Abstract
Four recently approved drugs (cabazitaxel, sipuleucel-T, abiraterone, and denosumab), along with emerging therapies, bone-building therapies, hormonal treatments, and immunotherapies, have all demonstrated promise in advanced prostate cancer. It appears that the best outcomes will be achieved from the sequential use of multiple agents.
INTRODUCTION
Prostate cancer is the most common cancer affecting men in the U.S. It is estimated that 241,740 new cases of prostate cancer will have been diagnosed during 2012, accounting for 29% of incident cancers in males.1 Age-adjusted and delay-adjusted analyses reveal declines in the incidence of prostate cancer from 183.66 per 100,000 in 2000 to 157.92 per 100,000 in 2008.2 Prostate cancer is second to lung cancer as a leading cause of cancer mortality in the U.S., with 28,170 deaths anticipated for 2012.1 Mortality rates have significantly declined in the previous two decades, from 38.34 per 100,000 in 1990 to 22.93 per 100,000 in 2008,2 and the 5-year relative survival rate for all stages of prostate cancer has increased from 66.4% in 1975 to 99.4% in 2003.3
Screening
Screening with the prostate-specific antigen (PSA) serum test is credited with the improved survival rates as a result of earlier detection of asymptomatic, clinically localized prostate cancers. However, prostate cancer screening has been an issue of controversy in the U.S. The most recent update to the 2008 U.S. Preventive Services Task Force guidelines recommends against PSA screening based on moderate or high certainty that it offers no net benefit or that the potential harms outweigh the benefits.4
The rationale for this recommendation is a high rate (approximately 80%) of false-positive results using cutoffs for serum PSA levels between 2.5 and 4.0 mcg/L. These false-positive results are often associated with unfavorable psychological effects and additional testing, including one or more biopsies in the following year compared with a negative PSA result.4 Prostate biopsies result in pain, fever, bleeding, infection, transient urinary problems, and additional clinical follow-up for about one-third of men.4 The updated guidelines are also based on evidence that PSA screening results in overdiagnosis and overtreatment of prostate cancers that are unlikely to become symptomatic.
The task force guidelines considered the magnitude of treatment-related harms to be at least moderate.4 However, the newly issued guidelines acknowledge that use of PSA screening has become a usual standard of care, and the decision to start or continue screening should be based on a process of shared decision-making between patients and physicians with a thorough discussion of the potential risks and benefits.4
Notably, in a recent survey of 125 primary care physicians, most respondents considered both patient age and estimated life expectancy when recommending PSA screening;5 however, they disagreed on the age at which to discontinue screening. About two-thirds of physicians (66.4%) indicated that it was difficult to assess life expectancy. The respondents also indicated several barriers to discontinuation of PSA screening; 74.4% cited patient expectations to continue yearly PSA tests, with 66.4% noting that it would take more time to explain reasons for not screening, compared with time required to simply continue screening, and 54.0% reported an increased sense of a risk for malpractice if they did not order a PSA test.5 These findings suggest that the controversy regarding PSA screening is likely to persist despite the recent update to the task force guidelines.
Prognosis
The most favorable prognosis is associated with low-risk disease, characterized by a PSA value of 10 ng/mL or less, a Gleason score of 6 or less, and clinical stage T1c or T2a.6,7 Patients at high risk of recurrence include those with clinically localized stage T3a disease with a Gleason score of 8 to 10 or a PSA level above 20 ng/mL.7 Although only 29.8% of men had a diagnosis of low-risk prostate cancer from 1989 to 1992, this rate increased to 45.3% by 2001.8 Patients with localized prostate cancer initially have favorable responses to active surveillance or treatment with either radical prostatectomy or radiation therapy. A multicenter longitudinal study of radical prostatectomy (N = 12,677) revealed an overall 12% prostate cancer–specific mortality (PCSM) rate, with a 95% confidence interval (CI) of 9% to 15%. Among patients with low-risk disease, the 15-year PCSM rate was 5% (95% CI, 3–7) compared with 38% (95% CI, 19–56) for men with high-risk disease.9
However, it is estimated that 20% to 40% of patients with a diagnosis of high-grade, clinically localized prostate cancer subsequently experience relapse,10–12 characterized by rising PSA levels following initial therapy. Furthermore, biochemical recurrence following prostatectomy, defined as a PSA level above 0.2 ng/mL,13 may progress to metastatic disease in 30% to 70% of men within 10 years of the initial diagnosis.10–12,14–18
The first-line treatment of patients with symptomatic, advanced-stage or metastatic prostate cancer relies on androgen-deprivation therapy (ADT). ADT can be accomplished by either surgical or medical castration with the continuous or intermittent administration of a luteinizing hormone–releasing hormone (LHRH) agonist with or without antiandrogen therapy.7 Although ADT is initially associated with a favorable response for most men, most patients eventually develop castrate-resistant (hormone-refractory) prostate cancer (CRPC). CRPC is characterized by serial increases in serum PSA levels, evidence of progression on radiographic evaluation, and the development of symptoms.19–21 The clinical course of CRPC is quite diverse; some patients develop nonmetastatic disease, whereas others experience asymptomatic or symptomatic metastatic CRPC.19,20
Multiple mechanisms are thought to promote progression to CRPC, including overexpression of the androgen receptor; mutations in androgen receptors that increase androgen sensitivity; and increased production of local androgens by prostate cells, attributed to the expression of steroidogenic enzymes.19,20 Other factors implicated in disease progression include (1) modulation of androgen receptor regulators, including co-activators (e.g., the p160 family of nuclear steroid receptor co-activators and NCOA2), (2) co-repressors (e.g., β-arrestin 2), and (3) the down-regulation of androgen receptor–related co-repressors.19,20
Activation of androgen receptor–independent pathways is also implicated in the development of CRPC, including the PI3K/Akt/mTOR pathway; the Ras/Raf/ERK pathway; and other pathways, such as transforming growth factor (TGF)-βR, WnT/β-catenin, Src kinase, and interleukin-6R.19,20 Evidence is also emerging to suggest that the processes of tumor cell growth, angiogenesis, and metastasis are related to an interaction between prostate cancer cells and the bone microenvironment.20 Patients at increased risk of progression to metastatic CRPC include those with rapid PSA doubling times and PSA levels above 20 ng/mL.14,15,22,23 Notably, the median survival of men with metastatic CRPC is 18 to 24 months.21,24,25 This article summarizes the efficacy and safety of newly approved and emerging systemic therapies for metastatic CRPC.
NEWLY APPROVED TREATMENTS
Most patients with metastatic CRPC require aggressive treatment to slow disease progression and to prevent metastasis. Before 2004, chemotherapy for metastatic CRPC did not improve survival, although treatment with mitoxantrone (Novantrone, EMD Serono) and prednisone or hydrocortisone was effective for alleviating pain associated with bone metastases.26,27 However, significant advances have been made during the decade in the development of alternative treatments for metastatic CRPC that are safe and effective, with the choice of treatment guided by the presence or absence of symptoms.7
Currently, four systemic agents have been shown to improve overall survival for patients with metastatic CRPC and have received approval by the FDA for this indication: docetaxel (Taxotere, Sanofi), sipuleucel-T (Provenge, Dendreon), cabazitaxel (Jevtana, Sanofi), and abiraterone acetate (Zytiga, Janssen).7 Favorable results for other systemic treatments for metastatic CRPC also have been reported and are pending submission to or approval by the FDA.
Docetaxel (Taxotere)
Docetaxel is the most frequently administered therapy for chemotherapy-naive patients with symptomatic, metastatic CRPC. Approved by the FDA in 2004, docetaxel is a semisynthetic taxane that inhibits microtubular depolymerization and phosphorylates Bcl-2, which leads to cell apoptosis.28,29 Two phase 3 trials established the efficacy of docetaxel for the treatment of metastatic CRPC: TAX 327 and SWOG 9916 (Southwest Oncology Group) (Table 1).28,30
Table 1.
Summary of Primary Endpoints for TAX 327 and Southwest Oncology Group (SWOG) 9916 Trials
| Trial | Treatment Group | Death Rate (%) | HR for Survival (95% CI) | Median Survival Duration (Months) |
|---|---|---|---|---|
|
| ||||
| TAX 327a | • Docetaxel 75 mg/m2 every 3 weeks | 50.0 | 0.76 (0.62–0.94) | 18.9 (17.0–21.2)* |
| • Docetaxel 30 mg/m2 every week | 57.0 | 0.91 (0.75–1.11) | 17.4 (15.7–19.0)* | |
| • Mitoxantrone 12 mg/m2 every 3 weeks | 60.0 | Reference | 16.5 (14.4–18.6)* | |
|
| ||||
| SWOG 9916b | • Docetaxel 60–70 mg/m2 estramustine 280 mg, and dexamethasone 60 mg every 3 weeks | 64.0 | 0.80 (0.67–0.97) | 17.5 |
| • Mitoxantrone 12–14 mg/m2 and prednisone 5 mg every 3 weeks | 70.0 | Reference | 15.6 | |
The TAX 327 Trial
TAX 327, a non-blinded, international study, enrolled patients with confirmed adenocarcinoma of the prostate gland that had progressed to metastatic disease while they were receiving ADT. Patients were randomly assigned to one of three groups: docetaxel 75 mg/m2 every 3 weeks (n = 335), docetaxel 30 mg/m2 once weekly (n = 334), or mitoxantrone 12 mg/m2 every 3 weeks (n = 337). All three groups also received oral prednisone or prednisolone 5 mg twice daily starting on day 1.
The median duration of follow-up was 20.8 months for patients who received docetaxel every 3 weeks and 20.7 months for those in the other two treatment arms. Median survival time was 18.9 months for docetaxel 75 mg/m2, 17.4 months for docetaxel 30 mg/m2, and 16.5 months for mitoxantrone. The difference in survival rates between the docetaxel 30-mg/m2 group and the placebo group was not statistically significant (P = 0.36). However, the hazard ratio (HR) for death in the combined docetaxel groups, compared with mitoxantrone, was 0.83 (95% CI, 0.70–0.99; P = 0.04).30
An updated analysis of survival in TAX 327 included follow-up results through March 2007 and confirmed that the docetaxel regimen maintained superior efficacy compared with mitoxantrone.31 The median survival rate for patients in the docetaxel 75 mg/m2 group was 19.2 months compared with 16.3 months for patients receiving mitoxantrone plus prednisone (HR, 0.79; 95% CI, 0.67–0.93; P = 0.004). The difference in median survival between these two groups was 2.9 months; 18.6% of patients receiving docetaxel 75 mg/m2 survived at least 3 years, compared with 16.8% of those treated with docetaxel 30 mg/m2 and 13.5% of those receiving mitoxantrone plus prednisone.31
The SWOG 9916 Trial
In the multicenter SWOG 9916 study, 770 men were randomly assigned to one of two treatment groups; 674 patients met study eligibility criteria, which included adenocarcinoma of the prostate and progressive metastatic disease following ADT.28 A total of 338 patients received estramustine (Emcyt, Pfizer) 280 mg three times daily, 1 hour before or 2 hours following meals on days 1 through 5, plus docetaxel 60 mg/m2 preceded by oral dexamethasone 60 mg in three divided doses with the first dose taken the night before docetaxel. Alternatively, patients (n = 336) received mitoxantrone 12 mg/m2 on day 1 plus prednisone 5 mg twice daily. Both regimens were given in 21-day cycles. The doses of docetaxel and mitoxantrone were increased to 70 mg/m2 and 14 mg/m2, respectively, if there were no occurrences of grade 3 or 4 adverse events during the first cycle.28
The intention-to-treat (ITT) analysis revealed a median survival of 17.5 months for docetaxel compared with 15.6 months for mitoxantrone and prednisone (P = 0.02). The HR for death was 0.80 (95% CI, 0.67–0.97) in favor of docetaxel. The median time to progression was 6.3 months for patients in the docetaxel arm compared with 3.2 months for patients in the mitoxantrone plus prednisone arm (P < 0.001).28 The results from TAX 327 and SWOG 9916 led to the FDA’s approval of docetaxel in 2004 for the treatment of chemotherapy-naive patients with metastatic CRPC.21
Cabazitaxel (Jevtana)
Cabazitaxel, which was approved for the treatment of metastatic CRPC by the FDA in 2010, is a tubulin-binding taxane that has established efficacy for the treatment of solid tumors that are resistant to docetaxel.32,33 An international, multicenter, randomized, open-label, phase 3 trial compared cabazitaxel plus prednisone with mitoxantrone plus prednisone for metastatic CRPC that had progressed following treatment with a docetaxel-based regimen.
Patients received either 21-day cycles of cabazitaxel 25 mg/m2 (n = 378) or mitoxantrone 12 mg/m2 (n = 377). Both groups also received prednisone 10 mg.33 The median follow-up period was 12.8 months. Median overall survival rates were 15.1 months (95% CI, 14.1–16.3) for cabazitaxel and 12.7 months (95% CI, 11.6–13.7) for mitoxantrone. This difference reflected a 30% reduction in relative risk of death for cabazitaxel (HR, 0.70; 95% CI, 0.59–0.83; P < 0.0001).
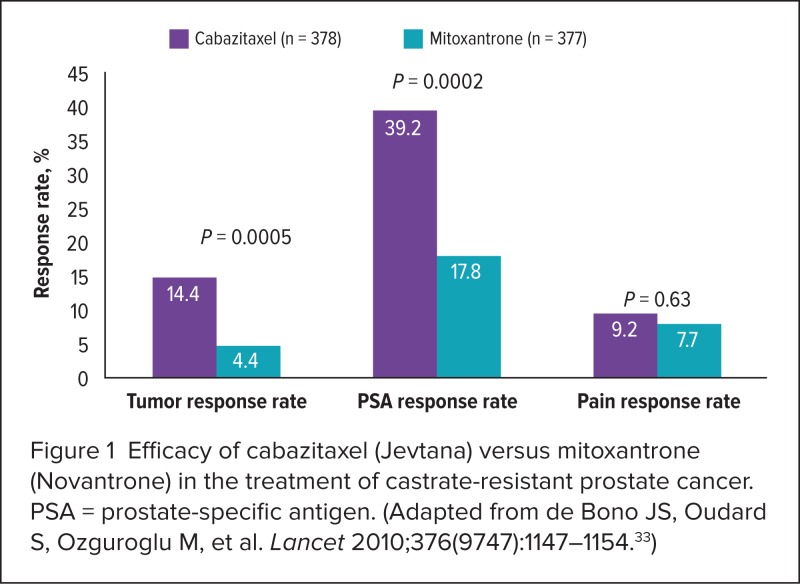
Median progression-free survival also favored cabazitaxel at 2.8 months (95% CI, 2.4–3.0) compared with 1.4 months (95% CI, 1.4–1.7) for mitoxantrone (HR, 0.74; 95% CI, 0.64–0.86; P < 0.0001). Additional endpoints regarding rates of tumor response, PSA progression, and pain response are presented in Figure 1 and Table 2.33
Figure 1.
Efficacy of cabazitaxel (Jevtana) versus mitoxantrone (Novantrone) in the treatment of castrate-resistant prostate cancer. PSA = prostate-specific antigen. (Adapted from de Bono JS, Oudard S, Ozguroglu M, et al. Lancet 2010;376(9747):1147–1154.33)
Table 2.
Disease Progression With Cabazitaxel (Jevtana) and Mitoxantrone (Novantrone)
| Treatment Group | ||||
|---|---|---|---|---|
| Progression endpoint | Cabazitaxel | Mitoxantrone | Hazard Ratio (95% CI) | P Value |
| Median time to tumor progression, months | 8.8 | 5.4 | 0.61 (0.49–0.76) | <0.0001 |
| Median time to PSA progression, months | 6.4 | 3.1 | 0.75 (0.63–0.90) | 0.001 |
| Median time to pain progression, months | Not reached | 11.1 | 0.91 (0.69–1.19) | 0.52 |
CI = confidence interval; PSA = prostate-specific antigen.
Adapted from de Bono JS, Oudard S, Ozguroglu M, et al. Lancet 2010;376(9747):1147–1154.33
Mortality rates owing to toxicities within 30 days following the last dose of study drug were higher for cabazitaxel (4.9%) than for mitoxantrone (2.4%).33 The most frequently reported hematological toxicities were as follows:
neutropenia, grade 3 or higher: 81.7% for cabazitaxel, 58.0% for mitoxantrone
leukopenia: 68.2% for cabazitaxel, 42.3% for mitoxantrone
anemia: 10.5% for cabazitaxel, 4.9% for mitoxantrone
Diarrhea (grade 3 or higher) affected 6.2% of cabazitaxel patients and fewer than 1.0% of men receiving mitoxantrone. Rates were comparable for nonhematological toxicities (grade 3 or above) between the two treatment groups.33
These results underscore the importance of monitoring, prophylactic interventions, and therapy for patients receiving cabazitaxel.33 Cabazitaxel causes nausea, vomiting, and severe diarrhea in some patients.32 Recommended supportive care for patients experiencing gastrointestinal toxicities include rehydration, antiemetic medications, and antidiarrheal agents, as well as adherence to current guidelines for administration of prophylactic white blood cell growth factor.7,32 In addition, treatment delays or dose reductions to 20 mg/m2 may be required for patients experiencing grade 3 or higher diarrhea.32
Cabazitaxel received FDA approval in 2010 for the treatment of men with metastatic CRPC who previously had been treated with a docetaxel-based regimen.32 The medication is discussed in this month’s Drug Forecast article on page 440.
Sipuleucel-T (Provenge)
Sipuleucel-T is an autologous active cellular immunotherapy consisting of peripheral blood mononuclear cells, including antigen-presenting cells (APCs), that have been activated with prostatic acid phosphatase (PAP) and granulocyte–macrophage colony-stimulating factor (GM–CSF), a recombinant human protein.34 Although the precise mechanism of action is not yet understood,34 it is hypothesized that the activated APCs promote endogenous T cells to destroy PAP-bearing prostate cancer cells.35 Sipuleucel-T is the first in a new class of cancer immunotherapeutic agents to be approved by the FDA (in 2010) for the treatment of asymptomatic or minimally symptomatic metastatic CRPC.20,34,35
A phase 3, multicenter, randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of sipuleucel-T compared with placebo.36 Eligible study participants included men with minimally symptomatic or asymptomatic metastatic CRPC who were expected to live at least 6 months. Patients were randomly assigned, in a 2:1 ratio, to receive sipuleucel-T (n = 341) or placebo (n = 171). Treatment was administered every 2 weeks for three cycles. The primary endpoint was overall survival, and the time to objective disease progression was a secondary endpoint.36
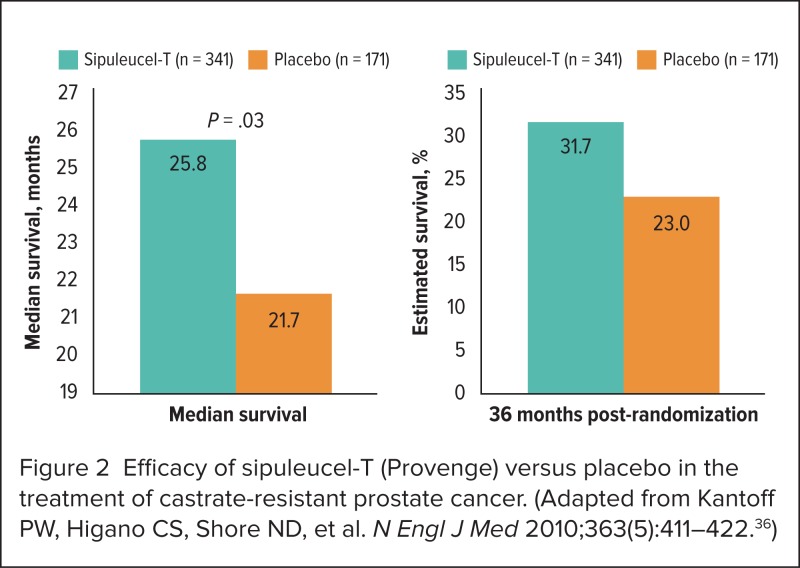
At the median follow-up of 34.1 months, a 22% reduction in mortality risk was evident for patients receiving sipuleucel-T (adjusted HR, 0.78; 95% CI, 0.61–0.98; P = 0.02). Median survival time and the estimated probability of survival 36 months following randomization are shown in Figure 2, which illustrates the superiority of sipuleucel-T compared with placebo.36 The most common adverse events affecting patients in the two treatment arms are summarized in Table 3.36
Figure 2.
Efficacy of sipuleucel-T (Provenge) versus placebo in the treatment of castrate-resistant prostate cancer. (Adapted from Kantoff PW, Higano CS, Shore ND, et al. N Engl J Med 2010;363(5):411–422.36)
Table 3.
Most Frequent Adverse Events in 20% or More Patients Receiving Sipuleucel-T (Provenge) or Placebo
| Adverse Event | Sipuleucel-T (n = 338) No. (%) | Placebo (n = 168) No. (%) | ||
|---|---|---|---|---|
| All Grades | Grade 3–5 | All Grades | Grades 3–5 | |
| Any | 334 (98.8) | 107 (31.7 | 162 (96.4) | 59 (35.1) |
| Chills | 183 (54.1) | 4 (1.2) | 21 (12.5) | 0 (0.0) |
| Fatigue | 132 (39.1) | 4 (1.2) | 21 (12.5) | 3 (1.8) |
| Back pain | 116 (34.3) | 12 (3.6) | 61 (36.3) | 8 (4.8) |
| Pyrexia | 99 (29.3) | 1 (0.3) | 23 (13.7) | 3 (1.8) |
| Nausea | 95 (28.1) | 2 (0.6) | 35 (20.8) | 0 (0.0) |
| Arthralgia | 70 (20.7) | 7 (2.1) | 40 (23.8) | 5 (3.0) |
| Citrate toxicity* | 68 (20.1) | 0 (0.0) | 34 (20.2) | 0 (0.0) |
Citrate toxicity is associated with leukapheresis. Paresthesia and oral paresthesia are considered to be likely symptoms of citrate toxicity.
Adapted from Kantoff PW, Higano CS, Shore ND, et al. N Engl J Med 2010;363(5):411–422.36
An earlier phase 3 trial enrolled 127 patients with asymptomatic CRPC and failed to achieve the primary endpoint of time to disease progression, with a median time to progression of 11.7 weeks (95% CI, 9.1–16.6) for sipuleucel-T and 10.0 weeks (HR, 1.45; 95% CI, 8.7–13.3; 95% CI, 0.99– 2.11 for placebo; P = 0.52).37 However, sipuleucel-T demonstrated a statistically significant survival advantage at the 36-month follow-up, with a median overall survival of 25.9 months (95% CI, 20.0–31.9) compared with 21.4 months (95% CI, 12.3–25.8) for the placebo group (HR, 1.70; 95% CI, 1.13–2.56; P = 0.01). In addition, estimated survival rates were 34% for those in the sipuleucel-T arm compared with 11% for those treated with placebo (P = 0.005) at the last assessment prior to censoring at the 36-month follow-up.37
Sipuleucel-T is recommended for patients with good performance status (i.e., a score of 0–1, according to Eastern Cooperative Oncology Group [ECOG] criteria), an estimated life expectancy of at least 6 months, no evidence of visceral disease, and no (or minimal) symptoms.7,34 Currently, sipuleucel-T is not considered an appropriate therapy for rapidly progressive prostate cancer.7
Abiraterone Acetate (Zytiga)
Abiraterone acetate is a selective inhibitor of cytochrome P450 C17 (CYP17), an enzyme required for the production of testosterone in the testis, adrenal glands, and prostate.20,21,38 It offers the convenience of oral administration. Results from phase 1 and 2 trials suggested that it was efficacious for the treatment of chemotherapy-naive patients with CRPC as well as those who had previously received a docetaxel-based regimen.20,21
A phase 3, randomized, international, double-blind, placebocontrolled trial compared abiraterone 1 g once daily plus prednisone 5 mg twice daily with placebo plus prednisone 5 mg twice daily.39 Eligible participants included men with confirmed metastatic CRPC who had previously received a docetaxel-based regimen and who had evidence of disease progression.34 The primary study endpoint was overall survival from randomization to death from any cause. Prespecified secondary endpoints included PSA response rate, time to PSA progression, and radiographically confirmed progression-free survival. A total of 1,195 patients were assigned, in a 2:1 ratio, to receive either abiraterone (n = 797) or placebo (n = 398).39
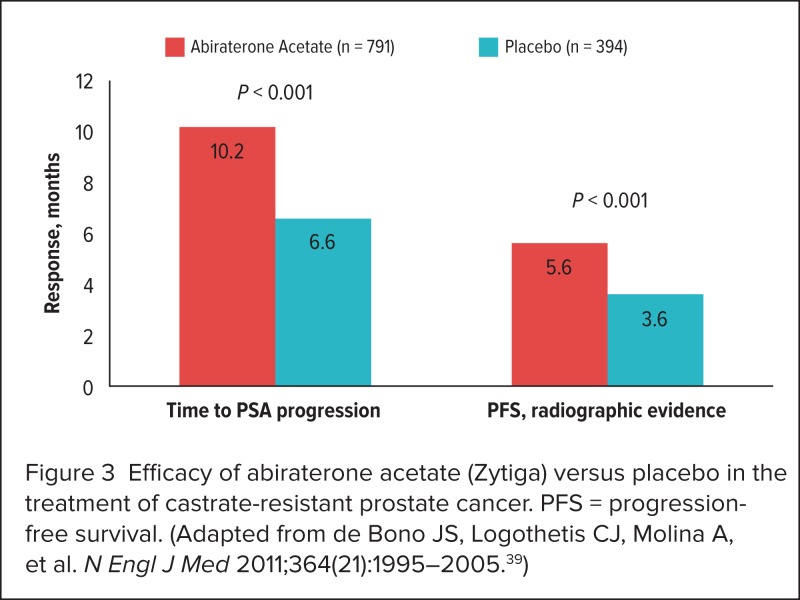
The preplanned interim analysis revealed a 35.4% decrease in mortality rates for abiraterone compared with placebo (HR, 0.65; 95% CI, 0.54–0.77; P < 0.001). Significant improvements for patients in the abiraterone arm were also reported for time to radiographic progression and PSA progression (Figure 3).39 The HR for time to PSA progression was 0.58 (95% CI, 0.46–0.73; P < 0.01), and the HR for radiographically confirmed progression-free survival was 0.67 (95% CI, 0.59–0.78; P < 0.001).
Figure 3.
Efficacy of abiraterone acetate (Zytiga) versus placebo in the treatment of castrate-resistant prostate cancer. PFS = progression-free survival. (Adapted from de Bono JS, Logothetis CJ, Molina A, et al. N Engl J Med 2011;364(21):1995–2005.39)
The PSA response rate for the abiraterone-treated patients was 29.0%, compared with 6.0% for the placebo group (P < 0.001). Adverse events affecting 20% or more of patients enrolled in the trial are presented in Table 4.39 The results of the interim analysis led to a recommendation by the data monitoring committee to unblind the study and to the FDA’s approval of abiraterone plus prednisone for the treatment of metastatic CRPC following therapy with a docetaxel-based regimen.21,38
Table 4.
Most Frequent Adverse Events Affecting 20% or More Patients Receiving Abiraterone Acetate (Zytiga) or Placebo
| Adverse Event | Abiraterone Acetate (n = 791) No. (%) | Placebo (n = 394) No. (%) | ||
|---|---|---|---|---|
| All Grades | Grades 3 and 4 | All Grades | Grades 3 and 4 | |
| Anemia | 178 (22.5) | 59 (7.5) | 104 (26.4) | 29 (7.4) |
| Thrombocytopenia | 28 (3.5) | 11 (1.4) | 13 (3.3) | 2 (0.5) |
| Diarrhea | 139 (17.6) | 5 (0.6) | 53 (13.5) | 5 (1.3) |
| Fatigue | 346 (43.7) | 66 (8.3) | 169 (42.9) | 39 (9.9) |
| Asthenia | 104 (13.1) | 18 (2.3) | 52 (13.2) | 8 (2.0) |
| Back pain | 233 (29.5) | 47 (5.9) | 129 (32.7) | 38 (9.6) |
| Nausea | 233 (29.5) | 13 (1.6) | 124 (3.1) | 10 (2.5) |
| Vomiting | 168 (21.2) | 14 (1.8) | 97 (24.6) | 11 (2.8) |
| Constipation | 206 (26.0) | 8 (1.0) | 120 (30.5) | 4 (1.0) |
| Arthralgia | 215 (27.2) | 33 (4.2) | 89 (22.6) | 16 (4.1) |
| Bone pain | 194 (24.5) | 44 (5.6) | 110 (27.9) | 29 (7.4) |
| Fluid retention and edema | 241 (30.5) | 18 (2.3) | 77 (19.5) | 4 (1.0) |
Adapted from de Bono JS, Logothetis CJ, Molina A, et al. N Engl J Med 2011;364(21):1995–2005.39
The COU-AA-302 Trial
COU-AA-302, an international, randomized, double-blind, phase 3 trial, was undertaken to compare abiraterone acetate 1,000 mg plus prednisone 5 mg twice daily with placebo.40 A total of 1,088 patients with metastatic CRPC who had not previously been treated with chemotherapy were assigned to one of the two study groups.
Co-primary endpoints included radiographic progression-free survival and overall survival. Results from a planned interim analysis revealed a statistically significant improvement in both primary endpoints for abiraterone as well as median time to opiate use, time to chemotherapy initiation, time to deterioration in ECOG performance status scores, and time to PSA progression.40
Notably, median time to radiographic progression-free survival was not reached for the abiraterone arm, compared with 8.3 months for the placebo group (HR, 0.43; 95% CI, 0.35–0.52; P < 0.0001), and median overall survival was not reached for patients receiving abiraterone (27.2 months for placebo) (HR, 0.75; 95% CI, 0.61–0.93; P = 0.0097).40
Abiraterone was approved in April 2011 in combination with prednisone for the treatment of patients with CRPC who had received prior chemotherapy with docetaxel. At the time of this publication, abiraterone was under review for approval by the FDA for the treatment of chemotherapy-naive patients with CRPC.
THERAPIES FOR BONE METASTASES
Zoledronic Acid (Zometa)
Metastatic prostate cancer frequently affects the bone and is associated with significant skeletal-related events (SREs), characterized by pathological fractures and spinal cord compression often necessitating radiation or surgery to provide pain relief. Until recently, the bisphosphonate zoledronic acid (Zometa, Novartis) was the mainstay of treatment for patients with bone metastases associated with CRPC.
A randomized, placebo-controlled, phase 3 trial was designed to compare the efficacy and safety of zoledronic acid at dosages of 4 mg and 8 mg with placebo administered once every 3 weeks. The study protocol was subsequently amended to reduce the dose of zoledronic acid to 4 mg because of renal toxicity associated with the 8-mg dose. All patients also received supplemental calcium 500 mg and vitamin D 400 to 500 IU daily. SREs occurred in 44% of patients receiving placebo and in 33.2% of those receiving zoledronic acid 4 mg, for a difference of −11.0% (95% CI, −20.3 to −1.8; P = 0.21).41
Importantly, zoledronic acid is excreted primarily via the renal system, and the risk of renal toxicities is increased for patients with impaired renal function. Zoledronic acid is contraindicated for patients with severe renal dysfunction, such as a baseline creatinine clearance (CrCl) below 30 mL/minute. Dose reductions are required for patients with baseline CrCl levels below 60 mL/minute.42
Denosumab (Xgeva)
Denosumab (Xgeva, Amgen) is a human monoclonal antibody that binds to the human receptor activator of nuclear factor kappa-B ligand (RANKL), a protein that contributes to the formation, function, and survival of osteoclasts. Denosumab inhibits the RANKL on the surface of osteoclasts, thereby preventing bone destruction.20,21,43 Denosumab has been approved for the prevention of SRE in patients with bone metastases caused by solid tumors, including prostate cancer.43
A randomized, multicenter, international, blinded, double dummy phase 3 trial compared denosumab with zoledronic acid.44 All patients had current or prior radiographic evidence of at least one bone metastasis and failure to respond to at least one prior hormonal therapy. A total of 1,904 patients were assigned, in a 1:1 ratio, to the two treatment groups: 950 received denosumab 120 mg plus intravenous placebo, and 951 received zoledronic acid 4 mg plus subcutaneous placebo, administered every 4 weeks. Dose adjustments for zoledronic acid, as recommended in the prescribing information, were made at baseline according to the Cockcroft–Gault formula for CrCl values.44
The median time to the first SREs in the denosumab group was 20.7 months (95% CI, 18.8–24.9) compared with 17.1 months for the zoledronic acid group (95% CI, 15.0–19.4). This reflected an 18% reduction in the time to the first SRE among patients treated with denosumab (HR, 0.82; 95% CI, 0.71–0.95; P = 0.0002 for non-inferiority and P = 0.0008 for superiority). A significant delay between the time of first and subsequent SREs was also evident for denosumab, compared with zoledronic acid, with a total of 494 SREs reported for denosumab and 584 SREs reported for zoledronic acid (rate ratio, 0.82; 95% CI, 0.71–0.94; adjusted P = 0.008).44
Overall rates of adverse events, serious adverse events, and fatal adverse events were comparable between the two treatment groups, although the rate of grade 3 or 4 adverse events was significantly higher for those in the denosumab arm (72%) compared with those in the zoledronic acid arm (66%) (P = 0.01). Of the patients who received denosumab, 13% experienced hypocalcemia, compared with 6% of those who received zoledronic acid (P < 0.0001).44
EMERGING THERAPIES IN PHASE 3 TRIALS
In addition to the four new treatment options for CRPC described earlier, several agents are in phase 3 trials, and preliminary results suggest that treatment options for CRPC will continue to expand in the near future. Two novel hormonal therapies—MDV3100 (enzalutamide, Medivation/Astellas) and TAK-700 (orteronel, Millennium Takeda Oncology)— target the androgen receptor pathway.20,21
Phase 3 trials are also under way for two immunotherapeutic agents: ipilimumab (Yervoy, Bristol-Myers Squibb), which was approved in 2011 for the treatment of melanoma, and ProstVac (Bavarian Nordic), a vaccine. Radium-223 chloride (Alpharadin, Algeta/Bayer) is under evaluation in a phase 3 trial for patients with symptomatic CRPC and bone metastases.18,19
Hormonal Therapies
MDV3100 (Enzalutamide)
MDV3100 is an oral, second-generation, selective androgen receptor antagonist with favorable preclinical and early phase clinical trial results for the treatment of metastatic CRPC. Two phase 3 trials are ongoing: PREVAIL (Efficacy and Safety Study of Oral MDV3100 in Chemotherapy-Naive Patients With Progressive Metastatic Prostate Cancer Who Have Failed Androgen Deprivation Therapy) and AFFIRM (Safety and Efficacy Study of MDV3100 in Patients With Castration-Resistant Prostate Cancer Who Have Been Previously Treated With Docetaxel-Based Chemotherapy).
The PREVAIL Trial. Results are pending from PREVAIL. This study is being conducted to evaluate of MDV3100 in men with CRPC who have not been previously treated with chemotherapy.45
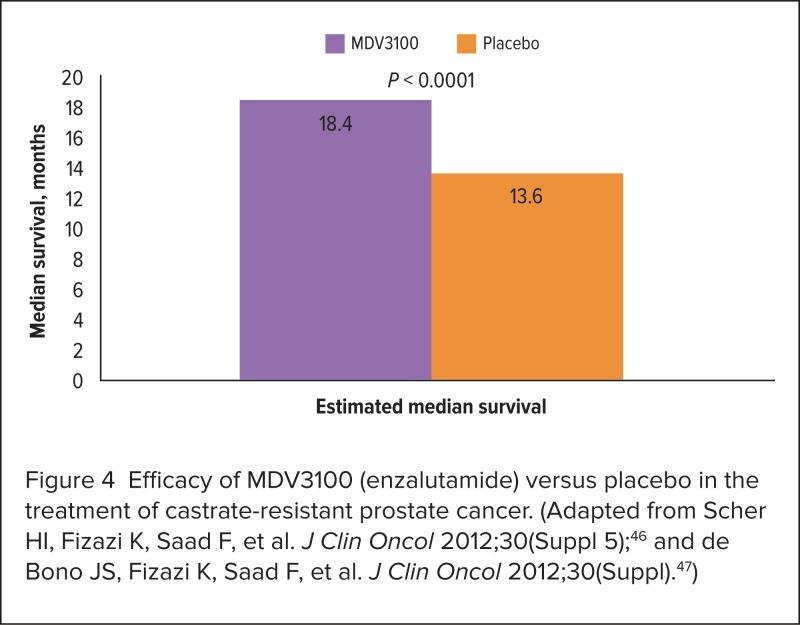
The AFFIRM trial. In this randomized, double-blind, placebo-controlled, international study, 1,199 patients were assigned, in a 2:1 ratio, to receive MDV3100 160 mg/day or placebo. The planned interim analysis, at 520 deaths, revealed a 37% reduction in mortality risk with MDV3100 compared with placebo (HR, 0.631; P < 0.0001).46,47 Figure 4 illustrates the estimated median survival times for both groups, revealing a median difference in overall survival of 4.8 months.46,47
Figure 4.
Efficacy of MDV3100 (enzalutamide) versus placebo in the treatment of castrate-resistant prostate cancer. (Adapted from Scher HI, Fizazi K, Saad F, et al. J Clin Oncol 2012;30(Suppl 5);46 and de Bono JS, Fizazi K, Saad F, et al. J Clin Oncol 2012;30(Suppl).47)
Progression-free survival, based on radiographic results, was 60% longer for the MDV3100 group at 8.3 months, compared with 2.9 months for the placebo group (HR = 0.404; 95% CI, 0.350–0.466; P < 0.0001). Median time to PSA progression was 8.3 and 3.0 months for MDV3100 and placebo, respectively (HR, 0.248; 95% CI, 0.204–0.303; P < 0.0001). Partial responses were evident for 25.1% of patients treated with MDV3100 and for 2.9% of those treated with placebo.
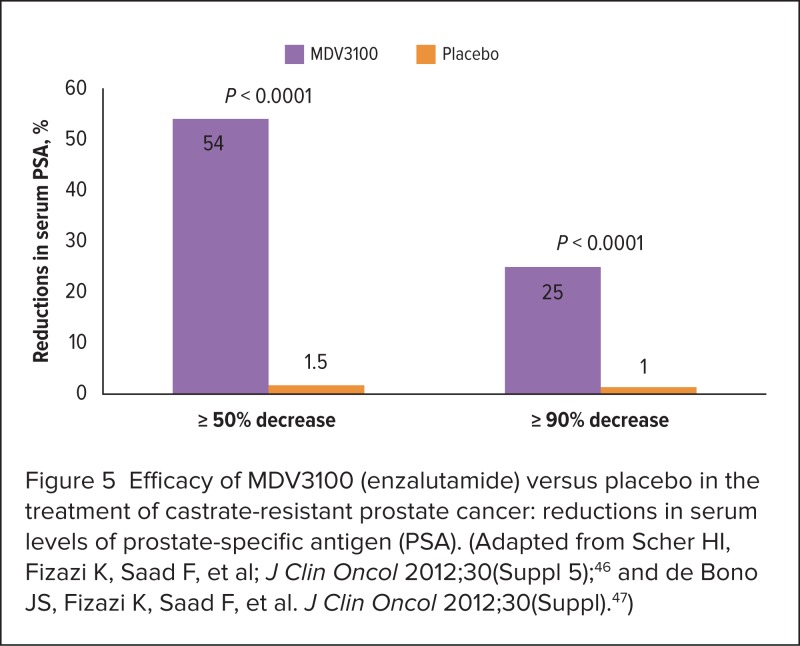
Complete responses were reported for 3.8% of patients in the MDV3100 group and for 1.0% of the placebo group (P < 0.0001).47 Patients who received MDV3100 also experienced significant reductions in serum PSA levels (Figure 5).
Figure 5.
Efficacy of MDV3100 (enzalutamide) versus placebo in the treatment of castrate-resistant prostate cancer: reductions in serum levels of prostate-specific antigen (PSA). (Adapted from Scher HI, Fizazi K, Saad F, et al; J Clin Oncol 2012;30(Suppl 5);46 and de Bono JS, Fizazi K, Saad F, et al. J Clin Oncol 2012;30(Suppl).47)
Higher rates of fatigue, diarrhea, and hot flushes (all grades) were reported for those in the MDV3100 arm. Assessment of grade 3 or higher adverse events revealed cardiac disorders (0.9% for MDV3100 and 2.0% for placebo), fatigue (6.0% with MDV3100 vs. 7.0% with placebo), seizures (0.6% with MDV3100 and 0.0% with placebo), and abnormal liver function test results (0.4% with MDV3100 and 0.8% with placebo).47
TAK-700 (Orteronel)
TAK-700, an oral inhibitor of CYP17, has demonstrated reductions in serum PSA levels and partial tumor responses in a phase 1/2 trial of 96 men with metastatic CRPC.48 Based on these favorable responses, Millennium is recruiting participants for two multinational randomized, double-blind phase 3 studies—ELM–PC (Evaluation of the Lyase inhibitor in Metastatic Prostate Cancer).49
The C21004 Trial. C21004 is designed to compare TAK-700 with placebo for chemotherapynaive patients with metastatic CRPC. This randomized, double-blind, multicenter trial is expected to recruit 1,454 study participants. Primary endpoints are radiographic progression-free survival and overall survival. Secondary endpoints are PSA response at 12 weeks, changes in circulating tumor cell counts, and time to pain progression.50
The C21005 Trial. TAK-700 is combined with prednisolone or placebo to treat men with metastatic CRPC that has progressed despite previous docetaxel-based therapy.50,51 Approximately 1,083 men will be recruited. The primary endpoint is overall survival; secondary endpoints are PSA response and pain response at 12 weeks as well as radiographic progression-free survival from randomization to disease progression or death.51
Immunotherapies
Ipilimumab (Yervoy)
Ipilimumab is a human anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) monoclonal antibody that inhibits activation of CTLA-4, which promotes a T-cell–mediated immune response.20,21 Results from a phase 2 trial demonstrated greater reductions in serum PSA levels at 3 months for patients with advanced prostate cancer who were treated with ADT plus ipilimumab (55%) compared with those who received ADT alone (38%).52
Two randomized, double-blind, multicenter, phase 3 trials are currently under way to further evaluate the role of ipilimumab for the treatment of advanced CRPC. The first trial expects to enroll an estimated 800 patients with CRPC and at least one bone metastasis following progression after docetaxel therapy to compare ipilimumab with placebo following radiation therapy. The primary study endpoint is overall survival, and secondary endpoints include progression-free survival, pain response, and safety.53
The second randomized, double-blind, phase 3 trial is being conducted to evaluate the efficacy and safety of ipilimumab compared with placebo in asymptomatic or minimally symptomatic patients with metastatic CRPC who have not received prior chemotherapy.54 Overall survival has been established as the primary study endpoint. Progression-free survival, pain response, and safety are included as secondary endpoints.
Approximately 600 patients are expected to be enrolled and observed from randomization to date of death.54
ProstVac
ProstVac is a novel cancer vaccine consisting of fowlpox and vaccinia PSA vaccines administered in combination with three co-stimulatory molecules, including intracellular adhesion molecule-1, B7, and leukocyte function-associated antigen.20,55 A phase 2 trial was conducted with 122 patients who had mildly symptomatic metastatic CRPC. These patients were randomly assigned to receive either ProstVac (n = 82) with GM–CSF or a control vaccine (n = 40). Although progression-free survival did not differ significantly between the two treatment arms (P = 0.60), patients receiving ProstVac demonstrated better overall survival; 30% were still living at the 3-year follow-up compared with 17% of the control group. In addition, median survival was 8.5 months longer with ProstVac than with the control vaccine (25.1 vs. 16.6 months, respectively; HR = 0.56; 95% CI, 0.37–0.85; log-rank P = 0.0061).55
These promising results led to the initiation of a randomized, double-blind phase 3 trial of ProstVac alone, ProstVac plus GM–CSF, or placebo for men with asymptomatic or minimally symptomatic metastatic CRPC. A total of 1,200 patients will be recruited for trial enrollment with overall survival the primary study endpoint. A secondary endpoint is the proportion of patients in each of the two ProstVac groups who do not experience radiological progression of disease, pain progression, initiation of chemotherapy, or death at 6 months compared with placebo.56
Bone-Targeting Agents
Radium-223 (Alpharadin)
Results from a phase 2 trial of radium-223, a bone-seeking radionuclide, offer a promising treatment alternative for bone metastases associated with CRPC.57 Patients received four injections of radium-223 (n = 33) or placebo (n = 31) on 4-week cycles. The primary endpoints were changes in bone–alkaline phosphatase concentrations and time to SREs. Secondary endpoints focused on safety, serum markers of bone turnover, time to PSA progression, and overall survival.57
The median relative change in bone–alkaline phosphatase levels, from baseline to 4 weeks following the last study injection, was −65.6% (95% CI, −69.5 to −57.7) for the radium-223 group compared with 9.3% (95% CI, 3.8–60.9) for controls (P < 0.0001). The adjusted HR for time to the first SRE was 1.75 (95% CI, 0.96–3.19; P = 0.065).
The median times to the first SRE were 14 weeks for the radium-223 group (95% CI, 9–30) and 11 weeks for the control group (95% CI, 5–25).
The median times to PSA progression were 26 weeks for the radium-223 group (95% CI, 16–39) and 8 weeks for the control group (95% CI, 4–12) (P = 0.048). The adjusted HR for overall survival was 2.12 (95% CI, 1.13–3.98; P = 0.020). Median overall survival was 65.3 weeks for patients treated with radium-223 compared with 46.4 weeks for those in the control arm (log-rank P = 0.66).57
The ALSYMPCA Trial. ALpharadin in SYMptomatic Prostate CAncer, a phase 3, double-blind, randomized, international trial, is being conducted to compare radium-223 plus best standard of care with placebo and best standard of care for patients with bone metastases associated with CRPC. In a 2:1 ratio, patients receive six injections of radium-223 (50 kilobecquerels ([kBq/kg]) or placebo every 4 weeks. A total of 921 patients were included (614 receiving radium-223 and 307 receiving placebo).58
Significant improvements were reported for overall survival in the radium-223 arm at 14.9 months compared with 11.3 months for the placebo arm (HR, 0.695; 95% CI, 0.581–0.832; P = 0.00007). In addition, the time to the first SRE was 15.6 months for radium-223 and 9.8 months for placebo (HR, 0.658; 95% CI, 0.552–0.830; P = 0.00037). The safety profile was favorable for radium-223, with 2.2% of patients experiencing grade 3 or 4 myelosuppression compared with 0.7% of those treated with placebo. Rates of grade 3 or 4 thrombocytopenia were 6.3% for the radium-223 group and 2.0% for the placebo arm.58
In additional analyses of ALSYMPCA, radium-223 significantly delayed time to the first SRE (13.6 months) compared with placebo (8.4 months) (HR, 0.610; 95% CI, 0.461–0.807; P = 0.00046).59 Radium-223 has been granted a fast-track approval designation by the FDA for the treatment of bone metastases associated with CRPC.
CONCLUSION
Tremendous strides have been made in the identification of new treatments for CRPC and bone-related metastases. Four new agents, including cabazitaxel, sipuleucel-T, abiraterone acetate, and denosumab received FDA approval for CRPC or SRE since 2010, and five new treatments have demonstrated positive results in phase 3 trials, suggesting that additional therapies may soon be approved for the management of metastatic CRPC. However, research has not yet clarified which patients will achieve optimal benefit from these agents. Specifically, there are no predictive models or biomarkers to identify patients who are likely to benefit from approved and anticipated new regimens, including those that target skeletal complications that are characteristic of metastatic CRPC.20,21
Of particular concern is the determination of the appropriate sequencing for administration of newly approved and emerging therapies.19–21 Algorithms for risk stratification and development of nomograms to estimate individual probabilities of survival will play a key role in facilitating physicians’ efforts to select the optimal therapy for individual patients.20
Research is also needed to develop and refine the application of biologic and clinical measures of treatment response, including tumor imaging; PSA measures such as doubling time; and biomarkers of angiogenesis, inflammatory cytokines, and bone turnover. These measures should be augmented by assessments of patient quality of life such as pain, the use of medications for pain, and patient-reported quality-of-life measures.20
It is likely that the most favorable clinical and safety outcomes will be achieved from the sequential use of multiple agents. Accomplishing this goal will require multidisciplinary health care teams, including urologists, medical oncologists, and radiation oncologists, to achieve continuity and a continuum of care. Assessment of patient preferences regarding treatment options, as well as regular communication among all members of the care team, will be essential to achieve the best clinical outcomes for all patients.20
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Research Program, National Cancer Institute; Fast Stats: An interactive tool for access to SEER cancer statistics. Available at: http://seer.cancer.gov/faststats. Accessed June 2012. [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2008. Bethesda, Md: National Cancer Institute; Available at: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission. Accessed June 2012. [Google Scholar]
- 4.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012 May 21; doi: 10.7326/0003-4819-157-2-201207170-00459. (online). [DOI] [PubMed] [Google Scholar]
- 5.Pollack CE, Platz EA, Bhavsar NA, et al. Primary care providers’ perspectives on discontinuing prostate cancer screening. Cancer. 2012 Apr 19; doi: 10.1002/cncr.27577. (online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology Prostate Cancer. Version 3.2012. Washington, D.C.: Available at: http://guidelines.nccn.org. Accessed July 11, 2012. [Google Scholar]
- 8.Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: Trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27(26):4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 11.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169(2):517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 12.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291(22):2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 13.Cookson MS, Aus G, Burnett Al, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177(2):540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 14.Antonarakis ES, Trock BJ, Feng Z, et al. The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: 25-year follow-up (Abstract 5008) J Clin Oncol. 2009;27(Suppl.) [Google Scholar]
- 15.D’Amico AV, Cote K, Loffredo M, et al. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20(23):4567–4573. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Roach M, Lu J, Pilepich MV, et al. Four prognostic groups predict long-term survival from prostate cancer following radiotherapy alone on Radiation Therapy Oncology Group clinical trials. Int J Radiat Oncol Biol Phys. 2000;47(3):609–615. doi: 10.1016/s0360-3016(00)00578-2. [DOI] [PubMed] [Google Scholar]
- 17.Schellhammer PF, Moriarty R, Bostwick D, et al. Fifteen-year minimum follow-up of a prostate brachytherapy series: Comparing the past with the present. Urology. 2000;56(3):436–439. doi: 10.1016/s0090-4295(00)00711-1. [DOI] [PubMed] [Google Scholar]
- 18.Uchio EM, Aslan M, Wells CK, et al. Impact of biochemical recurrence in prostate cancer among U.S. veterans. Arch Intern Med. 2010;170(15):1390–1395. doi: 10.1001/archinternmed.2010.262. [DOI] [PubMed] [Google Scholar]
- 19.Antonarakis ES, Armstrong AJ. Emerging therapeutic approaches in the management of metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2011;14(3):206–218. doi: 10.1038/pcan.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George D, Moul JW. Emerging treatment options for patients with castration-resistant prostate cancer. Prostate. 2012;72(3):338–349. doi: 10.1002/pros.21435. [DOI] [PubMed] [Google Scholar]
- 21.Higano CS, Crawford ED. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol. 2011;29(6 Suppl):S1–S8. doi: 10.1016/j.urolonc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351(2):125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 23.Potters L, Morgenstern C, Calugaru E, et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2008;179(5 Suppl):S20–S24. doi: 10.1016/j.juro.2008.03.133. [DOI] [PubMed] [Google Scholar]
- 24.Mostaghel EA, Montgomery B, Nelson PS. Castration-resistant prostate cancer: Targeting androgen metabolic pathways in recurrent disease. Urol Oncol. 2009;27(3):251–257. doi: 10.1016/j.urolonc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: Locking up the molecular escape routes. Clin Cancer Res. 2009;15(10):3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 26.Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: Results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17(8):2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 27.Tannock IF, Osaba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 28.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 29.Pienta KJ, Fisher EI, Eisenberger MA, et al. A phase II trial of estramustine and etoposide in hormone refractory prostate cancer: A Southwest Oncology Group trial (SWOG 9407) Prostate. 2001;46(4):257–261. doi: 10.1002/1097-0045(20010301)46:4<257::aid-pros1031>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 31.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 32.Jevtana (cabazitaxel), prescribing information. Bridgewater, N.J.: Sanofi-Aventis; Jun, 2010. [Google Scholar]
- 33.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomized openlabel trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 34.Provenge (sipuleucel-T), prescribing information. Seattle, Wash.: Dendreon Corp.; Jun, 2011. [Google Scholar]
- 35.Garcia JA, Dreicer R. Immunotherapy in castration-resistant prostate cancer: Integrating sipuleucel-T into our current treatment paradigm. Oncology (Williston Park) 2011;25(3):242–249. [PubMed] [Google Scholar]
- 36.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 37.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 38.Zytiga (abiraterone acetate), prescribing information. Horsham, Pa.: Janssen/Centocor Ortho Biotech Inc.; Apr, 2011. [Google Scholar]
- 39.de Bono JS, Logothetis CJ, Molina A, et al. for the COU-AA-301 Investigators Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan CJ, Smith MR, De Bono JS, et al. for the COU-AA-302 Investigators Interim analysis (IA) results of COU-AA-302, a randomized, phase III study of abiraterone acetate (AA) in chemotherapy-naive patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) (Abstract LBA4518) J Clin Oncol. 2012;30(Suppl) [Google Scholar]
- 41.Saad F, Gleason DM, Murray R, et al. for the Zoledronic Acid Prostate Cancer Study Group A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 42.Zometa (zoledronic acid), prescribing information. East Hanover, N.J.: Novartis; 2012. [Google Scholar]
- 43.Xgeva (denosumab), prescribing information. Thousand Oaks, Calif.: Amgen Inc.; 2010. [Google Scholar]
- 44.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377(9768):813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medivation. PREVAIL: Pre-chemotherapy MDV3100 Prostate Cancer Trial. Available at: http://yourprostatecancer.com. Accessed June 2012.
- 46.Scher HI, Fizazi K, Saad F, et al. for the AFFIRM Investigators Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer post docetaxel: Results from the phase III AFFIRM study (Abstract LBA1) J Clin Oncol. 2012;30(Suppl 5) [Google Scholar]
- 47.de Bono JS, Fizazi K, Saad F, et al. for the AFFIRM Investigators Primary, secondary, and quality-of-life endpoints from the phase III AFFIRM study of MDV3100, an androgen receptor signaling inhibitor (Abstract 4519) J Clin Oncol. 2012;30(Suppl) [Google Scholar]
- 48.Agus DB, Stadler WM, Shevrin DH, et al. Safety, efficacy, and pharmacodynamics of the investigational agent TAK-700 in metastatic castration resistant prostate cancer (mCRPC): Updated data from a phase I/II study. ASCO Annual Meeting, Abstract 4531. J Clin Oncol. 2011;29(Suppl) [Google Scholar]
- 49.Takeda. Anti-Prostate Cancer Agent Orteronel (TAK-700) Enters into Phase III Clinical Trials in Japan Anti-prostate Cancer Agent. Jan 27, 2012. Available at: www.takeda.com/press/article_44988.html. Accessed June 2012.
- 50.ClinicalTrials.gov. Study comparing orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT01193244?term=TAK-700+AND+prostate+cancer&phase=2&rank=3. Accessed June 2012. [DOI] [PubMed]
- 51.ClinicalTrials.gov. Study comparing orteronel plus prednisone in patients with metastatic castration-resistant prostate cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT01193257?term=TAK-700+AND+prostate+cancer&phase=2&rank=2. Accessed June 2012.
- 52.Tollefson MK, Karnes RJ, Thompson RH, et al. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer. American Society of Clinical Oncology Genitourinary Cancers Symposium; San Francisco. March 5–7, 2010. [Google Scholar]
- 53.ClinicalTrials.gov. Study of immunotherapy to treat advanced prostate cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT00861614?term=ipilimumab+and+prostate&phase=2&rank=2. Accessed June 2012.
- 54.ClinicalTrials.gov. Phase 3 study of immunotherapy to treat advanced prostate cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT01057810?term=ipilimumab+and+prostate&phase=2&rank=1. Accessed June 2012.
- 55.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviralbased PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ClinicalTrials.gov. A phase 3 efficacy study of a recombinant vaccinia virus vaccine to treat metastatic prostate cancer (prospect) Available at: http://clinicaltrials.gov/ct2/show/NCT01322490?term=Prostvac+and+prostate&phase=2&rank=1. Accessed June 2012.
- 57.Nilsson S, Franzen L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomized, multicenter, placebo-controlled phase II study. Lancet Oncol. 2007;8(7):587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 58.Parker C, Nilsson S, Heinrich D, et al. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA) J Clin Oncol. 2012;30(Suppl) LBA4512. [Google Scholar]
- 59.Sartor AO, Heinrich D, O’Sullivan JM, et al. Radium-223 chloride (Ra-223) impact on skeletal-related events (SREs) and ECOG performance status (PS) in patients with castration-resistant prostate cancer (CRPC) with bone metastases: Interim results of a phase III trial (ALSYMPCA) (Abstract 4551) J Clin Oncol. 2012;30(Suppl) [Google Scholar]