Abstract
Aquaporin-4 (AQP-4), the most important water channel in the brain, is expressed by astrocyte end feet abutting microvessels. Altered expression levels of AQP-4 and redistribution of the protein throughout the membranes of cells found in glioblastoma multiforme (GBM) lead to development of the edema often found surrounding the tumor mass. Dysregulation of AQP-4 also occurs in hippocampal sclerosis and cortical dysplasia in patients with refractory partial epilepsy. This work reports on analysis of the relationship between AQP-4 expression and the incidence of epileptic seizures in patients with GBM. Immunohistochemical and polymerase chain reaction techniques were used to evaluate AQP-4 in biopsy specimens from 19 patients with GBM, 10 of who had a history of seizures before surgery. AQP-4 mRNA levels were identical in the two groups of patients, but AQP-4 expression was more frequently detected on the GBM membranes from specimens of patients with seizures than from individuals without (10 versus 2, P < 0.001). We conclude that reduced expression of cell surface AQP-4 is characteristic of GBM patients without seizures, likely attributable to a posttranslational mechanism.
INTRODUCTION
Epileptic seizures complicate the clinical course of approximately 20–40% of patients with glioblastoma multiforme (GBM) (1–3). The pathogenesis of the seizures associated with malignant intracranial glial tumors is still uncertain and probably differs between GBM and other glial tumors (2). Recent reports that the incidence of seizures is correlated to glutamine synthetase levels provide the first evidence that specific biochemical abnormalities may cause seizures in patients with GBM (4). Aquaporins (AQPs) are hydrophobic integral membrane proteins that act as water channels and contribute to maintaining proper homeostasis of fluids in various cells and tissues. AQP-4 is the most widely expressed member of the AQP family in the brain (5), where it plays a crucial role in the control of extra-cellular space volume. AQP-4 regulates water influx and efflux as well as potassium scavenging after high-frequency neuronal activity (5). Disruption of AQP-4 expression on astrocytes was associated with edema in brain tumors, including GBM (6,7). Dysregulation of AQP-4 expression was also increasingly associated with epilepsy (8–10). In this study, we investigated the relationship between the AQP-4 expression in patients with GBM and the occurrence of seizures.
MATERIALS AND METHODS
Patients
Surgical specimens from 19 patients were selected among 88 GBM patients who underwent primary tumor resection between 1 April 2007 and 1 July 2009. Ten patients had a history of at least one seizure before surgery; the other nine did not. Patients were selected and put into two groups (with and without a history of seizures), with age, disease duration before surgery and tumor size assessed (tumor size was determined by magnetic resonance imaging [MRI]). All GBM lesions were located supratentorially. A complete past neurological examination before surgery, a Karnofsky score and in-traoperative and postoperative course and survival data were available for each patient. Survival was calculated as of 1 November 2010.
Diagnosis of seizures was made on the basis of clinical and electroencephalographic features. Seizure type was defined in accordance with the classification system proposed by the International League Against Epilepsy (ILAE) Classification Core Group (11). The study was approved by the Local Ethic Committee.
MRI Analysis
Imaging was performed using a 1.5-T MRI system (Signa HDx, GE Healthcare, Waukesha, WI, USA). For each patient, the standard protocol consisted of sagittal T1 followed by axial DP-T2, diffusion, fluid-attenuated inversion recovery (FLAIR) and preweighted gradient echo planar images during the first pass of a standard bolus (0.1 mmol/kg) of gadopentetate dimeglumine (Magnevist), followed by multiplanar (axial and coronal) T1 weighted images. To correlate the expression of AQP-4 to the degree of peritumoral edema, we calculated the edema index. The edema index (6) describes the degree of peritumoral brain edema compared with tumor volume, and it was obtained dividing the total volume of the tumor and the surrounding edema by the volume of the tumor itself. We calculated the edema index for each lesion using the largest diameters of the lesion on the axial image and on the coronal plane.
Surgical Procedures and Neurophysiologic Evaluation
Sixteen patients underwent microsurgical resection under general anesthesia. A stereotactic biopsy was performed in two patients while they were awake. The remaining patients underwent surgery while awake for resection of a left-sided parietotemporal GBM. All of the patients underwent intraoperative neurophysiologic monitoring (12). Five patients admitted to the intensive care unit (ICU) after surgery underwent continuous electroencephalogram (EEG) monitoring.
Histological Examination and Immunohistochemistry
Surgically removed tumor specimens were fixed in Carnoy fluid, paraffin embedded and stained with hematoxylineosin by using standard techniques. Histological diagnoses were classified according to the 2007 publication WHO Classification of Tumours of the Central Nervous System (13).
Immunostaining was routinely performed using an UltraVision LP Detection System: HRP Polymer/DAB Plus Chromogen kit (catalog no. TL-125-HD; Thermo Scientific, part of Thermo Fisher Scientific, Waltham, MA, USA). Briefly, selected blocks were cut at 5 μm, and sections were picked up on activated slides to enhance adhesion. After paraffin extraction and rehydration, antigen retrieval was performed with boiling EDTA (ethylenediaminetetraacetic acid) buffer for 20 min. After endogenous peroxidase was blocked, sections were incubated with a primary antibody: either polyclonal anti-AQP-4 antibody (diluted 1:200) (Chemicon, Temecula, CA, USA) or anti-p53 prediluted primary antibody clone DO7 (Ventana Medical Systems [now a member of the Roche Group; current location: Tucson, AZ, USA]). Appropriate positive and negative controls were used on control slides.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) for Identification of AQP-4
RNA (ribonucleic acid) was extracted from fixed human brain slices derived from samples of GMB by using the Qiagen RNeasy® FFPE extraction kit (Hilden, Germany) and following the manufacturer’s instructions.
For AQP-4 mRNA expression analysis, cDNA was synthesized using the High-Capacity cDNA Reverse Transcription kit starting from 200 ng RNA. Specific primers were commercially available (TaqMan Gene Expression Assay). All above reagents were purchased from Life Technologies Italia (Monza, Italy). Because of the extreme sensitivity of the technique, control genes need to be carefully selected to account for differences in the quantity and quality of starting RNA and in cDNA synthesis efficiency. These genes are called endogenous controls. The main requisite is that their expression levels should not significantly vary among tissues and experimental situations analyzed. TATA-box binding protein (TBP) and hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) were used as endogenous controls, and their expression was determined on GBM specimens from the patients on the basis of available data, indicating that, in GBM tissues, these two genes are among the best-suited endogenous controls (14). All the above reagents were purchased from Applied Biosystems (Monza, Italy). qRT-PCR was conducted using the 7900 HT Fast Real-Time PCR System (SDS2.3 Software; Applied Biosystems). All reactions were performed in triplicate; triplicates were from the same cDNA reaction and therefore represent technical replicates. For each sample, the cycle threshold (CT) value of the gene under analysis was normalized with the following formula: ΔCT = CT of the gene under analysis – CT endogenous control gene. For relative expression, the mean ΔCT was determined, and the relative expression of the gene under analysis was calculated with the expression 2−ΔCT. Evaluation of the methylation status of the O-6-methylguanine-DNA methyltransferase (MGMT) promoter was also carried out by PCR as previously described (15).
Statistical Analysis
Categorical data were compared in a more conservative way by using a Fisher exact test. Continuous data were compared using the Student t test. Correlations were investigated by using the Spearman log-rank test. The log-rank test was used to compare survival of patients with seizures versus those without. Statistical analysis was carried out by using the Statistical Package for the Social Sciences software, version 9.0 (SPSS, Chicago, IL, USA).
RESULTS
Clinical, MRI and Neurophysiologic Features
Demographic and clinical characteristics of the patients are summarized in Table 1. Two patients with seizures manifested generalized tonic-clonic seizures, whereas the others experienced clinically focal seizures. Seizures were the presenting symptom that prompted neuroradiological evaluation in 8 of 10 patients.
Table 1.
Demographic and clinical characteristics of patients.
| Patient | Age | Sex | Seizure type | Antiepileptic drugs | Symptoms at presentation |
|---|---|---|---|---|---|
| 1 | 60 | F | FC | VPA | Seizure |
| 2 | 64 | M | GTC | VPA | Seizure |
| 3 | 63 | F | FC | CBZ | Seizure |
| 4 | 58 | M | Focal aphasic | OXC | Seizure |
| 5 | 48 | M | FC | LVT | Seizure |
| 6 | 66 | M | FS | LVT | Motor aphasia |
| 7 | 42 | M | FS | OXC | Headache |
| 8 | 57 | M | Focal inhibitory | LVT | Seizure |
| 9 | 77 | M | FC | LVT | Seizure |
| 10 | 64 | M | GTC | LVT | Seizure |
| 11 | 68 | M | None | None | Headache |
| 12 | 36 | F | None | None | Headache |
| 13 | 58 | M | None | None | Motor aphasia |
| 14 | 71 | M | None | None | Headache |
| 15 | 37 | F | None | None | Headache |
| 16 | 65 | M | None | None | Headache |
| 17 | 61 | M | None | None | Left hemiparesis |
| 18 | 58 | F | None | None | Headache |
| 19 | 84 | F | None | None | Headache |
CBZ, carbamazepine; FC, focal clonic; FS, focal sensory with elementary symptoms; GTC, generalized tonic-clonic; LVT, levetiracetam; OXC, oxcarbazepine; VPA, valproic acid.
The time elapsed from the onset of symptoms to surgery (44.2 ± 21 versus 26 ± 25.6 d, P = 0.08) as well as survival (22.1 ± 9.1 versus 22.1 ± 9.6 months, P = 0.138) did not differ between the groups. The mean tumor size among patients with and without seizures was also not significantly different (36 ± 14.9 versus 42.3 ± 11.9 mm, P = 0.109). The edema index did not differ among patients with seizures and without (3.87 versus 3.48, P = 0.808). MRI revealed bleeding in one patient per group. Cortical involvement was present in nine patients with seizures and in nine without seizures. In the group with seizures, the tumor involved the frontal lobe in four patients, the temporal lobe in four patients and the parietal lobe in two patients. In the group without seizures, the tumor involved the frontal lobe in five patients, the temporal lobe in one patient and the parietal lobe in three patients.
Neurophysiologic monitoring showed no significant modifications throughout the surgical procedures of each patient, except for one patient, who had convulsive seizures with generalized EEG spike discharges.
One of the five patients monitored with continuous EEG while in ICU had convulsive status epilepticus, with EEG evidence of intermittent bilateral prolonged spike discharges. Both the patient with seizures during surgery and the one with status epilepticus during ICU admission had a history of seizures before surgery.
Histological Examination and Immunohistochemistry
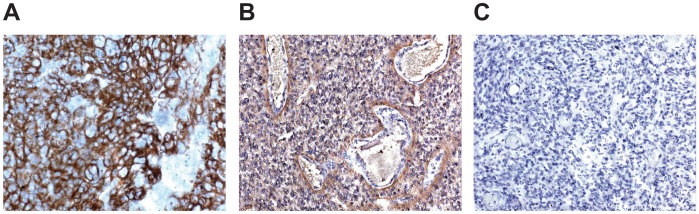
AQP-4 immunostaining was detected in all the samples from patients with a history of epileptic seizures and in two of nine patients without (P = 0.001). Immunostaining for AQP-4 was localized on the cellular membrane of GBM cells. In patients with epilepsy, the distribution of AQP-4 immunostaining was diffuse in six patients (++) and perivascular in four patients (+). In patients with no history of seizures, staining was either undetectable or revealed a diffuse pattern of expression (++). Examples of positive and negative immunostaining for AQP-4 are provided in Figure 1. There was no difference in the frequency of positive immunostaining for p53 between patients with and without seizures (5 of 10 versus 3 of 9, P = 0.65)
Figure 1.
Examples of immunostaining for AQP-4 in biopsy specimens from patients with GBM. (A) Diffuse positivity. (B) Perivascular positivity. (C) Absence of staining. (A) and (B) show biopsy specimens from patients with a history of seizures. (C) shows specimens from a patient without a history of seizures.
qRT-PCR Evaluation
qRT-PCR data showed that GBM patients express high levels of AQP-4 mRNA. No differences in transcript expression were observed between patients with or without a history of seizures. There was no difference in the frequency of hypermethylation of the MGMT promoter between patients with and without seizures (7 of 10 versus 3 of 9, P = 0.179).
Correlation Between AQP-4 Expression and Edema Index
To evaluate the relationship between AQP-4 expression and peritumoral edema, edema index was compared between patients with and without positive AQP-4 immunostaining. No significant difference was evident between patients with positive AQP-4 immunostaining and those without (4.1 ± 3.3 versus 2.8 ± 1.4, P = 0.395)
DISCUSSION
Epileptic seizures may complicate the course of patients with GBM. The pathogenesis of the seizures in these patients is unknown but is likely imputable to a number of different factors (16). These include alterations in extracellular pH, bleeding and modified synthesis and catabolism of neurotransmitters (2,16,17). Cortical location of GBM is a risk factor for seizures. In contrast, large tumor size and increased age seem to be associated with reduced risk (1). The current study investigated whether variations of AQP-4 expression on GBM cell membrane might be linked to the pathogenesis of seizures in GBM. Age, tumor size, bleeding and cortical involvement were not significantly different between patients with and without seizures. Therefore, the majority of confounding factors appear not to be significantly relevant in our series.
Under physiological conditions, AQP-4 is present on the astrocytic cell membrane, where molecules appear to be concentrated at the tip of astrocytic end feet in contact with microvessels and, to a lesser extent, on the rest of the cell membrane (5). AQP-4 is the main component of the so-called ortogonal array of particles (OAPs), which are preferentially located at the tip of astrocytic end feet. Furthermore, in the context of OAPs, AQP-4 forms part of a macromolecular complex, including dystrophyn, dystroglycan and voltage-gated potassium channel type 4.1 (18–20). Functional coupling of AQP-4 was also suggested with chloride channels and the electroneural cotransporter potassium-chloride cotransporter-1 (KCC-1) (19). Both OAPs and coupling with chloride and potassium currents are critical for the control of water homeostasis and asrocytic volume. Control of cell volume is linked to the regulation of (a) extracellular space volume, (b) extracellular potassium concentration and (c) efflux of glutamate and other osmolytes such as taurine (19). It was demonstrated that as a consequence of cells swelling (for instance after high frequency neuronal firing or during systemic hyponatriemia), there is a compensatory efflux of potassium, chloride and glutamate from the cells (19,20). The net effect of increased extracellular potassium and glutamate concentration is a depolarization of neuronal membrane, with increased excitability (19,20). Stimulation of astrocytes as well as modulation of asrocytic volume and chloride currents are important in determining synchronization of neuronal firing (19,20). Increased excitability along with hypersynchrony are critical factors in the development of epileptic seizures (15,21). The bidirectional water channel AQP-4 seems to play a critical role in these mechanisms of control of astrocyte volume; therefore, its dysregulation has an effect on the neuronal excitability.
Changes in the quantitative and qualitative expression of AQP-4 is typical in patients with GBM (6,18). Quantitatively, both overproduction and lack of expression (6,7) have been reported. Qualitatively, AQP-4 expression in GBM is usually more uniform and lacks the focal upregulation at the tip of the end feet (6,18). Moreover, these tumors have few OAPs, if any at all (22).
Until now, in GBM, AQP-4 expression was generally related to the degree of peritumoral vasogenic edema (6). In the present study, the edema index did not differ significantly between patients with positive and negative immunostaining for AQP-4. This finding probably is explained by the choice of including patients with similar lesion dimensions, since the aim of this study was to determine if AQP-4 is related to the development of seizures independently from its action on edema.
The potential role of AQP-4 in epilepsy is also sustained by recent reports. Altered AQP-4 expression and loss of perivascular upregulation are reported in biopsy specimens from patients with refractory epilepsy associated with hippocampal sclerosis (8) and focal cortical dysplasia (10). AQP-4 gene polymorphisms have been associated with temporal lobe epilepsy and febrile seizures (23). In animal models, the relationship between AQP-4 expression and epileptic seizures is complex, even if the results are rather homogeneous. AQP-4 knockout mice had increased resistance to development of induced seizures (20). However, when induced seizures occur, their severity is greater than in wild-type mice (20). Furthermore, after traumatic brain injury, AQP-4 knockout mice had decreased latency to seizures after treatment with pentylenetetrazole (24). Taken together, these results suggest that a lack of AQP-4 may increase the resistance to induced seizures in mice lacking brain injuries, but in turn increased seizure susceptibility in the presence of traumatic brain injury or increased seizure duration and severity when they occur in the absence of traumatic injury. As mentioned above AQP-4 is related to the scavenging of extracellular potassium and may influence the efflux of glutamate from the cells. Therefore, a dysregulation of AQP-4 may have an important effect on the excitability of neurons and probably on the synchronization of neuronal activity. Our results suggest that the absence or reduced expression of AQP-4 may be associated with a reduced occurrence of seizures in patients with GBM. The distribution of AQP-4 in our patients with a history of seizures is mainly located throughout the glial membrane, confirming previous reports (6,7,18). In contrast, only two patients without seizures had AQP-4 expression on the GBM membrane. This result supports the hypothesis that the absence of AQP-4 represents a protective factor against the development of seizures. Because epileptic seizures may have a multi-factorial genesis in GBM, an imbalance between inducing and protective factors may determine the onset of seizures.
The apparent discordance between our results and those in knockout mice for AQP-4 after traumatic brain injury should be considered, taking into account that, in our patients without seizures, AQP-4 is lacking only on the membrane of GBM cells, whereas, in knockout mice, AQP-4 is not expressed in the whole brain. Second, both knockout mice for AQP-4 and patients with absent AQP-4 may be similar in that they are resistant to seizure induction. We have no data on the possible severity of seizure if occurring in GBM patients lacking AQP-4. Third, in patients with seizures, AQP-4 is mainly distributed throughout the GBM cell membrane, and it is possible that the loss of AQP-4 polarized expression is important for seizures genesis.
Whereas AQP-4 membrane expression was different in our patients with and without seizures, the levels of AQP-4 mRNA were not. The lack of correlation between membrane expression and mRNA levels may be due to a leaky scanning mechanism during translation of AQP-4 mRNA (25). Consistent with this hypothesis, different isoforms of AQP-4 have been identified, but only the M23 isoform is capable of forming OAPs, leading to polarized expression of the protein on astrocytic end feet. The M1 isoform, instead, is unable to form OAPs. In AQP-4–M1 mRNA, two possible translation initiation signals were identified and may lead to the possible formation of M1 as well as M23 isoforms from the same mRNA (25). It is therefore possible that a shift in balance between the two isoforms underlies the difference in cell membrane expression, despite unchanged levels of mRNA. The limitations of our study are the small sample size to perform multivariate analysis and the study type, which is not a case control, even if the patients with and without seizures were similar for age, disease duration before surgery, survival and tumor size judged on MRI scans.
Further studies are warranted to elucidate the relationship between AQP-4 expression and the incidence of epileptic seizures in patients with GBM, to understand how AQP-4 dysregulation, potassium channel function and glutamate turnover are involved in the genesis of epileptic seizures in these patients.
CONCLUSION
Our results suggest that reduced expression of AQP-4, probably as a result of posttranslational mechanisms, is associated with a reduced risk of seizures in patients with GBM.
ACKNOWLEDGMENTS
This study was supported in part by the Compagnia di San Paolo (Progetto triennale per il management integrato del paziente adulto portatore di tumore del sistema nervoso centrale).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Chaichana KL, Parker SL, Olivi A, Quinones-Hinojosa A. Long-term seizure outcome in adult patients undergoing primary resection of malignant brain tumours. J Neurosurg. 2009;111:282–92. doi: 10.3171/2009.2.JNS081132. [DOI] [PubMed] [Google Scholar]
- 2.Rosati A, et al. Epilepsy in cerebral glioma: timing of appearance and histological correlates. J Neurooncol. 2009;83:395–400. doi: 10.1007/s11060-009-9796-5. [DOI] [PubMed] [Google Scholar]
- 3.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumours: current concepts and review of the literature. Neuro Oncol. 2002;4:278–99. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosati A, et al. Epilepsy in glioblastoma multiforme: correlation with glutamine synthetase levels. J Neurooncol. 2009;93:319–24. doi: 10.1007/s11060-008-9794-z. [DOI] [PubMed] [Google Scholar]
- 5.Nagelhus EA, Mathisen TM, Ottersen OP. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with Kir4.1. Neuroscience. 2004;129:905–13. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 6.Nico B, et al. Aquaporin-4 contributes to the resolution of peritumoural brain ooedema in human glioblastoma multiforme after combined chemotherapy and radiotherapy. Eur J Cancer. 2009;45:3315–25. doi: 10.1016/j.ejca.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psichiatry. 2002;72:262–5. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid T, et al. Loss of perivascular aquaporin 4 may underlie deficient water and K homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci U S A. 2005;102:1193–8. doi: 10.1073/pnas.0409308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu MS, Lee DJ, Binder DK. Potential role of the glial water channel aquaporin-4 in epilepsy. Neuron Glia Biol. 2007;3:287–97. doi: 10.1017/S1740925X08000112. [DOI] [PubMed] [Google Scholar]
- 10.Medici V, Frassoni C, Tassi L, Spreafico R, Gabelli R. Aquaporin 4 expression in control and epileptic human cerebral cortex. Brain Res. 2011;167:330–9. doi: 10.1016/j.brainres.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Engel J. Report of the ILAE Classification Core Group. Epilepsia. 2006;47:1558–68. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 12.Neuloh G, Shramm J. Intraoperative neurophysiological mapping and monitoring for supratential procedures. In: Deletis V, Shils JL, editors. Neurophysiology in Neurorsurgery. Academic Press; London: 2002. pp. 341–401. [Google Scholar]
- 13.Louis DN, Ohgaki H, Wiestler OD, Cavene WK, editors. WHO Classification of Tumours of the Central Nervous System. 4th edition. IARC; Lyon: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valente V, et al. Selection of suitable housekeeping genes for expression analysis in glioblastoma using quantitative RT-PCR. BMC Mol Biol. 2009;10:17. doi: 10.1186/1471-2199-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman JC, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CPG islands. Proc Natl Acad Sci U S A. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Breemen MMS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours. Lancet Neurol. 2007;6:421–30. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 17.Beaumont A, Whittle IR. The pathogenesis of tumour associated epilepsy. Acta Neurochirur (Wien) 2000;142:1–15. doi: 10.1007/s007010050001. [DOI] [PubMed] [Google Scholar]
- 18.Warth A, Mittelbronn M, Wolburg H. Redistribution of the water channel protein aquaporin-4 and the K+ channel protein Kir4.1 differs in low- and high-grade human brain tumours. Acta Neuropathol. 2005;109:418–26. doi: 10.1007/s00401-005-0984-x. [DOI] [PubMed] [Google Scholar]
- 19.Pasantes-Morales H, Cruz-Rangel S. Brain volume regulation: osmolytes and aquaporin perspectives. Neuroscience. 2010;168:871–84. doi: 10.1016/j.neuroscience.2009.11.074. [DOI] [PubMed] [Google Scholar]
- 20.Binder DK, Nagelhus EA, Ottersen OP. Aquaporin-4 and epilepsy. Glia. 2012;60:1203–14. doi: 10.1002/glia.22317. [DOI] [PubMed] [Google Scholar]
- 21.Niedermeyer E. Epileptic seizure disorders. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography Basic Principles, Clinical Applications and Related Fields. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 505–619. [Google Scholar]
- 22.Warth A, Kroger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain basal capillary laminae. Acta Neuropathol. 2004;107:311–8. doi: 10.1007/s00401-003-0812-0. [DOI] [PubMed] [Google Scholar]
- 23.Heuser K, et al. Variants of the genes encoding AQP-4 and Kir4.1 are associated with subgroups of patients with temporal lobe epilepsy. Epilepsy Res. 2010;88:55–64. doi: 10.1016/j.eplepsyres.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Lu DC, Zador Z, Yao J, Fazlollahi F, Manley GT. Aquaporin-4 reduces post-traumatic seizure susceptibility by promoting astrocytic glial scar formation in mice. J Neurotrauma. 2011 2011 Sep 22; doi: 10.1089/neu.2011.2114. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pisani F, Rossi A, Nicchia GP, Svelto M, Frigeri A. Translational regulation mechanism of aquaporin-4 supramolecular organization in astrocytes. Glia. 2011;59:1923–32. doi: 10.1002/glia.21234. [DOI] [PubMed] [Google Scholar]