilization. The FDA approved cabazitaxel in combination with prednisone for patients with mCRPC who had already been treated with a docetaxel-containing regimen.14 Based on the positive results from clinical studies of cabazitaxel as second-line treatment in mCRPC, investigators are now comparing cabazitaxel with docetaxel as first-line therapy in this setting.28
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Uchio EM, Aslan M, Wells CK, et al. Impact of biochemical recurrence in prostate cancer among U.S. veterans. Arch Intern Med. 2010;170(15):1390–1395. doi: 10.1001/archinternmed.2010.262. [DOI] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Trock BJ, Feng Z, et al. The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: 25-year follow-up (Abstract) J Clin Oncol. 2009;27(Suppl):15s. [Google Scholar]
- 5.D’Amico AV, Cote K, Loffredo M, et al. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20(23):4567–4573. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 6.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgenindependent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 7.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 8.Sharifi N, Dahut WL, Steinberg SM, et al. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005;96:985–989. doi: 10.1111/j.1464-410X.2005.05798.x. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 11.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 12.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 13.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jun 18, 2010. FDA update. Cabazitaxel. Available at: www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm216214.htm. Accessed March 6, 2011.
- 15.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 16.Rao S, Krauss NE, Heerding JM, et al. 3′-(p-azidobenzamido) taxol photolabels the N-terminal 31 amino acids of beta-tubulin. J Biol Chem. 1994;269:3132–3134. [PubMed] [Google Scholar]
- 17.Galsky MD, Dritselis A, Kirkpatrick P, Oh WK. Cabazitaxel. Nat Rev Drug Discov. 2010;9(9):677–678. doi: 10.1038/nrd3254. [DOI] [PubMed] [Google Scholar]
- 18.Pal SK, Twardowski P, Sartor O. Critical appraisal of cabazitaxel in the management of advanced prostate cancer. Clin Interv Aging. 2010;5:395–402. doi: 10.2147/CIA.S14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 20.Jevtana (cabazitaxel) Injection, prescribing information. Bridgewater, N.J.: Sanofi; Jun, 2010. Available at: http://products.sanofi.us/jevtana/jevtana.html. Accessed March 6, 2012. [Google Scholar]
- 21.Pivot X, Koralewski P, Hidalgo JL, et al. A multicenter phase II study of XRP6258 administered as a 1-h IV infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann Oncol. 2008;19(9):1547–1552. doi: 10.1093/annonc/mdn171. [DOI] [PubMed] [Google Scholar]
- 22.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 23.Cabazitaxel at 20 mg/m2 compared to 25 mg/m2 with prednisone for the treatment of metastatic castration resistant prostate cancer (PROSELICA) Mar 1, 2012. Available at: http://clinicaltrials.gov/ct2/show/NCT01308580. Accessed March 6, 2012.
- 24.National Comprehensive Cancer Network (NCCN) NCCN clinical practice guidelines in oncology: Prostate cancer: Version 1. 2011. Available at: www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed February 1, 2012. [DOI] [PubMed]
- 25.Cabazitaxel with radiation and hormone therapy for prostate cancer. Jan 6, 2012. Available at: http://clinicaltrials.gov/ct2/show/NCT01420250. Accessed February 1, 2012.
- 26.Study of cabazitaxel plus bavituximab as second-line chemotherapy for patients with castration-resistant prostate cancer. Aug 2, 2011. Available at: http://clinicaltrials.gov/ct2/show/NCT01335204. Accessed February 1, 2012.
- 27.Cabazitaxel plus prednisone with octreotide for castration-resistant prostate cancer (CRPC) previously treated with docetaxel. Nov 9, 2011. Available at: http://clinicaltrials.gov/ct2/show/NCT01469338. Accessed February 1, 2012.
- 28.Cabazitaxel versus docetaxel both with prednisone in patients with metastatic castration resistant prostate cancer (FIRSTANA) Mar 1, 2012. Available at: http://clinicaltrials.gov/ct2/show/NCT01308567. Accessed March 6, 2012.
- 29.Cabazitaxel–PF induction chemotherapy. Nov 1, 2011. Available at: http://clinicaltrials.gov/ct2/show/NCT01379339. Accessed February 1, 2012.
- 30.Phase II study of cabazitaxel-XRP6258 in advanced non-small cell lung cancer. Jan 24, 2012. Available at: http://clinicaltrials.gov/ct2/show/NCT01438307. Accessed February 1, 2012.
- 31.Cabazitaxel in patients with urothelial carcinoma who have disease progression following platinum-based chemotherapy. Jan 3, 2012. Available at: http://clinicaltrials.gov/ct2/show/NCT01437488. Accessed February 1, 2012.
- 32.Chemotherapy for patients with gastroesophageal cancers who have progressed after one prior chemo regimen. Sep 21, 2011. Available at: http://clinicaltrials.gov/ct2/show/NCT01365130. Accessed February 1, 2012.
- 33.Villanueva C, Awada A, Campone M, et al. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: A phase I/II study. Eur J Cancer. 2011;47(7):1037–1045. doi: 10.1016/j.ejca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 34.de Bono JS, Sartor O. Author’s reply. Lancet. 2011;377:122. [Google Scholar]
- 35.National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. Jun 14, 2010. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed December 5, 2011.
- 36.Basch E, Restrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network (NCCN) NCCN clinical practice guidelines in oncology: Prevention and treatment of cancer-related infections: Version 2. 2011. Available at: www.nccn.org/professionals/physician_gls/pdf/infections.pdf. Accessed February 1, 2012. [DOI] [PubMed]
- 38.Manifold C. Cabazitaxel (Jevtana): Another arrow in the prostate cancer quiver. Informulary. 2010;3(6):1–2. [Google Scholar]
- 39.Ramsey SD, Clarke L, Kamath TV, Lubeck D. Evaluation of erlotinib in advanced non-small cell lung cancer: Impact on the budget of a U.S. health insurance plan. J Manag Care Pharm. 2006;12(6):472–478. doi: 10.18553/jmcp.2006.12.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Red Book. Montvale, N.J: Thomson Healthcare Inc.; 2010. [Google Scholar]