Abstract
The major cause for plaque instability in atherosclerotic disease is neoangiogenic revascularization, but the factors controlling this process remain only partly understood. Hedgehog (HH) is a morphogen with important functions in revascularization, but its function in human healthy vessel biology as well as in atherosclerotic plaques has not been well investigated. Hence, we determined the status of HH pathway activity both in healthy vessels and atherosclerotic plaques. A series of 10 healthy organ donor–derived human vessels, 17 coronary atherosclerotic plaques and 24 atherosclerotic carotid plaques were investigated for HH pathway activity. We show that a healthy vessel is characterized by a high level of HH pathway activity but that atherosclerotic plaques are devoid of HH signaling despite the presence of HH ligand in these pathological structures. Thus, a dichotomy between healthy vessels and atherosclerotic plaques with respect to the activation status of the HH pathway exists, and it is tempting to suggest that downregulation of HH signaling contributes to long-term plaque stability.
INTRODUCTION
Cardiovascular disease is the leading cause of death for both men and women in much of the Western world and is predicted to be the leading global killer by 2020 (1). Atherosclerosis is responsible for coronary heart disease, most strokes and limb ischemia. A large number of studies have shown thrombosis to result from plaque instability. Thrombotic events (that follow fissure or rupture of the fibrous cap with propagation of the thrombus upstream from the site of cap rupture) are the underlying basis for acute disease in the majority of cases involving atherosclerosis (2). Thus, understanding atherosclerotic plaque stability is highly relevant. Plaques that rupture show increased adventitial and plaque neovascularization, suggesting that neoangiogenesis contributes to their instability (3) and anti-neoangiogenic therapy is emerging as a promising novel avenue for treating plaque instability (4,5). This approach is hampered by lack of insight into the activity of endogenous angiogenic signaling pathways.
Among the molecular signals involved in revascularization, the Hedgehog (HH) pathway is featured prominently (6). HH constitutes a family of highly hydrophobic secreted morphogenetic hormones of which in humans three isoforms exist: Sonic (SHH), Indian (IHH) and Desert (DHH) (7). Their functions include maintenance of the endothelial compartment and orchestrating revascularization upon vessel occlusion–induced ischemia (8). After delivery to target cells, HH binds to its transmembrane receptor Patched-1 (PTCH1). Under HH-unligated conditions, PTCH1 keeps a second transmembrane receptor called Smoothened (SMO) inactive (9). In mammals, this probably involves translocation of a vitamin D3–like 3-β hydroxysteroid (10). After binding of HH to PTCH1, this inhibition is relieved and SMO subsequently initiates signaling through the glioma-associated (GLI) transcription factors, resulting in the transcription of target genes, including both GLI1 and PTCH1 (Figure 1) (10). The in vivo relevance of the induction of these genes was proven in ischemia experiments, in which intramyocardial gene transfer of SHH promoted recovery and preservation of left ventricular function in myocardial ischemia by enhanced neovascularization (11), supporting the notion that HH aids in ischemic tissue rescue and especially in its revascularization. In agreement, HH production is critical for VEGF production (9,12) and endothelial maintenance and growth (9,13–17). From this, we can infer that HH signaling may well play a detrimental role as well, causing revascularization-mediated plaque instability, and constitutes an attractive candidate for targeting pathological neovascularization (18). Importantly, the activity of the HH pathway in atherosclerotic structures has not been investigated.
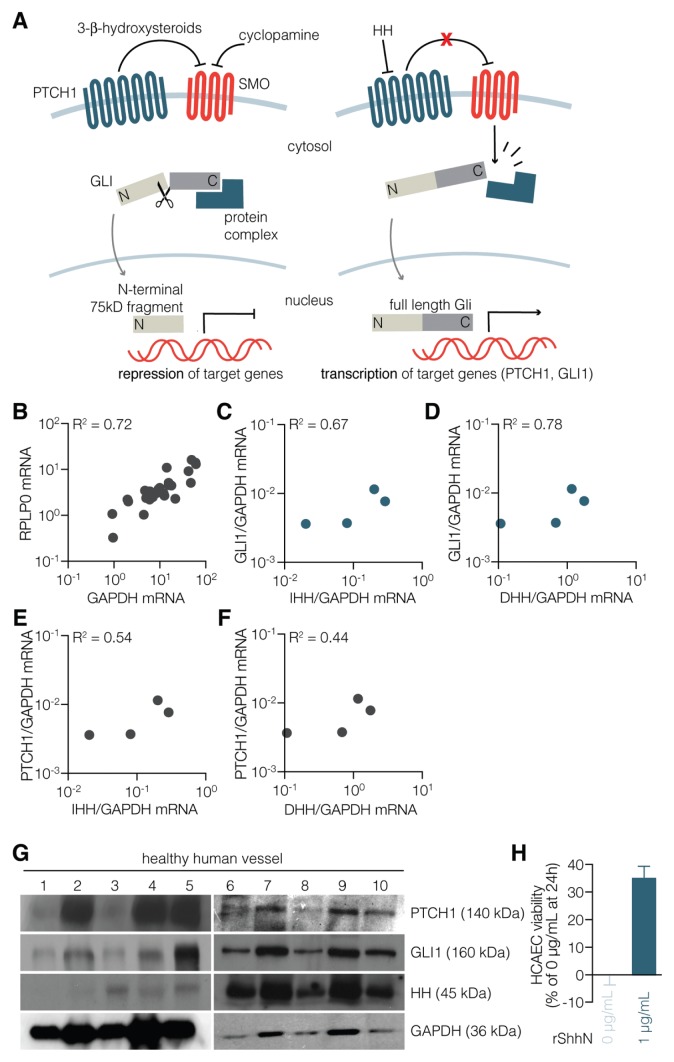
Figure 1.
High activity of the HH pathway is characteristic of healthy human vessels. (A) Schematic depiction of HH signal transduction in the absence (left panel) and presence of ligand (right panel). (B) Comparison of GAPDH and RFLP0 expression levels in healthy vessels and plaques. HH pathway target genes GLI1 (C, D) and PTCH1 (E, F) transcript levels were correlated with the expression levels of IHH (C, E) and DHH (D, F) in renal arteries from four healthy organ donors. (G) HH pathway activity in renal arteries from 10 healthy organ donors (sequentially numbered) was assessed using Western blot for the target genes. (H) Human coronary endothelial cells were cultured according to routine procedures for 24 h with either vehicle control or recombinant ShhN (1 μg/mL). Cell viability was evaluated by MTT reduction. Data are means ± standard error of the mean (n = 3) (p < 0.05). Because expression levels of the routinely used GAPDH housekeeping gene may be changed depending on HH pathway activity, we first compared GAPDH levels with RPLP0 levels, since the latter gene was suggested to be a better housekeeping gene when studying HH pathway activity (27). As shown in (B), GAPDH and RPLP0 levels correlate well in all samples used for this study, and we thus considered GAPDH an appropriate housekeeping gene.
These considerations prompted us to investigate the activity of the HH pathway in healthy human vessels as well as in atherosclerotic pathology. We found that normal vessels are characterized by substantial HH pathway activity in response to ligand present in this tissue, but that in atherosclerotic plaques, this activity is downregulated, leading to a functional dichotomy. We propose that the downregulation of HH activity in plaques, despite the presence of ligand, may serve as a protective response of the body to mitigate the risk posed by atherosclerotic disease by enhancing plaque stability.
MATERIAL AND METHODS
Clinical Material
To obtain healthy vessels, slices of renal arteries from family kidney transplantation donors were stored in cold preservation solution, and vessels were prepared by removing connective tissue, fat and lateral vessels. After opening each vessel lengthwise, the adventitia side was dried with sterile gauze. One layer of the gauze was left on the adventitia side and cut off around the vessel, after which material was stored at −80°C. Carotid plaques were obtained from subjects undergoing carotid endarterectomy for symptomatic high-grade stenosis of the internal carotid artery. Plaques were freshly harvested and divided into sections of 5-mm thickness and stored at −80°C. Percutaneous coronary atherectomy was performed in de novo lesions by means of a pullback atherectomy catheter (Arrow, Reading, PA, USA). Briefly, this 10-french compatible over-the-wire catheter is positioned beyond the lesion to treat. After the cutting blade is exposed and on-axis high-speed rotation is started, the catheter is gently withdrawn by manual pullback. This procedure allows complete circumferential cuts along the whole length of the lesion, thus providing in vivo plaque samples, suitable for analysis. In all patients, one biopsy was taken out of the culprit lesion. All studies were done according to national and institutional ethical guidelines.
Quantitative Reverse Transcriptase–Polymerase Chain Reaction
RNA isolation from clinical samples was performed using TRIzol according to the manufacturer recommendations. The RNA was dissolved in 30 μL RNAse-free water and stored at −80°C. RNA concentration was checked using a Nanodrop spectrophotometer (Isogen, Life Science, De Meern, the Netherlands). For cDNA synthesis, 1 μg RNA was used with an end volume of 20 μL. Subsequently, quantitative polymerase chain reactions (PCRs) detecting the expression level of different proteins were performed on a Roche LightCycler 480 system using the following primers (“h” stands for “human”): hRPLP0 (RPLP0: ribosomal protein, large, P0) forward, GGCAC CATTG AAATC CTGAG TGATG TG; hRPLP0 reverse, TTGCG GACAC CCTCC AGGAAGC; hGAPDH forward, AAGGT GAAGG TCGGA GTCAA C; hGAPDH reverse, TGGAA GATGG TGATG GGATT; hGLI1 forward, GTTCA CATGC GCAGA CACAC T; hGLI1 reverse, TTCGA GGCGT GAGTA TGACT T; hPTCH1 forward, CGGCA GCCGC GATAA G; hPTCH1 reverse, TTAAT GATGC CATCT GCATC C; hSHH forward, GCTCG GTGAA AGCAG AGAAC; hSHH reverse, CCAGG AAAGT GAGGA AGTCG; hIHH forward, CACCC CCAAT TACAA TCCAG; hIHH reverse, CGGTC TGATG TGGTG ATGTC; hDHH forward, ACCAA TCTAC TGCCC CTGTG; hDHH reverse, GTTGT AGTTG GGCAC GAGGT.
Cell Culture and MTT Assay
Primary human coronary arterial endothelial cultures (HCAECs) were maintained per provider suggestions (Lonza, Verviers, Belgium). In short, HCAECs were grown in EGM-2MV medium (Lonza, Verviers, Belgium) supplemented with 5% fetal calf serum, growth factors, hydrocortisone and gentamicine, at 37°C in a 5% CO2 humidified atmosphere. After treatment with recombinant human Shh-N (R&D Systems, Minneapolis, MN, USA), HCAEC viability was determined by a routine 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay as described by Queiroz et al. (13).
Western Blotting Analysis
Plaques and healthy vessels were lysed in lysis buffer (50 mmol/L Tris [Tris(hydroxymethyl)aminomethane]-HCl [pH 7.4], 1% Tween 20, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EGTA [ethylene glycol tetraacetic acid], 1 mmol/L o-vanadate, 1 mmol/L NaF and protease inhibitors [1 μg/mL aprotinin, 10 μg/mL leupeptin and 1 mmol/L 4-{2-amino-ethyl}-benzolsulfonyl-fluoride-hydrochloride]) on ice. Subsequently, samples were cleared by centrifugation and dissolved in an equal volume of 2× sodium dodecyl sulfide (SDS) gel loading buffer (100 mmol/L Tris-HCl [pH 6.8], 200 mmol/L dithiothreitol, 4% SDS, 0.1% bromophenol blue and 20% glycerol) and boiled for 5 min. Thereafter, proteins were resolved on molecular size using SDS polyacrylamide gel electrophoresis and analyzed by Western blot essentially as described previously (19). Briefly, membranes were blocked in 1% fat-free dried milk or bovine serum albumin (2%) in Tris-buffered saline (TBS)/0.1% Tween 20 (TBST) and incubated overnight at 4°C with the appropriate primary antibody. Primary antibodies against GLI1 (#2553) and PTCH1 (#2468) were obtained from Cell Signaling Technology (Beverly, MA, USA). Anti-HH (5E1; recognizing IHH, DHH and SHH) (20) antibody was purchased from the Developmental Hybridoma Bank (Iowa City, IA, USA), respectively. After incubation, membranes were washed in TBST and incubated with anti-rabbit, anti-goat and anti-mouse horseradish peroxidase–conjugated secondary antibodies at 1:2,000 dilutions in blocking buffer for 1 h. Blots were imaged using LumiLight Plus ECL (Roche, Basel, Switzerland). Despite the absence of calcium or zinc salts from our buffers (21), we observed efficient immunodetection of HH ligands using the 5E1 antibody, as also shown previously by Lee et al. (22) and Kohtz et al. (23).
Analysis of Vitamin D3 in Lipoproteins
Isolation of lipoproteins was performed as described by Queiroz et al. (13), and radioimmunoassay for measurement of vitamin D3 was performed as described earlier (24–26).
RESULTS
Healthy Human Vessel Is Characterized by Strong Constitutive Activation of HH Signaling
The vascular network is widely recognized to be targeted by HH ligands, since these morphogens constitute an important angiogenic signal after ischemic stress (11), but the activation status of the HH pathway in otherwise unchallenged healthy human vessel remains uncertain. Hence, we isolated the renal artery from healthy multiorgan donors and investigated these for HH signaling activity by assessing relative mRNA expression levels of the HH pathway targets PTCH1 and GLI1. Because expression levels of the routinely used GAPDH housekeeping gene may change depending on HH pathway activity, we first compared GAPDH levels with RPLP0 levels, since the latter gene has been suggested to be a better housekeeping gene when studying HH pathway activity (27). As shown in Figure 1B, GAPDH and RPLP0 levels correlate well in all samples used for this study, and we thus considered GAPDH to be an appropriate housekeeping gene.
Healthy vessels showed high expression of GLI1 and PTCH1, and these expression levels positively correlated with the expression levels of IHH and DHH (Figures 1C–F). SHH transcript levels were below detection levels. To confirm these observations, we analyzed PTCH1 and GLI1 protein levels in 10 healthy vessels by Western blot analysis (Figure 1G). PTCH1 and GLI1 protein levels were easily detectable in healthy vessels, although we did observe large interpatient variability. A subsequent study in aortic, carotid and femoral artery segments (29 samples obtained during autopsy) showed that the HH ligand was abundant in all arteries studied (data not shown). Interestingly, pharmacological inhibition of HH signaling using either cyclopamine (28) or vitamin D (29) was previously shown to compromise survival of primary coronary artery endothelial cells (13), suggesting that HH signaling is not only active in this cell type, but also required for endothelial maintenance. In line with these data, treatment of primary coronary artery endothelial cells with recombinant sonic hedgehog N-terminus (ShhN) induced increased cell viability relative to control treated cells (Figure 1H). This finding confirms a recent report showing that HH signaling is important for vascular integrity in the murine coronary circulation (30) and suggests that activation of HH signaling is a general feature of the human vasculature.
Presence of HH Ligand in Atherosclerotic Plaques
Subsequently, we investigated the presence of HH ligands in atherosclerotic structures. To this end, material obtained from 17 coronary plaques was collected and subjected to Western blot analysis for HH ligand (Figure 2A). This analysis revealed local production of HH during atherosclerotic plaque formation.
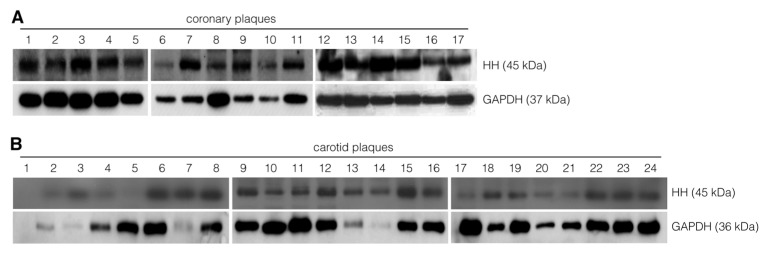
Figure 2.
Abundant presence of HH ligands in atherosclerotic plaques. (A) Coronary plaques were collected from 17 patients by catheterization, and protein levels of HH ligands were analyzed by Western blot. (B) Carotid plaques were collected by endarterectomy and analyzed for the levels of HH protein using Western blot. Immature forms (as deduced from apparent molecular weight) are abundantly present in plaques. Carotid plaques also contain abundant HH. Thus, atherosclerotic plaques are characterized by the presence of HH ligands.
The presence of HH ligand was confirmed by Western blot analysis on atherosclerotic carotid plaques (Figure 2B). Thus, locally produced HH ligand is present in substantial amounts in atherosclerotic structures in human vessels, raising questions about the activity of HH signal transduction in atherosclerotic plaques and how plaque stability can be maintained despite the neoangiogenic influence expected from the high levels of HH ligands detected in plaques.
Absence of HH Signaling Despite Ligand Expression in Atherosclerotic Plaques
We investigated whether the presence of HH ligand in atherosclerotic plaques correlated with HH pathway activity. To this end, atherosclerotic carotid plaques obtained from 24 patients were investigated for PTCH1 and GLI1 expression. Interestingly, PTCH1 and GLI1 expression (Figure 3A) was very low in atherosclerotic plaques, especially when compared with healthy vessels. More importantly, the expression levels of PTCH1 and GLI1 did not correlate with the expression levels of HH ligands, as shown in Figures 3B and C. In agreement, Western blot analysis indicated that these samples contain undetectable levels or only weak traces of HH pathway targets (Figure 3D). Thus, despite the presence of abundant HH ligand in atherosclerotic plaques, this presence does not translate into activation of the HH pathway. From this result, we can infer the existence of mechanisms that limit activation of this signaling in plaques.
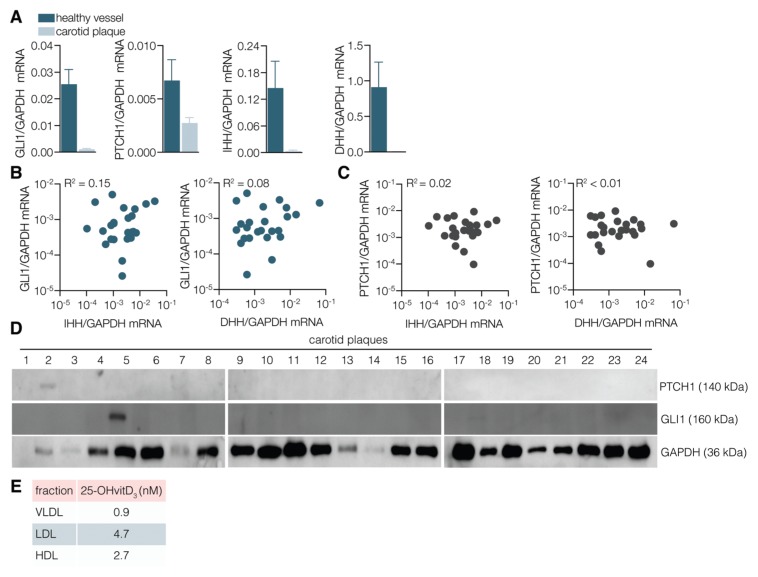
Figure 3.
Absence of response to HH ligands in atherosclerotic plaques. (A) Atherosclerotic plaques were collected from 24 patients undergoing carotid endarterectomy for symptomatic high-grade stenosis of the internal carotid artery. Renal arteries from four family kidney transplantation donors were used as healthy control vessels. HH ligand expression and pathway activity was assessed by quantitative reverse transcriptase PCR. Expression of HH pathway targets GLI1 (B) and PTCH1 (C), as determined by quantitative reverse transcriptase PCR, was correlated with the expression level of HH ligands in atherosclerotic plaques. (D) The low levels or absence of HH target gene products were confirmed by Western blotting analysis in the same 24 subjects. (E) Radioimmunoassay analysis of three major plasma lipoproteins reveals that LDL contains the highest concentrations and VLDL the lowest levels of the endogenous HH signaling antagonist 25-OH vitamin D3 (25-OHvitD3).
Lipoproteins are important constituents of atherosclerotic plaques and are potentially important carriers of endogenous HH antagonists (31). Consequently, we investigated the presence of vitamin D3 in the different lipoprotein components of the blood of healthy volunteers (Figure 3E). Interestingly, low-density lipoprotein (LDL) contains significant levels of Smoothened inhibitory vitamin D3. Because LDL is important in plaque formation, it is possible that the thus-derived local availability of vitamin D3 (or alternative hydroxysterols, which inhibit HH signaling irrespective of the presence of HH [29]) exerts a local negative effect on HH signaling, explaining the remarkable dichotomy in HH signaling between healthy vessels and atherosclerotic plaque. Because HH is an important mediator of revascularization and revascularization is the major cause of plaque instability, it is possible that the lipoprotein compartment exerts multilevel influence on the pathological consequences of thrombotic complications. Hence, we propose that enhanced understanding of the interaction between HH signaling and vascular biology will substantially contribute to our comprehension of the pathogenesis of cardiovascular disease.
DISCUSSION
Although most people develop atherosclerotic plaque from a young age (32), most often these plaque do not rupture. Among the factors that predispose individuals to rupture are neovascularization, and thus understanding the mechanisms that control such neoangiogenesis are important. HH signaling is among the most important signals controlling neoangiogenesis, and this consideration prompted us to investigate HH signaling in both healthy vessels and atherosclerotic plaque. We observed strong constitutive activation of HH signal transduction in healthy human vessels, which is required for survival of primary human endothelial cultures. This result is in perfect agreement with the observation that HH is important for the maintenance of the endothelial compartment per se in experimental rodents (30). Importantly, however, activation of HH signaling seems to be dichotomous; in atherosclerotic plaques, such signaling is not observed. Because the absence of HH signaling in plaques is expected to inhibit revascularization of such plaques, we propose that the inhibition of HH signaling in plaque material contributes to plaque stability and constitutes a protective signal (33).
CONCLUSION
The mechanism maintaining low activity of HH signaling in plaque remains unclear, but does not involve the absence of HH ligand, since ample amounts of HH protein and RNA are detected in atherosclerotic structures. Interestingly, we did observe that (especially on the mRNA level) HH levels in healthy vessels are increased compared with atherosclerotic plaques. One could thus argue that the amount of HH in plaques is not enough to robustly activate the pathway. However, such a threshold hypothesis is not likely for morphogens that are expected to act in response to low gradients of ligand. More likely, the low activity of HH signaling in plaques must constitute inhibition downstream of the primary HH receptor Patched (7,9). Smoothened antagonists can inhibit HH signal transduction downstream of Patched and are thus obvious candidates to participate in the HH signaling dichotomy. As lipoproteins constitute important components of the plaque structure, the presence of HH antagonists in lipoproteins may negatively regulate HH pathway activity. Interestingly, we did find high levels of the Smoothened antagonist 25-OH vitamin D3 in LDL. It is tempting to propose that LDLs are Janus-faced particles in atherosclerotic disease. On one hand, LDLs contribute to plaque formation, but on the other hand, they contribute to plaque stability by carrying antagonists of downstream HH signaling. It is important, however, to point out that other mechanisms, such as suppressor of fused (SUFU) expression (7,34), may also contribute to the functional dichotomy in HH signaling in the human vasculature. Disregarding the exact mechanism of HH signal transduction inhibition in atherosclerotic plaques, it is tempting to suggest that this inhibition constitutes an important antipathogenic signal and that enhanced knowledge of the mechanisms involved may contribute to better treatment and prevention of atherosclerotic disease.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Shah PK. Molecular mechanisms of plaque instability. Curr Opin Lipidol. 2007;18:492–9. doi: 10.1097/MOL.0b013e3282efa326. [DOI] [PubMed] [Google Scholar]
- 2.Constantinides P. Atherosclerosis: a general survey and synthesis. Surv Synth Pathol Res. 1984;3:477–98. doi: 10.1159/000156950. [DOI] [PubMed] [Google Scholar]
- 3.Tenaglia AN, Peters KG, Sketch MH, Annex BH. Neovascularization in atherectomy specimens from patients with unstable angina: implications for pathogenesis of unstable angina. Am Heart J. 1998;135:10–4. doi: 10.1016/s0002-8703(98)70336-9. [DOI] [PubMed] [Google Scholar]
- 4.Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 2007;49:2073–80. doi: 10.1016/j.jacc.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 5.Kolodgie FD, et al. Elimination of neoangiogenesis for plaque stabilization - Is there a role for local drug therapy? J Am Coll Cardiol. 2007;49:2093–101. doi: 10.1016/j.jacc.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 6.Bijlsma MF, Peppelenbosch MP, Spek CA. Hedgehog morphogen in cardiovascular disease. Circulation. 2006;114:1985–91. doi: 10.1161/CIRCULATIONAHA.106.619213. [DOI] [PubMed] [Google Scholar]
- 7.Kasper M, Jaks V, Fiaschi M, Toftgard R. Hedgehog signalling in breast cancer. Carcinogenesis. 2009;30:903–11. doi: 10.1093/carcin/bgp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavine KJ, Ornitz DM. Shared circuitry developmental signaling cascades regulate both embryonic and adult coronary vasculature. Circ Res. 2009;104:159–69. doi: 10.1161/CIRCRESAHA.108.191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svard J, Rozell B, Toftgard R, Teglund S. Tumor suppressor gene co-operativity in compound Patched1 and suppressor of fused heterozygous mutant mice. Mol Carcinog. 2009;48:408–19. doi: 10.1002/mc.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen MM. Hedgehog signaling update. Am. J. Med. Gen. Part A. 2010;152A:1875–914. doi: 10.1002/ajmg.a.32909. [DOI] [PubMed] [Google Scholar]
- 11.Kusano KF, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 12.Chen WW, et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9589–94. doi: 10.1073/pnas.1017945108. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Queiroz KCS, et al. Human plasma very low density lipoprotein carries Indian hedgehog. J Prot Res. 2010;9:6052–9. doi: 10.1021/pr100403q. [DOI] [PubMed] [Google Scholar]
- 14.Coultas L, et al. Hedgehog regulates distinct vascular patterning events through VEGF-dependent and -independent mechanisms. Blood. 2010;116:653–60. doi: 10.1182/blood-2009-12-256644. [DOI] [PubMed] [Google Scholar]
- 15.Renault MA, et al. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol. 2010;49:490–8. doi: 10.1016/j.yjmcc.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Beck A, Fraidenraich D. A direct, non-canonical pathway for Hedgehog proteins in the endothelium. Cell Cycle. 2010;9:647–8. [PubMed] [Google Scholar]
- 17.Lavine KJ, Long F, Choi K, Smith C, Ornitz DM. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 2008;135:3161–71. doi: 10.1242/dev.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavine KJ, Ornitz DA. Rebuilding the coronary vasculature: Hedgehog as a new candidate for pharmacologic revascularization. Trends Cardiovasc Med. 2007;17:77–83. doi: 10.1016/j.tcm.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Souza AC, et al. A promising action of riboflavin as a mediator of leukaemia cell death. Apoptosis. 2006;11:1761–71. doi: 10.1007/s10495-006-9549-2. [DOI] [PubMed] [Google Scholar]
- 20.Decker S, et al. Trisomy 12 and elevated GLI1 and PTCH1 transcript levels are biomarkers for Hedgehog-inhibitor responsiveness in CLL. Blood. 2011;119:997–1007. doi: 10.1182/blood-2011-06-359075. [DOI] [PubMed] [Google Scholar]
- 21.Maun HR, et al. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J Biol Chem. 2010;285:26570–80. doi: 10.1074/jbc.M110.112284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CS, Buttitta L, Fan CM. Evidence that the WNT-inducible growth arrest-specific gene 1 encodes an antagonist of sonic hedgehog signaling in the somite. Proc Natl Acad Sci U S A. 2001;98:11347–352. doi: 10.1073/pnas.201418298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohtz JD, et al. N-terminal fatty-acylation of sonic hedgehog enhances the induction of rodent ventral forebrain neurons. Development. 2001;128:2351–63. doi: 10.1242/dev.128.12.2351. [DOI] [PubMed] [Google Scholar]
- 24.Bijlsma MF, et al. Endogenous hedgehog expression contributes to myocardial ischemia-reperfusion-induced injury. Exp Biol Med. 2008;233:989–96. doi: 10.3181/0711-RM-307. [DOI] [PubMed] [Google Scholar]
- 25.Versteeg HH, et al. VIIa/tissue factor interaction results in a tissue factor cytoplasmic domain-independent activation of protein synthesis, p70, and p90 S6 kinase phosphorylation. J Biol Chem. 2002;277:27065–72. doi: 10.1074/jbc.M110325200. [DOI] [PubMed] [Google Scholar]
- 26.Dunaeva M, Voo S, van Oosterhoud C, Waltenberger J. Sonic hedgehog is a potent chemoattractant for human monocytes: diabetes mellitus inhibits Sonic hedgehog-induced monocyte chemotaxis. Basic Res Cardiol. 2010;105:61–71. doi: 10.1007/s00395-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin KJ, et al. High-sensitivity array analysis of gene expression for the early detection of disseminated breast tumor cells in peripheral blood. Proc Natl Acad Sci U S A. 2001;98:2646–51. doi: 10.1073/pnas.041622398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 29.Bijlsma MF, et al. Repression of smoothened by patched-dependent (Pro-) Vitamin D3 secretion. PLoS Biol. 2006;4:1397–410. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavine KJ, Kovacs A, Ornitz DM. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest. 2008;118:2404–14. doi: 10.1172/JCI34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaliullina H, et al. Patched regulates Smoothened trafficking using lipoprotein-derived lipids. Development. 2009;136:4111–21. doi: 10.1242/dev.041392. [DOI] [PubMed] [Google Scholar]
- 32.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bijlsma MF, et al. Endogenous hedgehog expression contributes to myocardial ischemia-reperfusion-induced injury. Exp Biol Med (Maywood) 2008;233:989–96. doi: 10.3181/0711-RM-307. [DOI] [PubMed] [Google Scholar]
- 34.Svard J, et al. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev. Cell. 2006;10:187–97. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]