Abstract
Isocorydine (ICD), an anticancer agent under current evaluation, decreased the percentage of side population (SP) cells significantly in hepatocellular carcinoma (HCC) cell lines. ICD treatment sensitized cancer cells to doxorubicin (DXR), a conventional clinical chemotherapeutic drug for HCC. We found that ICD decreased the percentage of SP cells in HCC cell lines by preferentially killing SP cells. In the early stage of treatment, ICD inhibited SP cell growth by arresting cells in G2/M; later, it induced apoptosis. Our xenograft model confirmed that ICD selectively reduced the size and weight of SP-induced tumor masses in vivo. Furthermore, it was found that programmed cell death 4 (PDCD4), a tumor suppressor gene, was relatively low when expressed in SP cells compared with non-SP cells, and its expression level was remarkably elevated when cells were treated with ICD. Taken together, these data suggest that ICD is a drug that may target the SP cells of HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer mortality worldwide, resulting in 600,000 deaths annually (1). Resistance to conventional chemotherapy is acknowledged as a major obstacle for the effective treatment of cancer. There are multiple reasons for the drug resistance of HCC, among which is overproduction of some ATP-binding cassette (ABC)-dependent transporter family members (2). ATP-binding cassette-dependent subfamily G member 2 (ABCG2) is an important member of this family that correlates with drug resistance. This transporter can efflux many conventional drugs out of cancer cells, rendering chemotherapy ineffective. ABCG2 expression is also known to be essential for the side population (SP) phenotype (3).
SP, which was first identified in murine hematopoietic stem cells, refers to a small fraction of cells that can efflux the fluorescent dye Hoechst 33342 and can therefore be detected by flow cytometry (4). SP cells exist in many normal tissues and organs and exhibit tissue- and organ-specific stem cell–like characteristics (5). SP cells have also been identified in various tumors, including breast cancer (6), neuroblastoma (7), lung cancer (8), glioma tumors (9), nasopharyngeal carcinoma, hepatoma and colon cancer (10) and are considered a subpopulation enriched in cancer stem cells (CSCs). As in HCC, SP cells harbor cancer stem cell–like features, including self-renewal, tumorigenicity and multidifferentiation (11–13). In addition to the characteristics described above, we have reported that SP cells in HCC correlate with drug resistance, which serves as more evidence that these cells have CSC characteristics. SP cells in the MHCC-97L cell line possess a greater capacity to efflux doxorubicin (DXR), one of the most commonly used chemotherapeutic drugs, than non-SP cells. Thus, SP cells show a reduced intracellular accumulation of DXR, and ABCG2 confers characteristics of the SP phenotype in HCC cells (14).
Because the SP is a group of drug- resistant cells in HCC and commonly used anticancer drugs fail to kill them efficiently, it is important to explore strategies for using new drugs that target SP cells. Isocorydine (ICD) is an alkaloid monomer from Papaveraceae spp. plants, including Dactylicapnos scandens Hutchins and Dicranostigma leptopodum (Maxim) Fedde (DLF). DLF was studied for the treatment of pulmonary tuberculosis and was recently found to inhibit the hepatoma cell line SMMC-7721, both in vitro and in vivo by inducing apoptosis (15). We demonstrated that ICD is an active ingredient in DLF that prohibited the proliferation of HCC cell lines both in vitro and in vivo by inducing G2/M cell cycle arrest (16). In this study, we focused on investigating the mechanism by which ICD selectively inhibits the growth of SP HCC cells.
PDCD4, which was originally identified as being upregulated during apoptosis, was recently found to function as a tumor suppressor gene (17), and down-regulation of the PDCD4 protein was observed in HCC tissues compared to matched noncancerous liver tissues (18). This finding suggests that PDCD4 plays an essential role in the apoptosis of HCC cells (19). In this study, we explored whether PDCD4 is involved in the drug resistance of SP cells. Using cDNA microarray, we found that PDCD4 was upregulated in HCC cell lines treated with ICD; we also confirmed this upregulation by Western blot. Here, we report that PDCD4 may be important in SP cells and may participate in ICD-induced apoptosis of SP cells.
MATERIALS AND METHODS
Cell Lines and Reagents
The MHCC-97L, MHCC-97H and MHCC-LM3 cell lines were obtained from Zhongshan Hospital, Fudan University (Shanghai, China); PLC/PRF/5 and SNU-449 were purchased from ATCC. MHCC-97L, MHCC-97H, MHCC-LM3 and PLC/PRF/5 were cultured in Dulbecco’s modified Eagle medium (DMEM) (Gibco; Life Technologies, Carlsbad, CA, USA), and SNU449 was cultured in RPMI 1640 medium (Gibco; Life Technologies). All medium contains 10% fetal bovine serum (FBS) (Thermo Scientific; Logan, UT, USA) that was heat-inactivated at 56°C for 30 min. The media for the above cells were supplemented with 100 IU/mL penicillin G and 100 μg/mL streptomycin (Sigma-Aldrich, St. Louis, MO, USA), and cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. ICD isolated from Dactylicapnos scandens Hutchins was purchased from the Shanghai Zhanshu Chemical Sci-Tech Company (Shanghai, China). All other reagents were from Sigma-Aldrich unless otherwise noted.
Side Population Analysis and Sorting by Flow Cytometry
According to the Goodel et al. protocol (4), cells were trypsinized from dishes, washed in phosphate-buffered saline (PBS) and then suspended in 10 mmol/L hydroxyethylpiperazine-2-ethanesulfonic acid (HEPES)-buffered DMEM containing 2% FBS. Cell suspensions at concentrations of 1 × 106 cells/mL were stained with Hoechst 33342 (Invitrogen, Carls-bad, CA, USA) in a 37°C water bath for 90 min (gently shaking at intervals of 10 min), with or without 10 μmol/L Fumitremorgin C as a negative control. After incubation, cell suspensions were centrifuged at 4°C and then resuspended in precooled Hanks balanced salt solution (HBSS) (Invitrogen) containing 2 μL/mL propidium iodide (PI). Side population analysis and sorting were performed by an Epics Altra Flow Cell Sorter (Beckman Coulter; Fullerton, CA, USA) with a 488-nm argon laser and an INNOVA 90-CA5 ultraviolet laser (Coherent; Santa Clara, CA, USA). The Hoechst dye was excited by a 351-nm ultraviolet laser, and the fluorescence emission was collected through 450DF20 (Hoechst blue) and 675ALP filters (Hoechst red).
Cell Cycle Analysis
A total of 200,000 cells were seeded in six-well culture plates and were allowed to recover for 24 h. The cells were then synchronized with 1 mmol/L thymidine for another 24 h. The cells were subsequently treated with 150 μg/mL ICD (diluted in DMEM with 10% FBS) for the indicated times. The cells (including dead cells in the supernatant) were collected, washed in PBS and fixed in precooled 70% ethanol at −20°C overnight. Before analysis by flow cytometry, the cells were washed three times with PBS, re-suspended in 500 μL precooled PI/TritonX-100 buffer and incubated at room temperature in the dark for 30 min.
Apoptosis Analysis
Cells were collected, washed in PBS and then resuspended in binding buffer to a concentration of 1 × 106 cells/mL. Cell suspensions (100 μL) were added to tubes, mixed with 5 μL annexin V and 5 μL 7-AAD (both available from BD Biosciences, San Jose, CA, USA) and incubated at room temperature for 15 min. An additional 400 μL binding buffer was added to each sample, which was mixed gently for analysis by flow cytometry.
To detect the apoptosis in xenograft tissue, TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP- biotin nick end labeling) assay was performed according to the manual of the TUNEL Apoptosis Assay Kit (Roche Diagnostics, Mannheim, Germany).
Cell Proliferation Assays
A total of 5,000 cells were plated in 96-well culture plates for 24 h and were then treated with reagents at the indicated concentrations for the indicated times. BrdU assay was performed according to the manufacturer’s manual (Roche Diagnostics). Optical density (OD) values were measured by an ELISA (enzyme-linked immunosorbent assay) reader (Multiskan MK3; Thermo Scientific) at 450 nm.
Western Blotting
Cell lysates were prepared with the T-PER tissue protein extraction reagent (Pierce, Rockford, IL, USA) with a cocktail of proteinase inhibitors and phosphatase inhibitors (both available from Roche Diagnostics). The total protein was quantified by the modified Bradford assay according to the manufacturer’s instructions. Cell lysates (20–40 μg protein loaded for each lane) were resolved by 10–12% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) (depending on the molecular weight) and were then transferred to nitrocellulose or polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The electroblotted membranes were blocked by PBS containing 5% non-fat milk (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and were probed with primary antibodies overnight at 4°C (Supplementary Table S1). After washing, the membranes were incubated with secondary antibodies (Supplementary Table S1) at room temperature for 1 h, followed by chemiluminescence detection using the SuperSignal West Femto Maximum Sensitivity Substrate Kit (Pierce). Finally, the membranes were exposed to X-ray film (Kodak, Rochester, NY, USA) for the desired times.
Immunofluorescence of Cultured Cells
Cultured cells were maintained in a chamber slide system for 24 h and were fixed with 4% paraformaldehyde in PBS (pH 7.4) for 30 min at room temperature. For expression analysis of the ABCG2 protein, immunodetection was performed as in our previous report (14). The cells were observed and photographed under an Axioskop 2 microscope (Carl Zeiss, Oberkochen, Germany) with a DP70 charged-coupled device (CCD) system (Olympus, Tokyo, Japan).
Xenograft Experiments
Two different mouse models were used to observe in vivo effect of ICD on SP cells and non-SP cells. For the subcutaneous model, 8 × 104 SP cells or non-SP cells, freshly sorted as previously described, were inoculated subcutaneously into 6- to 8-wk-old male BALB/c (nu/nu) mice. Three weeks after injection, when tumors were observable, both SP and non-SP groups were divided into treatment groups and control groups. Mice in each treatment group were treated with 0.4 mg/kg ICD for 5 d per week through intraperitoneal injection, and each control group was given PBS. After 4 wks, mice were euthanized and tumor masses were subjected to analysis.
As for the liver orthotopic model, 8 × 104 sorted SP cells or non-SP cells isolated from the MHCC-97L cell line were transplanted into the livers of 6- to 8-wk-old male BALB/c (nu/nu) mice. One week after transplantation, both the SP group and non-SP group were divided into treatment and control group. Likewise, in the subcutaneous model, the treatment group was treated with ICD for 5 d per week, and control group was given PBS. After 6 wks, mice were sacrificed, and tumor masses were subjected to various analyses.
All of the mice used in this study were housed and manipulated according to protocols approved by the Shanghai Medical Experimental Animal Care Commission.
Cell Cytotoxity Assay
A total of 5,000 cells were planted on a 96-well plate and recovered for 24 h. The cells were treated with ICD at the indicated concentrations for the indicated times. When the incubation ended, the medium was replaced with fresh medium containing 0.5 μg/mL MTT and incubated at 37°C in the dark for 4 h. After removing the medium, 100 μL DMSO was added to each well. The plate was shook to ensure the formazon dissolved sufficiently. The OD value of the colored solution was measured at a wavelength of 570 nm by an ELISA reader.
Doxorubicin Efflux and Retention Assay
According to the protocol reported previously (20), cells were pretreated with ICD or DXR for 24 h and were then plated into 12-well culture plates and allowed to recover for 24 h. The cells were incubated with 20 μg/mL DXR at 37°C for 4 h. The cells were visualized and photographed at 0, 4 and 24 h after incubation under a microscope with a digital CCD system.
RNA Interference of PDCD4
siRNA fragments of PDCD4 (5′-AAG GUG GCU GGA ACA UCU AUU-3′; 5′-GGU GGC UGG AAC AUC UAU UTT-3′) were synthesized by GenePharma (Shanghai, China). The transfection of siRNAs was performed by Lipofectamine 2000 according to the manufacturer’s manual (Invitrogen). SP and non-SP cells were sorted from the MHCC-97L cell line and were then planted onto a six-well plate. After 24 h, SP and non-SP cells were transfected with siRNA of PDCD4. Twenty-four hours after transfection ended, ICD was added to treat cells for 48 h. Then the cells were collected and subjected to analyze apoptosis by flow cytometry.
Statistical Analysis
All data are expressed as mean ± standard deviation. Statistical analyses were performed with the Student t test. p < 0.05 was considered statistically significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
ICD Reduces the SP Fraction in HCC Cell Lines and Downregulates ABCG2
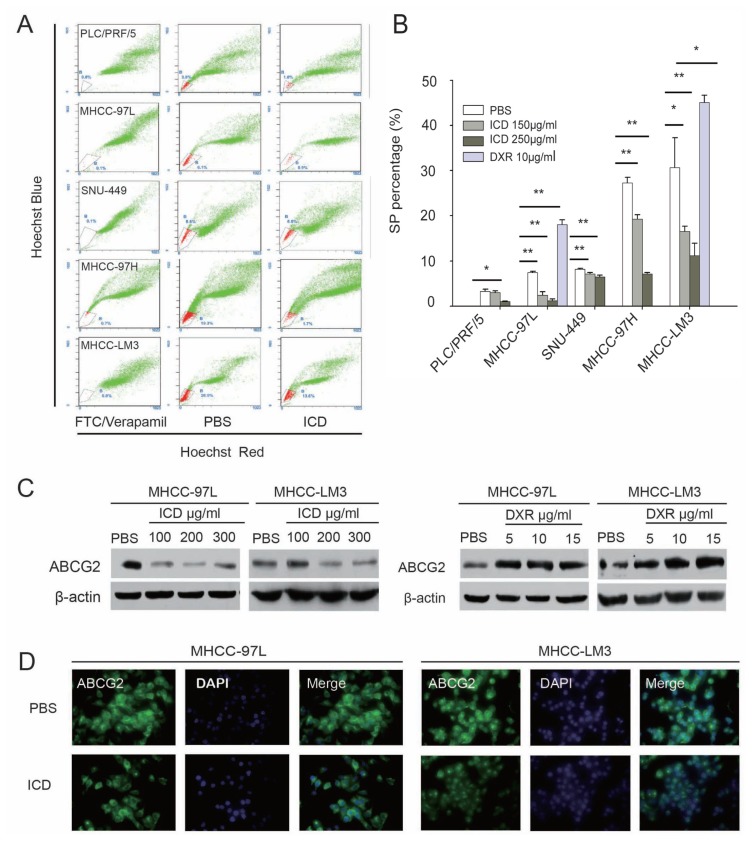
To investigate the effects of ICD on the fraction of SP cells in HCC cell lines, Hoechst sorting and analysis were performed in several HCC cell lines after ICD treatment. We found that ICD significantly decreased the fraction of SP cells in the PLC/PRF/5, MHCC-97L, SNU-449, MHCC-97H and MHCC-LM3 cell lines in both time-dependent and dose-dependent manners. In contrast to ICD, DXR increased the percentages of SP cells in the MHCC-97L and MHCC-LM3 cell lines (Figures 1A, B; Supplementary Figure S1). Furthermore, our results show that ICD decreased the expression of ABCG2 in a dose-dependent manner, but DXR increased ABCG2 expression, which was consistent with the percent change of SP cells (Figure 1C).
Figure 1.
Altered SP fraction and ABCG2 expression after treatment with ICD. (A) Representative flow cytometry histograms of SP analysis of PLC/PRF/5, MHCC-97L, SNU449, MHCC-97H and MHCC-LM3 cells after treatment with 250 μg/mL ICD for 24 h. (B) Bar graph of (A), showing statistical analysis (t test) of SP percentages of four HCC cell lines treated with 150 μg/mL and 250 μg/mL ICD for 24 h; for MHCC-97L and MHCC-LM3 cells, the effect of 10 μg/mL DXR on SP cell percentages was also used as a control. *p < 0.05, **p < 0.01. (C) Western blot of ABCG2 protein in MHCC-97L and MHCC-LM3 cells after being treated with ICD and DXR, respectively, for 24 h; β-actin is a loading control. (D) Immunofluorescence staining confirmed that ABCG2 is downregulated in MHCC-97L and MHCC-LM3 cells after treatment with 150 μg/mL ICD for 24 h. ABCG2 (green) was detected with a monoclonal antibody and labeled with fluorescein isothiocyanate (FITC)-conjugated secondary antibody; nuclei were counterstained with 6-diamidino-2-phenylindole (DAPI).
Immunofluorescence detection was used to confirm the downregulation of ABCG2 observed by Western blot. We found that in both the MHCC-97L and MHCC-LM3 cell lines, the fluorescence intensity of ABCG2 became weaker after exposure to 150 μg/mL ICD for 24 h. These results were in accordance with the Western blot result, demonstrating that expression of the ABCG2 protein decreased after ICD treatment (Figure 1D).
ICD Induces Apoptosis of SP Cells in the MHCC-97L Cell Line
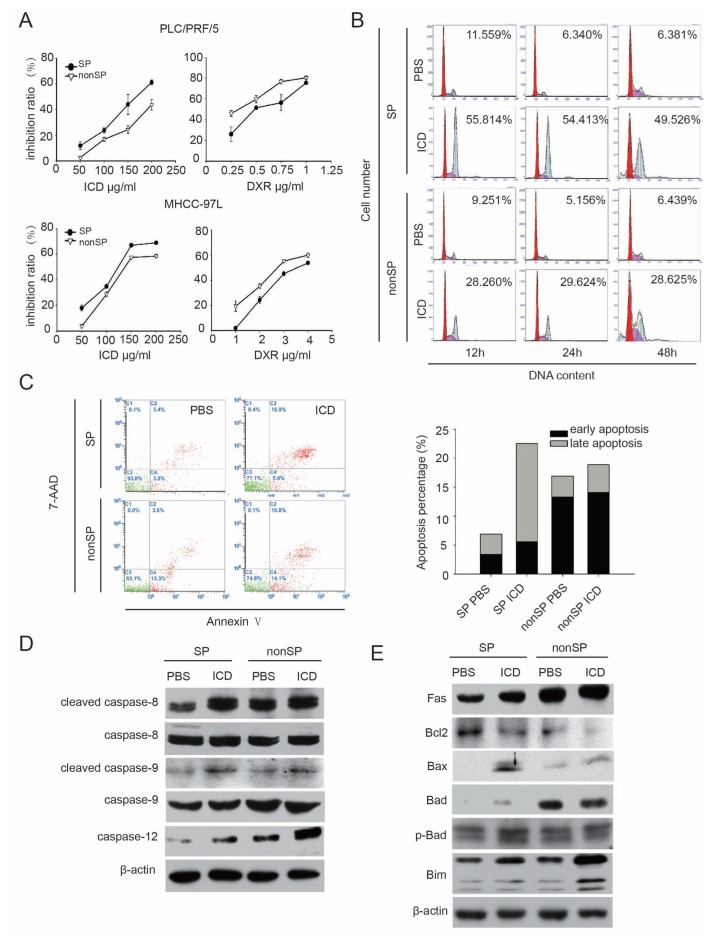
To understand the mechanism by which ICD decreases the percentage of SP cells, we assessed the effect of ICD on cell growth of SP/non-SP cells in vitro using a BrdU assay. The purity of SP sorting is shown in Supplementary Figure S2. We observed that ICD exerted stronger cell growth inhibition on both PLC/PRF/5-SP cells and MHCC-97L-SP cells compared with non-SP cells. The results revealed that increasing concentrations of ICD, ranging from 50 to 200 μg/ mL, all significantly suppressed SP cell viability, but the inhibitory effect on non-SP cells was weaker. In contrast, DXR was more effective in inhibiting non-SP cells, which is distinct from the ICD results (Figure 2A).
Figure 2.
ICD induces apoptosis in SP cells. (A) Cell proliferation was analyzed by BrdU assay in MHCC-97L and PLC/PRF/5 SP cells after treatment with ICD and DXR. (B) Representative flow cytometry histograms of SP and non-SP cells, in which G2/M arrest was induced by ICD treatment for 12, 24 and 48 h. The level of G2/M arrest in SP cells was more significant than the arrest in non-SP cells. (C) FACS analysis revealed that exposing MHCC-97L SP cells to ICD for 48 h induced apoptosis. Early apoptotic cells refer to cells labeled only by annexin V; late apoptotic cells refer to cells that have both signals of 7-AAD and annexin V (defined according to the manual of PE Annexin V Apoptosis Detection Kit from BD Biosciences). (D) Western blot of caspases in MHCC-97L SP and non-SP cells after treatment with 150 μg/mL ICD for 24 h. (E) Western blot of apoptosis proteins, which localized on the cellular and mitochondrial membranes in MHCC-97L SP and non-SP cells treated with 150 μg/mL ICD for 24 h.
Because ICD inhibited the growth of the HCC cell line by inducing G2/M arrest (16), we analyzed the cell cycle distribution of SP and non-SP cells exposed to ICD for 12, 24 and 48 h. No differences in the cell cycle distribution between SP and non-SP cells were noted in our previous work (14). When treated with ICD, G2/M arrest was observed in both cell populations. However, the number of cells in G2/M arrest in SP cells was greater than that in non-SP cells, which is in agreement with the results of the BrdU assay in this study (Figure 2B).
Further, we observed whereas ICD treatment for short time periods arrested SP cells in G2/M, long-term exposure led to apoptosis (16). Then we analyzed the SP/non-SP cells with an annexin V/ 7-AAD staining kit. We found that the percentage of cells undergoing apoptosis induced by ICD was more in SP cells than in non-SP cells, similar to the G2/M arrest induced by ICD (Figure 2C).
We further explored the molecular mechanism by which ICD induced apoptosis in SP cells. Western blotting showed that after ICD treatment, caspase-9, caspase-8 and caspase-12, which are known as initiator caspases of apoptosis, were activated, suggesting that all three apoptosis pathways (the death receptor pathway, the mitochondrial pathway and the endoplasmic reticulum pathway) were involved in apoptosis induced by ICD. To better understand the mechanism of apoptosis induced by ICD, other key cellular membrane and mitochondrial membrane proteins that are involved in positively or negatively regulating apoptosis were also analyzed, most of which were significantly altered in their expression levels (Figure 2E and Supplementary Figure S3).
In Vivo Studies Confirm That ICD Targets HCC SP Cells
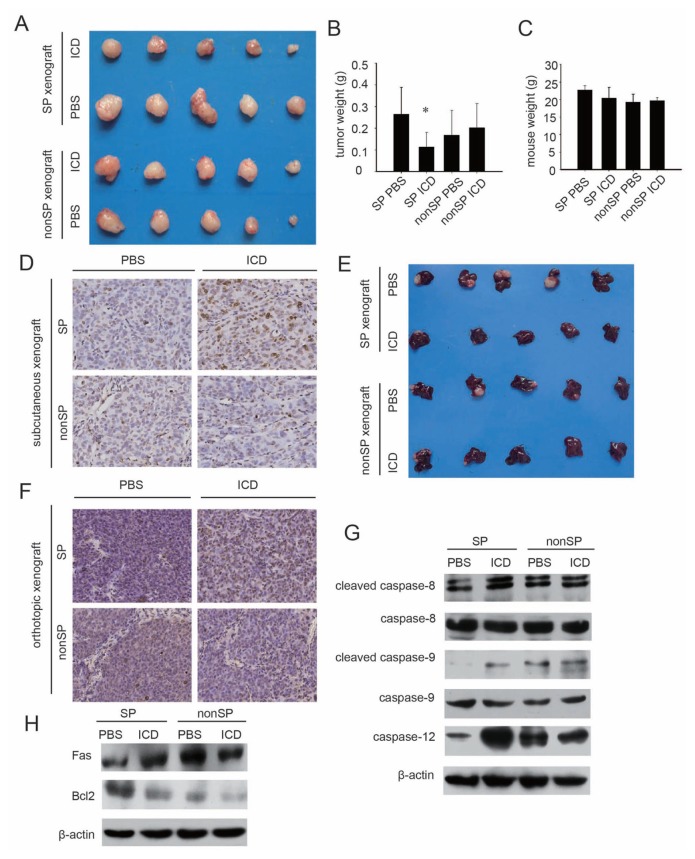
To validate our in vitro results, freshly sorted SP and non-SP cells were inoculated subcutaneously into nude mice. When visible tumor masses formed after 3 wks, the animals were randomly divided into a treatment group and a control group. The treatment group was intraperitoneally administered ICD for 5 d per week, and the control group was similarly given PBS. After 4 wks of treatment, all of the mice were euthanized and subjected to analysis.
For SP cell–induced tumor masses, ICD significantly reduced tumor sizes and tumor weights, whereas there were no significant differences between ICD and PBS treatment in the non-SP group, either in tumor size or tumor weight, confirming that ICD selectively inhibits the growth of SP cells (Figures 3A, B). It is worth noting that no difference in mouse weight was observed between the treatment group and the control group, suggesting that ICD has no adverse side effects on mouse growth (Figure 3C). Then TUNEL assay was performed to detect in vivo ICD-induced apoptosis in subcutaneous tumor tissues formed by SP and non-SP cells. The results showed that more apoptotic cells diffusely distributed in SP cells induced tumor tissues compared with the control group, which means that ICD reduced tumor volume through inducing apoptosis of treated SP cells in vivo (Figure 3D).
Figure 3.
Inhibitory effect of ICD on xenografts formed by SP and non-SP cells from the MHCC-97L line. (A) Tumor masses formed by MHCC-97L-SP or non-SP cells and treated with ICD or PBS in nude mice. (B) The weights of tumor masses induced by MHCC-97L SP or non-SP cells and treated with ICD or PBS (n = 5 each group, *p < 0.05). (C) The weights of tumor-bearing mice treated with ICD or PBS. (D) TUNEL assay of apoptotic cells in SP and non-SP cells induced subcutaneous tumor tissues of control groups (PBS) and ICD treatment groups. (E) Liver orthotopic SP and non-SP cells induced tumors treated with PBS or ICD. (F) TUNEL assay of apoptotic cells in SP and non-SP cells induced liver orthotopic tumor tissues of control groups (PBS) and ICD treatment groups. (G) Western blot of caspase proteins from the tumor tissues of the four groups. (H) Western blot of apoptosis-related proteins from the tumor tissues of the four subcutaneous xenograft groups.
Because the established cell line–induced tumors that were implanted subcutaneously have a different micro-environment from the tissue of origin of the tumors (28), liver orthotopic models were used to confirm the results of the subcutaneous xenograft above. We got similar results in the liver orthotopic model (Figure 3E). Likewise, in a subcutaneous xenograft model, TUNEL assay revealed that ICD was more effective in inducing apoptosis in SP cell–induced liver orthotopic tumor tissues than those of non-SP cells (Figure 3F).
Apoptosis-regulating proteins (Fas, Bcl-2 and caspase 8, 9 and 12) were also analyzed in xenograft tumors from SP and non-SP cells. The results revealed that ICD altered the expression of those proteins in similar patterns in vivo as were observed in vitro (Figures 3G, H).
ICD Enhances the Sensitivity of HCC Cell Lines to DXR
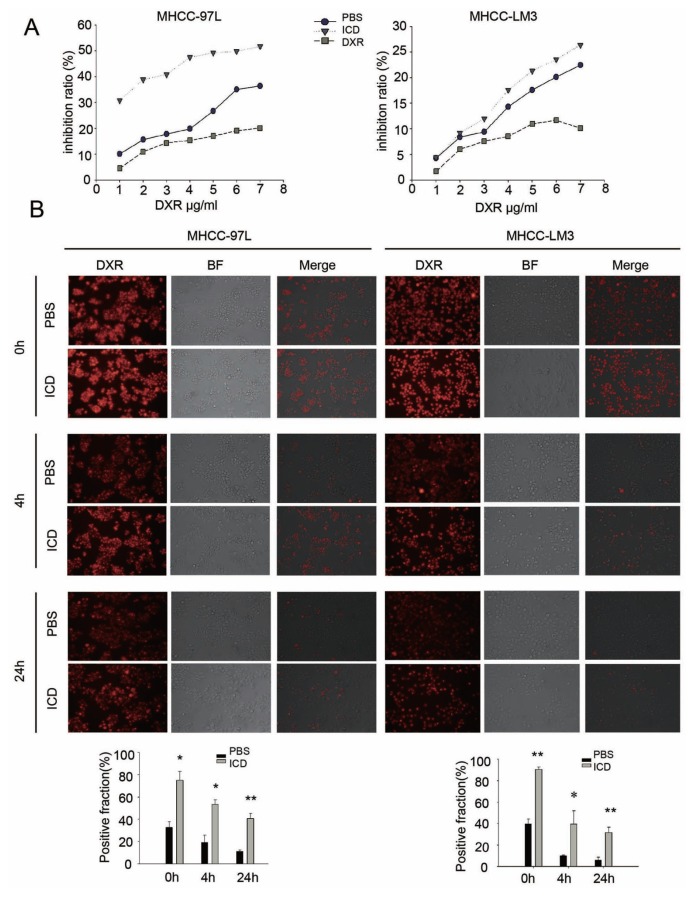
To determine whether a decrease in the SP fraction leads to enhanced drug sensitivity, we pretreated MHCC-97L and MHCC-LM3 cells with ICD or DXR for 24 h and then performed MTT assays to determine the sensitivity of the two cell lines to DXR. We found that compared with cells pretreated with PBS, the cells exposed to ICD were more sensitive to DXR, whereas cells pretreated with DXR were more resistant. This result suggests that continuous treatment with the same anticancer drug killed the sensitive cells, and only the drug-resistant cells remained (Figure 4A).
Figure 4.
ICD increases the sensitivity of HCC cell lines to DXR. (A) Cell cytotoxity detection with MTT assay; pretreatment with 150 μg/mL ICD increased the sensitivity of MHCC-97L and MHCC-LM3 cells to DXR treatment, and pretreatment with 10 μg/mL DXR decreased their sensitivity to DXR. (B) DXR efflux and retention assays showed that pretreatment with ICD reduces DXR efflux and prolongs its retention in both MHCC-97L and MHCC-LM3 cells. The left panel (red) shows the autofluorescence of DXR, the middle panel is the bright field image and the right panel is the merge of the previous two images. *p < 0.05; **p < 0.01.
To further understand the above observation, DXR efflux and retention assays were performed. Because DXR produces autofluorescence, its uptake and efflux by cells can be monitored by fluorescence microscopy. The results indicated that ICD pretreatment increased DXR influx (0 h), decreased DXR efflux (4 h) and caused DXR to enter the nuclei over time and interfere with DNA replication, thereby inhibiting tumor cell growth. The results were consistent in both MHCC-97L and MHCC-LM3 cells, suggesting ICD treatment followed by DXR showed stronger inhibitory effect on HCC cells than DXR treatment followed by DXR (Figure 4B).
PDCD4 Plays an Important Role in ICD-Induced Apoptosis
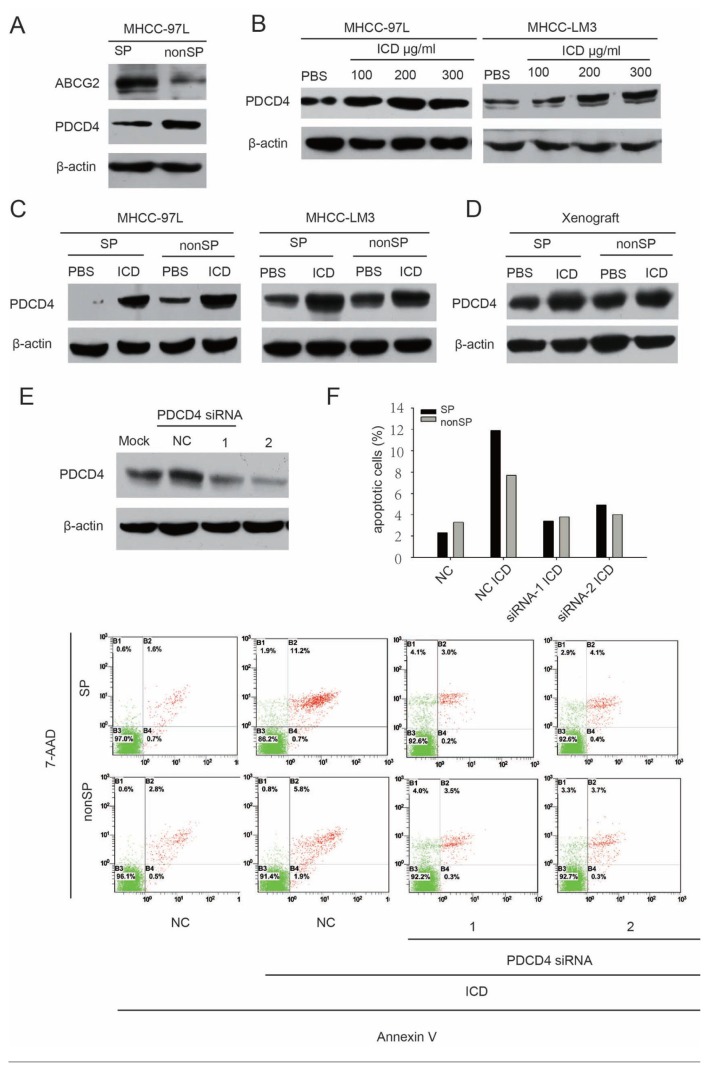
PDCD4, a tumor suppressor protein involved in apoptosis and drug resistance in HCC, was found to be low when expressed in SP cells (Figure 5A), and its expression was upregulated in MHCC-97L and MHCC-LM3 cells after treatment with ICD (Figure 5B). Further, up-regulated expression of the PDCD4 protein was also detected both in sorted SP and non-SP cells treated with ICD (Figure 5C). The upregulation level of PDCD4 expression was higher in SP cells than in non-SP cells. To confirm upregulation of PDCD4 expression caused by ICD treatment in vivo, we extracted protein from xenograft tissues induced by SP and non-SP cells after treatment with ICD or PBS. The results were in agreement with those in vitro: PDCD4 was more elevated in the treatment group compared to the control (Figure 5D). These data indicate that PDCD4 is likely involved in the sensitization of HCC cell lines to DXR through ICD.
Figure 5.
PDCD4 is a potential target of ICD. (A) Western blot of ABCG2 and PDCD4 in sorted SP and non-SP cells from the MHCC-97L cell line. (B) Western blot of PDCD4 in MHCC-97L and MHCC-LM3 cells exposed to ICD for 24 h. (C) Western blot of PDCD4 in sorted SP and non-SP cells from MHCC-97L and MHCC-LM3 lines treated with PBS or 150 μg/mL ICD for 24 h. (D) Western blot of PDCD4 in tumors induced by MHCC-97L SP or non-SP cells and treated with ICD or PBS, respectively. (E) Western blot of PDCD4 protein in MHCC-97L cells after RNA interference. NC, negative control. (F) Analysis of ICD-induced apoptosis in SP and non-SP cells after PDCD4 siRNA. After silencing of PDCD4, cells were treated with 150 μg/mL ICD for 48 h.
To further evaluate the action of PDCD4 in ICD treatment, we downregulated the expression of PDCD4 by using an RNA interference (Figure 5E). After silencing of PDCD4, ICD-induced apoptosis decreased correspondingly. Although PDCD4 downregulation alleviated apoptosis both in SP cells and non-SP cells, the alleviating effect in SP cells was more significant than that in non-SP cells (Figure 5F), indicating that PDCD4 plays an important role in ICD-induced apoptosis, especially in SP cells.
DISCUSSION
The SP phenotype has drawn enormous attention as a method to study stemlike cells in a variety of tissues and cancers (21). However, the data are still controversial regarding whether the SP in HCC possesses a stronger tumor-initiating capability. It has been reported that SP cells sorted from the HCC cell lines Huh7 and PLC/PRF/5 initiated tumors more effectively compared to their non-SP counterparts and that only 1,000 SP cells from the PLC/PRF/5 line formed tumors in vivo, showing that the SP was enriched with tumorigenic cells (12). However, in our previous study, SP cell derived from the MHCC-97L line displayed no differences in proliferative capability or clonogenicity in vitro (14). In this study, we observed that both SP and non-SP cells from the MHCC-97L line initiated tumors in vivo, and although SP-induced tumors were slightly bigger than non-SP-induced tumors, there was no statistical significance, which is consistent with our previous in vitro study. Two explanations have been suggested to account for the conflicting results between different labs: the heterogeneity of HCC cell lines and different Hoechst 33342 concentrations used for SP sorting. HCC cell lines differ greatly in tumor formation potential, and because of Hoechst 33342 toxicity to non-SP cells, the concentration of dye used also significantly affects SP sorting, making direct comparison of results from different labs difficult (22).
Despite the controversy of its tumor initiation capability, it is generally accepted that the SP is a drug-resistant subpopulation, due at least in part to the overexpression of ABC transporters (14). We found that MHCC-LM3 cells, which have a higher percentage of SP cells, efflux DXR more efficiently than MHCC-97L cells. Thus, the latter are more tolerant to DXR, adding evidence that the SP phenotype correlates with drug resistance in HCC. Because the SP phenotype is thought to be responsible for decreased responses to chemotherapy, screening for agents that target SP cells is valuable for sensitizing tumors. Although progress has been made, most of the anti-SP drugs are focused on CSC-like features rather than drug resistance. For example, curcumin, a phytochemical from the Indian spice turmeric, decreased the percentage of SP cells in the rat glioma cell line C6 after daily treatment and also decreased the percentage of SP cells when co-incubated with Hoechst 33342. Thus, curcumin may be a dietary phytochemical that targets CSC-like SP cells (23). Mesenchymal stem cells engineered to express TNF-related apoptosis ligand (TRAIL) caused apoptosis, death and reduced colony formation of SP cells from squamous and adenocarcinoma lung cancer cells and synergistically induced apoptosis when combined with traditional chemotherapy (24). In multiple myeloma, lenalidomide, a clinically used drug, was found to decrease the tumorigenicity and clonogenicity of SP cells through inducing phosphorylation of Akt, GSK-3α/β, MEK1, c-Jun, p53 and p70S6K (25). Hydroxycamptothecin, another anti-cancer agent, was shown to inhibit MHCC-97–SP cell proliferation and invasion by inducing differentiation (26).
In our study, ICD was originally found to reduce the SP fraction in several HCC cell lines and also to reduce the expression of ABCG2. ICD reduced the SP fraction by preferentially inhibiting SP cells. ICD also induced apoptosis in SP cells by upregulating proapoptotic regulators, such as Fas, Bax, Bim, Bik, Bak and puma, and downregulating antiapoptosis molecules, such as Bcl-2, Bcl-xl and Mcl-1. In addition, ICD significantly reduced the size and weight of SP cell xenografts but had no obvious inhibitory effects on tumors formed by non-SP cells, further suggesting that ICD selectively targets SP cells. Because no significant differences in mouse weights were observed between the treatment group and the control group, ICD appears to have no side effects on mouse growth.
SP cells are preferentially killed by ICD, and ABCG2 is overexpressed in SP cells; thus, when overall ABCG2 expression decreases after treatment with ICD, the surviving cells are more sensitive to DXR. In contrast to ICD, DXR treatment increased the SP fraction and ABCG2 expression. One possible explanation for this result is that DXR kills only those cells with relatively low levels of ABCG2, leaving those cells with high levels of ABCG2, thus enriching SP cells and accordingly increasing drug resistance.
It is noteworthy that the antiapoptosis protein Bcl-2 is overexpressed in SP cells, whereas proapoptosis proteins, such as Fas, Bax, Bad and Bim, are overexpressed in non-SP cells. This result is consistent with reports that SP cells are more resistant to apoptosis because of high levels of antiapoptosis proteins (including Bcl-2) and low levels of proapoptosis factors (including Bax) (27). The balance of these factors, in addition to the expression of ABC transporters, may lead to SP drug resistance and tolerance to apoptosis.
As for an in vivo experiment of ICD effect, subcutaneous xenograft models of human HCC cells in immunodeficient mice were used. Because in vivo drug screening, a surgical orthotopic implantation (SOI), would provide the most convincing evidence (28,29), a conventional liver orthotopic model of sorted SP cells and non-SP cells from the MHCC-97L cell line was used to confirm previous observations in the subcutaneous xenograft model. Unexpectedly, in two different in vivo models, we witnessed similar or almost the same inhibitory effect of ICD on HCC cell growth, implying that ICD targets HCC SP cells with drug resistance both in vitro and in vivo.
PDCD4 is associated with apoptosis and was recently shown to suppress tumor promoter-induced neoplastic transformation; it is also highly expressed in bladder carcinoma and breast carcinoma tissues compared with matched normal tissues (30). Along with other malignancies, PDCD4 was also downregulated in HCC tissues compared with matched noncancerous tissues. Low levels of PDCD4 Low levels of PDCD4 play an important role in preventing HCC cell apoptosis by modulating caspase activation and mitochondrial changes (18). Additionally, PDCD4 also plays a role in drug sensitivity in multiple cancers, such as breast and renal cell cancers. PDCD4 was observed to increase MCF-7 and UO31 sensitivity to anticancer drugs, which was accompanied by enhanced G2/M arrest and apoptosis (31). In our expression microarray, PDCD4 was upregulated dramatically after treatment with ICD; this result was confirmed by Western blot in MHCC-97L and MHCC-LM3 cells. In addition, PDCD4 expression was relatively low in drug-resistant SP cells and was elevated both in sorted SP and non-SP cells after exposure to ICD. Taken together, these data suggest that PDCD4 is involved in increased drug sensitivity after ICD treatment. Despite the fact that, after treatment with ICD, PDCD4 expression increased in both subpopulations, the level in SP cells was much higher than the level in non-SP cells. The same results were obtained in SP and non-SP tumors. Thus, we suggest that PDCD4 is important for the SP phenotype and is involved in the selective effects of ICD on SP cells.
CONCLUSION
Our previous in vitro experiments proved that ICD exhibits a relatively low cytotoxicity to L-02, an immortalized human liver cell line, compared with HCC cell lines (16). In vivo experiments also confirmed that ICD did not have obvious side effects. All of this evidence suggests that ICD may be a potential anticancer drug for HCC clinical therapy.
Supplemental Data
ACKNOWLEDGMENTS
This work was funded by grants from the National Key Sci-Tech Special Project of China (2008ZX10002-022), the National Key Program for Basic Research of China (973) (2009CB521803), the Program of Shanghai Subject Chief Scientist (A) (09XD1403600) and Research Project of Shanghai Municipal Health Bureau (201169).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Gomaa AI, et al. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–8. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 4.Goodell MA, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvi AJ, et al. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5:R1–8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschmann-Jax C, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho MM, et al. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 9.Bleau AM, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haraguchi N, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–13. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- 11.Chiba T, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–51. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 12.Jing F, et al. Side population cells from hepatocellular carcinoma cells have bilateral differentiation ability. Journal of US-China Medical Science. 2007;4:1–8. [Google Scholar]
- 13.Zen Y, et al. Histological and culture studies with respect to ABCG2 expression support the existence of a cancer cell hierarchy in human hepatocellular carcinoma. Am J Pathol. 2007;170:1750–62. doi: 10.2353/ajpath.2007.060798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu C, et al. Analysis of ABCG2 expression and side population identifies intrinsic drug efflux in the HCC cell line MHCC-97L and its modulation by Akt signaling. Carcinogenesis. 2008;29:2289–97. doi: 10.1093/carcin/bgn223. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Lv M, Hai J, Wang Q, Wang Q. Dicranostigma leptopodum (maxim) fedde induced apoptosis in SMMC-7721 human hepatoma cells and inhibited tumor growth in mice. Natural Science. 2010;2:457–63. [Google Scholar]
- 16.Sun H, et al. Isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/M cell cycle arrest and apoptosis. PLoS One. 2012;4:e36808. doi: 10.1371/journal.pone.0036808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshinaga H, et al. Novel human PDCD4 (H731) gene expressed in proliferative cells is expressed in the small duct epithelial cells of the breast as revealed by an anti-H731 antibody. Pathol Int. 1999;49:1067–77. doi: 10.1046/j.1440-1827.1999.00995.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, et al. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25:6101–12. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- 19.Li J, et al. miR-183 inhibits TGF-beta1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer. 2010;10:354. doi: 10.1186/1471-2407-10-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, et al. Regulation of the stability of P-glycoprotein by ubiquitination. Mol Pharmacol. 2004;66:395–403. doi: 10.1124/mol.104.001966. [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 22.Golebiewska A, et al. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–47. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Fong D, et al. Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett. 2010;293:65–72. doi: 10.1016/j.canlet.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loebinger MR, et al. TRAIL-expressing mesenchymal stem cells kill the putative cancer stem cell population. Br J Cancer. 2010;103:1692–7. doi: 10.1038/sj.bjc.6605952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubikova J, et al. Lenalidomide targets clonogenic side population in multiple myeloma: pathophysiologic and clinical implications. Blood. 2011;117:4409–19. doi: 10.1182/blood-2010-02-267344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, et al. Differentiation-inducing activity of hydroxycamptothecin on cancer stem-like cells derived from hepatocellular carcinoma. Dig Dis Sci. 2011;56:2473–81. doi: 10.1007/s10620-011-1601-6. [DOI] [PubMed] [Google Scholar]
- 27.Fan J, et al. Effect of Bcl-2 and Bax on survival of side population cells from hepatocellular carcinoma cells. World J Gastroenterol. 2007;13:6053–9. doi: 10.3748/wjg.v13.45.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–59. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 30.Cmarik JL, et al. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci U S A. 1999;96:14037–42. doi: 10.1073/pnas.96.24.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen AP, et al. Characterization of programmed cell death 4 in multiple human cancers reveals a novel enhancer of drug sensitivity. Mol Cancer Ther. 2004;3:103–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.