Abstract
Background and purpose
Diabetes mellitus (DM) is a major stroke risk factor and is associated with poor recovery compared with nondiabetic stroke patients. In the present study, we investigated the effects of tissue plasminogen activator (tPA) treatment of stroke in diabetic and non-diabetic rats.
Methods
Type-1 diabetes (T1DM) was induced by injection of streptozotocin. Non-T1DM and T1DM rats were subjected to embolic middle cerebral artery occlusion (MCAo) and treated with or without tPA 2 h after MCAo. Functional outcomes and immunostaining for advanced glycation endproducts receptor (RAGE), matrix metalloproteinase-9 (MMP-9) and toll-like receptor 4 (TLR4) and Western blotting were performed.
Results
tPA treatment of WT-MCAo rats significantly improved the functional outcome and reduced the lesion volume compared with non-treatment WT-MCAo rats (p < 0.05). There was no significant difference between treatment with or without tPA in the WT-MCAo group in brain hemorrhage, BBB leakage and expression of inflammatory mediators, RAGE, MMP-9 and TLR4. However, tPA treatment in T1DM-MCAo rats (T1DM-MCAo + tPA) significantly enlarged brain hemorrhage, augmented BBB leakage, and failed to decrease lesion volume and improve functional outcome after stroke compared to T1DM-MCAo control. tPA treatment also significantly increased the expression of RAGE, MMP-9 and TLR4 in the ischemic brain in T1DM-MCAo rats compared with T1DM-MCAo control rats (p < 0.05). Brain hemorrhage was significantly correlated with functional deficit and RAGE and TLR4 expression, respectively.
Conclusions
Treatment of stroke with tPA increased brain hemorrhage, BBB leakage and failed to improve functional outcome in T1DM rats. The increased inflammatory response may contribute to the failed neuroprotective effects of tPA treatment in T1DM rats.
Keywords: type-1 diabetes rats, Stroke, RAGE, tPA, TLR4, MMP-9
INTRODUCTION
Diabetes mellitus (DM) is a severe health problem associated with both microvascular and macrovascular diseases and leads to threefold to fourfold higher risk of experiencing ischemic stroke and arteriosclerosis (Mast et al., 1995). DM is associated with more severe strokes, in-hospital mortality, and poor recovery compared with nondiabetic individuals (Capes et al., 2001). Ischemic stroke patients with DM exhibit a distinct risk-factor and etiologic profile and a worse vascular prognosis than non-DM patients (Putaala et al., 2011). Tissue plasminogen activator (tPA) is the only established treatment for acute ischemic stroke (Albers et al., 2000; Hacke et al., 2004). tPA treatment of stroke in diabetes and hyperglycemia rats significantly increases blood–brain barrier (BBB) permeability and brain hemorrhage in the ischemic brain (Won et al., 2011; Fan et al., 2012). tPA treatment of stroke in hyperglycemia stroke patients also increases the incidence of intracerebral hemorrhage and worsens neurological outcomes (Alvarez-Sabin et al., 2003). However, the mechanisms underlying the adverse effects of tPA treatment of the diabetic population have not been fully elucidated.
Inflammatory mediators may contribute to the increase of vascular damage and complications, and promote atherosclerosis in diabetic patients (Jialal et al., 2002; Devaraj et al., 2006; Basta, 2008). Post stroke inflammatory activation in the ischemic penumbra contributes to the progression of the neuronal injury (Barone and Feuerstein, 1999; Chamorro and Hallenbeck, 2006) and may increase vascular damage associated with diabetes (Williams and Nadler, 2007). We have previously reported that high-mobility group box 1 (HMGB1), a potent proinflammatory cytokine-like factor, was increased in the diabetic animals after stroke (Ye et al., 2011). Receptor for advanced glycation endproducts (RAGE) is one of the HMGB1 primary receptors. When activated by HMGB1, RAGE promotes the proinflammatory phenotype (Fiuza et al., 2003). RAGE is associated with neurodegenerative diseases and inflammatory disorders (Barile and Schmidt, 2007; Maillard-Lefebvre et al., 2009). Matrix metalloproteinase-9 (MMP-9) is a potentially destructive enzyme for the neurovascular unit, and can lead to acute inflammatory response after stroke when upregulated via toll-like receptor 4 (TLR4) (Qiu et al., 2010). Upregulation of MMP-9 causes BBB degradation and hemorrhagic transformation (Tu et al., 2011). In addition, TLR4, as one of the receptors of HMGB1, is important in the activation of the innate immune system that contributes to inflammation and may increase neuroinflammation and exacerbate stroke injury (Buchanan et al., 2010). In this study, we investigated the effects of tPA treatment of stroke in diabetic and non-diabetic rats on functional outcomes, and the expression of inflammatory mediators RAGE, MMP-9 and TLR4 in the ischemic brain.
EXPERIMENTAL PROCEDURES
Diabetes induction
Adult Male Wistar rats (250–275 g) purchased from Charles River (Wilmington, MA) were used. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ, 60 mg/kg, Sigma Chemical Co., St. Louis, MO) to rats. The fasting blood glucose level was measured 10 days after STZ injection by using a glucose analyzer (Accu-Chek Compact System; Roche Diagnostics, Indianapolis, IN). Diabetes was defined by fasting blood glucose more than 300 mg/dl. Animals were subjected to right embolic middle cerebral artery occlusion (MCAo) 2 weeks after diabetes induction (Wu and Huan, 2008).
Rat MCAo model
We employed a model of embolic stroke as described previously (Zhang et al., 1997). Briefly, femoral arterial blood from a donor rat was withdrawn into 20 cm of PE-50 tubing and retained in the tube for 2 h to clot at room temperature, and subsequently retained for 22 h at 4 °C. Five centimeters of the PE-50 tube containing clot was cut and attached at each end to a 20-cm PE-10 tube interconnected by a syringe filled with saline. The clot was shifted by continuous alternating movement from one syringe to the other for 5 min. A single clot was transferred to a modified PE-50 catheter with a 0.3-mm outer diameter filled with saline.
The animals were anesthetized with 4% isoflurane during induction of stroke and then maintained with 2% isoflurane in a mixture of 30% O2 and 70% N2O. Body temperature was monitored and maintained at 37 °C using a feedback-regulated water heating system. Under the operating microscope (Carl Zeiss), the right common carotid artery (CCA), the right external carotid artery (ECA), and the internal carotid artery (ICA) were isolated via a midline incision. A modified PE-50 catheter with a 0.3-mm outer diameter was gently advanced from the ECA into the lumen of the ICA. When the catheter was 2–3 mm from the origin of the MCA, the clot (~4 cm) along with 2–3 μl of 0.9% saline was gently injected into the ICA. The catheter was withdrawn immediately after injection, and the right ECA was ligated.
Experimental protocols
Recombinant human tPA (tPA, Genentech, San Francisco, CA, USA) was infused intravenously, via the tail vein, 2 h after ischemia at a dose of 10 mg/kg (10% bolus and the remainder at a continuous infusion over a 30-min interval using a syringe infusion pump; Harvard Apparatus, Holliston, MA, USA). This dose of tPA (10 mg/kg) is commonly used for investigating the effect of fibrinolysis in the rodent (Niessen et al., 2002). Two hours after MCAo, the type-1 diabetes (T1DM) and non-DM rats were randomly separated into four groups and were infused with or without tPA (10 mg/kg, iv) via tail vein for 30 min.
WT-MCAo control group: animals were infused with saline at 2 h after MCAo as control (n = 8).
WT-MCAo-tPA treatment group: animals were infused with tPA (10 mg/kg) at 2 h after MCAo (n = 8).
T1DM-MCAo control group: animals were infused with saline at 2 h after MCAo (n = 8).
T1DM-MCAo-tPA treatment group: animals were infused with tPA (10 mg/kg) at 2 h after MCAo (n = 7).
Neurological functional tests
A battery of functional tests including foot-fault test, adhesive removal test and a modified neurological severity score (mNSS) test were performed prior to MCAo, and at 2 h (before treatment), 24 h, 48 h after MCAo by an investigator who was blinded to the experimental groups (Chen et al., 2001a,b). All animals were sacrificed at 48 h after MCAo for immunostaining. mNSS is a composite of motor, sensory, balance and reflex tests (Markgraf et al., 1992; Borlongan et al., 1995). Rats were excluded if mNSS was <6 or >13 before treatment. The foot-fault test evaluates placement dysfunction of forelimbs (Barth et al., 1990; Schallert et al., 2000). In addition, the adhesive removal test was performed both pre- and postoperatively (Schallert et al., 1982) (n = 8 or 7 rats/group).
Histological and immunohistochemical assessment
At 48 h after MCAo, all animals were sacrificed and brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. Seven coronal sections of tissue were processed and stained with hematoxylin and eosin (H&E) for calculation of volume of cerebral infarction (Swanson et al., 1990). The indirect lesion area, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere, was calculated using the Global Lab Image analysis system (Data Translation, Marlboro, MA) (Swanson et al., 1990). Lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere. Brain hemorrhage volume was measured by using H&E staining under light microscopy in all animals including all the animals that died within 48 h after stroke (n = 7 or 8 rats/group).
Immunohistochemical staining
A standard paraffin block was obtained from the center of the lesion (bregma −1 mm to +1 mm). A series of 6-μm-thick sections was cut from the block. Every 10th coronal section for a total of 5 sections was used for immunohistochemical staining. Antibody against RAGE (1:400; Dako, Carpenteria, CA, USA), MMP-9 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and TLR4 (goat polyclonal IgG; dilution 1:100; Cruz Biotech Inc., Santa Cruz, California) immunostaining were performed. Control experiments consisted of staining brain coronal tissue sections as outlined above, but the primary antibodies were omitted, as previously described (Li et al., 1998) (n = 7 or 8 rats/group).
Immunostaining quantitation
For measurement of RAGE, MMP-9, and TLR4, five slides from each brain, with each slide containing eight fields of cortex and striatum from the ischemic border area (IBZ) were digitized under a 20× objective, using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID computer imaging analysis system (Imaging Research, St. Catharines, Canada) (Calza et al., 2001; Chen et al., 2003a,b). The data are presented as percentage of positive area in the IBZ, respectively (Cui et al., 2011) (n = 7 or 8 rats/group).
Quantitative evaluation of Evans Blue dye extravasation
To test BBB permeability, an additional set of rats (n = 4/group) were sacrificed at 48 h after MCAo. Evans Blue dye (2%) in saline was injected intravenously as a BBB permeability tracer at 4 h before sacrifice. Evans Blue dye fluorescence intensity was determined by a microplate fluorescence reader (excitation 620 nm and emission 680 nm). Calculations were based on the external standards dissolved in the same solvent (Ishii et al., 2002; Zhang et al., 2002).
Western blot
Rats were sacrificed at 48 h after MCAo and brain tissues (n = 4/ group) were extracted from the ischemic brain. Equal amounts of cell lysate were subjected to Western blot analysis, as previously described (Chen et al., 2003b). Specific proteins were visualized using a SuperSignal West Pico chemiluminescence kit (Pierce). The following primary antibodies were used: anti-β-actin (1:20000; Sigma, St. Louis, MO, USA), and anti-MMP-9 (1:500, Santa Cruz, Santa Cruz, CA, USA), anti-RAGE 1:1000, R&D Systems, Minneapolis, MN, USA), anti-TLR4 (1:500, Santa Cruz, Santa Cruz, CA, USA).
Statistical analysis
Animals subjected to MCAo were randomized into four groups including WT, WT with tPA, T1DM, T1DM with tPA in the study. Three functional tests, adhesive removal, foot-fault, and mNSS tests were performed before MCAo and at 2 h (before tPA treatment), 24 and 48 h after MCAo. The study measured the effects of tPA on functional recovery as well as on the expression of inflammatory mediators (RAGE, TLR4 and MMP-9) at 48 h after stroke. Data were evaluated for normality. As a result, ranked data were used for the functional (mNSS, foot-fault and adhesive removal tests) measurements.
A 2-factorial design was considered with two independent factors of DM and tPA (presence/absence). The Global test (Lu et al., 2003; Goeman et al., 2004) was used to test the tPA effect on the group difference on functional recovery, measured from the multiple behavior tests in the 24 h and 48 h after MCAo. A 2-way analysis of variance (ANOVA) was used to study the tPA effect on the lesion volume, brain hemorrhage and expression of inflammatory mediator measurement. Analysis would start testing the tPA treatment effect on DM rats and WT rats interaction, followed by the subgroup analysis, if the interaction was detected at the 0.05 level. Spearman correlation coefficients were calculated to study the correlation between functional tests and lesion volume, brain hemorrhage and expression of inflammatory mediators.
RESULTS
Neurological functional outcome, lesion volume
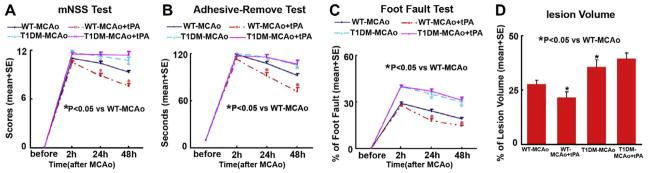
To test the effect of tPA treatment in T1DM rats after stroke on functional outcome, mNSS, adhesive-removal test and foot-fault test were performed. Global test statistical analysis was used to detect the differences in functional recovery among the four groups (WT, WT-tPA, DM and DM-tPA). A significant interaction indicates that tPA effects in DM rats differed from the effect in WT rats based on the Global test (p < 0.05) at 24 and 48 h (each time point) after MCAo. tPA treatment in WT-MCAo rats significantly improves functional outcome at 24 and 48 h after MCAo (p < 0.05, Global test) and significantly decreases lesion volume compared with non-treatment WT-MCAo control rats (p < 0.05, Fig. 1). However, T1DM-MCAo rats exhibit significantly increased functional deficit at 24 and 48 h after MCAo compared to WT-MCAo rats (p < 0.05, Global test, Fig. 1). In addition, tPA treatment of T1DM-MCAo rats did not improve functional outcome at 24 and 48 h after MCAo and decrease lesion volume compared with non-treatment T1DM-MCAo rats. These data suggest that tPA treatment promotes functional outcome and decreases lesion volume in WT-MCAo rats, but does not improve functional outcome nor reduce lesion volume in T1DM-MCAo rats.
Fig. 1.
Functional tests and lesion volume measurements: tPA treatment of stroke in WT rats significantly improves functional outcome and reduces lesion volume compared to WT-MCAo rats. T1DM-MCAo rats exhibit significantly attenuated functional outcome and increased lesion volume compared to WT-MCAo rats. tPA treatment of stroke in T1DM rats does not improve functional outcome or reduce lesion volume. (A) mNSS test. (B) Adhesive removal test. (C) Foot-fault test. (D) Lesion volume measurement.
Blood–brain barrier leakage, brain hemorrhage volume
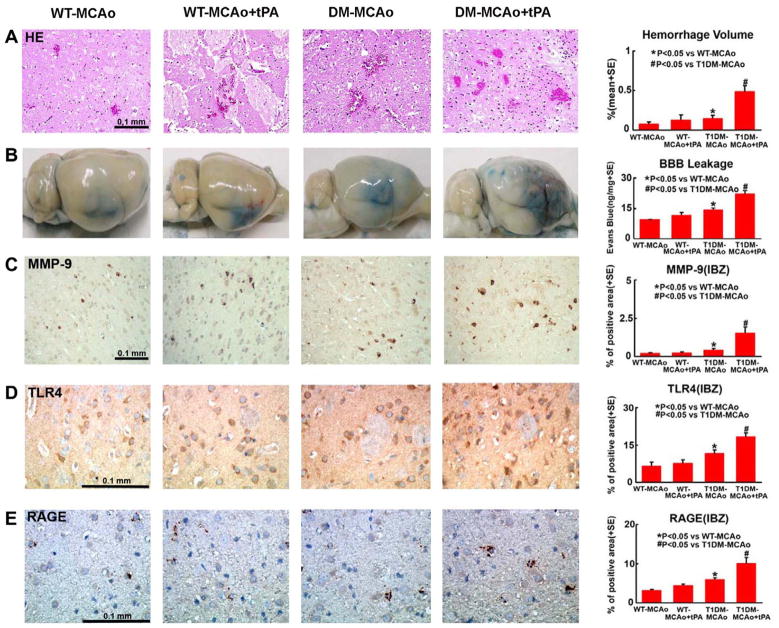
To test the mechanisms underlying the failure of tPA treatment to reduce functional outcome after stroke in T1DM rats, BBB leakage and brain hemorrhagic transformation were measured at 48 h after MCAo. Using Evans Blue assay, we found that T1DM-MCAo significantly increased brain hemorrhage volume (Fig. 2A) and BBB leakage (Fig. 2B) compared with WT-MCAo rats (p < 0.05). tPA treatment 2 h after MCAo in WT-MCAo rats did not increase brain hemorrhage and BBB leakage compared with WT-MCAo control rats (p > 0.05). However, tPA treatment significantly increased BBB leakage and brain hemorrhage volume in T1DM-MCAo rats compared with non-treatment T1DM-MCAo control rats (p < 0.05).
Fig. 2.
BBB leakage, brain hemorrhage and RAGE, TLR4 and MMP-9 expression in the ischemic brain: T1DM-MCAo rats show increased BBB leakage, brain hemorrhage volume, and MMP-9, TLR4 and RAGE expression after stroke compared to WT-MCAo rats. tPA treatment increases BBB leakage, brain hemorrhage volume, and MMP-9, TLR4 and RAGE expression in T1DM-MCAo rats compared to non-treatment T1DM-MCAo rats. (A) Brain hemorrhage measurement. (B) Evans Blue dye assay for BBB leakage. (C–E) MMP-9, TLR4 and RAGE immunostaining and quantitative data in IBZ. Scale bar in C–E = 0.1 mm.
RAGE, MMP-9 and TLR4 expression
To investigate the effect of tPA treatment on the inflammatory reaction in the ischemic brain, RAGE, MMP-9 and TLR4 expression were measured. Fig. 2 shows that RAGE, MMP-9 and TLR4 expression were higher in T1DM-MCAo rats compared with WT-MCAo rats, which is consistent with our previous studies (Ye et al., 2011). We found that there was no significant difference in RAGE, MMP-9 and TLR4 expression between treatment with or without tPA in WT-MCAo groups. However, tPA treatment significantly increased RAGE, MMP-9 and TLR4 expression in the ischemic brain in T1DM-MCAo rats compared to non-treated T1DM-MCAo rats (p < 0.05).
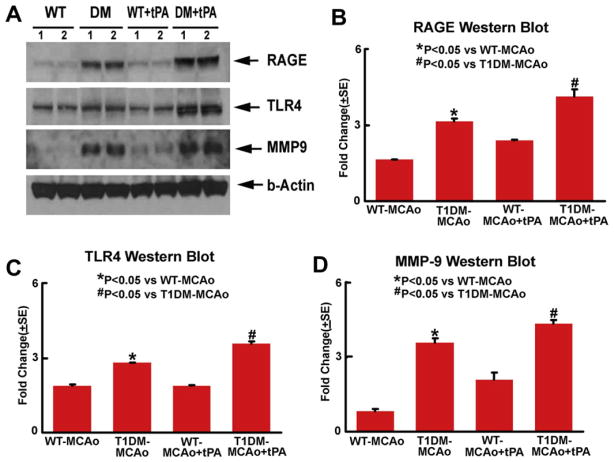
Consistent with the immunostaining, the Western blot assay also shows that tPA treatment of T1DM-MCAo rats significantly increased TLR4, RAGE and MMP-9 expression in the ischemic brain compared to non-treatment T1DM-MCAo rats (Fig. 3). These data suggest that tPA treatment in T1DM-MCAo rats increases inflammatory mediators in the ischemic brain.
Fig. 3.
Western blot assay: T1DM-MCAo rats exhibit increased brain tissue TLR4, RAGE and MMP-9 levels after stroke compared to WT-MCAo rats. tPA treatment significantly increases brain tissue TLR4, RAGE and MMP-9 levels in T1DM-MCAo rats compared to non-treatment T1DM-MCAo rats (p < 0.05).
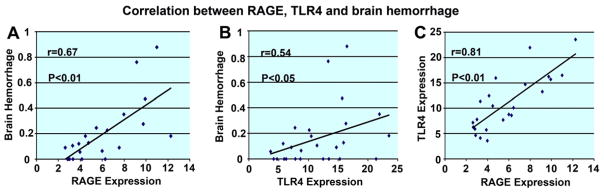
To understand the contributing role of the inflammatory factors in the tPA-induced failed functional outcome after stroke in T1DM rats, correlation analyses among brain RAGE, TLR4 and MMP expression and functional outcome, lesion volume and brain hemorrhage were assessed. Using the Spearman correlation analysis, Fig. 4 shows that brain hemorrhage significantly correlated with RAGE (r = 0.67, p < 0.01) and TLR (r = 0.54, p < 0.05) expression in the ischemic brain. RAGE expression was also strongly correlated with TLR4 expression (r = 0.81, p < 0.01) in the ischemic brain. Brain hemorrhage was significantly correlated with functional outcomes measured by mNSS test (r = 0.53, p < 0.05) and foot-fault test (r = 0.52, p < 0.05) 48 h after MCAo. These data suggest that increasing brain hemorrhage may be related with the failed functional outcome after stroke in T1DM stroke animals, and the increased expression of RAGE and TLR4 may play an important role in tPA-induced brain hemorrhage transformation in the ischemic brain in T1DM rats.
Fig. 4.
Correlation analysis: brain hemorrhage significantly correlated with RAGE and TLR expression in the ischemic brain. RAGE expression is strongly correlated with TLR4 expression in the ischemic brain. (A) Correlation analysis of brain hemorrhage with RAGE. (B) Correlation analysis of brain hemorrhage with TLR4. (C) Correlation analysis of RAGE with TLR4.
DISCUSSION
The results of the present study demonstrate that treatment of stroke in diabetic rats with tPA initiated at 2 h after MCAo fails to improve functional outcome and significantly increases brain hemorrhage, BBB leakage and the expression of inflammatory mediators RAGE, MMP-9 and TLR4. The increasing inflammatory response, such as RAGE and TLR4, significantly correlated with brain hemorrhage which may contribute to the reduced functional outcome in tPA-treated T1DM rats.
The inflammatory response is progressive after stroke and it contributes to the evolution of brain tissue injury (Barone and Feuerstein, 1999) and is associated with vascular disease (Chamorro and Hallenbeck, 2006). RAGE amplifies immune and inflammatory responses (Schmidt et al., 2001) and has been implicated in chronic diseases such as diabetes, atherosclerosis, neurodisorders, as well as aging. RAGE is a sensor of necrotic cell death and promotes inflammation and ischemic brain damage (Muhammad et al., 2008; Hassid et al., 2009). The upregulation of RAGE plays an essential role in the pathogenesis of diabetic atherosclerosis (Ihara et al., 2007) and has been shown to be involved in both micro-diabetic (Yamamoto et al., 2001) and macrodiabetic (Yan et al., 2003) vascular complications and impairment of angiogenesis in diabetes and in Abeta-induced neuronal and synaptic dysfunction (Origlia et al., 2009). In addition, diabetes regulates the advanced glycation end-product (AGE) (Schwenger et al., 2001; Vlassara and Palace, 2002), and the interaction of AGE with RAGE in endothelial cells activates the transcription factor nuclear factor-Kappa B (NF-κB), subsequently leading to increased expression of proatherogenic mediators, such as monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) (Schmidt et al., 1995). RAGE may also mediate MMP-9 and increase apoptosis in diabetic animals (Ishibashi et al., 2010).
MMP-9 is a potentially deleterious neurovascular protease (Qiu et al., 2010), associated with BBB disruption, neuronal apoptosis and survival (Copin et al., 2005; Kumari et al., 2011; Murase and McKay, 2012) and is significantly increased in the ischemic brain of diabetic mice after stroke (Chen et al., 2011). Diabetic mice with stroke have significantly increased neutrophil invasion, increased degradation of occludin and collagen IV and subsequently, increased BBB permeability (Kumari et al., 2011). Additionally, action of TLR4 signaling may lead to worse neurological functional recovery from stroke and increased infarct volumes and inflammatory response (Buchanan et al., 2010), while TLR4-deficient mice show smaller infarct volumes, better neurological outcomes and less inflammatory response after cerebral ischemia (Caso et al., 2007).
Our previous study has found that T1DM-MCAo rats exhibit significantly increased RAGE, TLR4 and MMP-9 expression in the ischemic brain compared to WT-MCAo rats (Ye et al., 2011). In this study, we demonstrate that tPA treatment significantly increased RAGE, MMP-9 and TLR4 expression in T1DM-MCAo rats compared to non-treatment T1DM-MCAo animals. The increased expression of RAGE and TLR4 significantly correlated with brain hemorrhage in the ischemic brain in T1DM rats. Therefore, the increased immune/inflammatory response may lead to increased brain hemorrhage and BBB leakage, which may contribute to the failed neuroprotective effects of tPA treatment in T1DM rats.
CONCLUSIONS
T1DM increases brain hemorrhage, BBB leakage and RAGE, TLR4 and MMP-9 expression after stroke. Treatment of stroke with tPA at 2 h after MCAo enlarged brain hemorrhage, augmented BBB leakage and exacerbated functional outcome in T1DM rats. The increased immune/inflammatory response as indicated by the increase of RAGE and TLR4 and MMP-9 expression may contribute to the failed neuroprotective effects of tPA treatment in T1DM rats. These data suggest that the use of tPA in the diabetic population after stroke warrants further investigation.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIA RO1 AG031811 (J.C.) and American Heart Association Grant 09GRNT2300151 (J.C.).
Abbreviations
- AGE
advanced glycation end-product
- BBB
blood–brain barrier
- DM
diabetes mellitus
- ECA
external carotid artery
- H&E
hematoxylin and eosin
- HMGB1
high-mobility group box 1
- IBZ
ischemic border area
- ICA
internal carotid artery
- MCAo
middle cerebral artery occlusion
- MMP-9
matrix metalloproteinase-9
- mNSS
modified neurological severity score
- RAGE
advanced glycation endproducts receptor
- T1DM
type-1 diabetes
- TLR4
toll-like receptor 4
Footnotes
DISCLOSURES
None.
References
- Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283:1145–1150. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator-treated patients. Stroke. 2003;34:1235–1241. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- Barile GR, Schmidt AM. RAGE and its ligands in retinal disease. Curr Mol Med. 2007;7:758–765. doi: 10.2174/156652407783220778. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Barth TM, Grant ML, Schallert T. Effects of MK-801 on recovery from sensorimotor cortex lesions. Stroke. 1990;21:III153–III157. [PubMed] [Google Scholar]
- Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res. 1995;676:231–234. doi: 10.1016/0006-8993(95)00150-o. [DOI] [PubMed] [Google Scholar]
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003a;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003b;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copin JC, Goodyear MC, Gidday JM, Shah AR, Gascon E, Dayer A, Morel DM, Gasche Y. Role of matrix metalloproteinases in apoptosis after transient focal cerebral ischemia in rats and mice. Eur J Neurosci. 2005;22:1597–1608. doi: 10.1111/j.1460-9568.2005.04367.x. [DOI] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- Fan X, Qiu J, Yu Z, Dai H, Singhal AB, Lo EH, Wang X. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke. 2012;43:567–570. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- Goeman JJ, van de Geer SA, de Kort F, van Houwelingen HC. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics. 2004;20:93–99. doi: 10.1093/bioinformatics/btg382. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hassid BG, Nair MN, Ducruet AF, Otten ML, Komotar RJ, Pinsky DJ, Schmidt AM, Yan SF, Connolly ES. Neuronal RAGE expression modulates severity of injury following transient focal cerebral ischemia. J Clin Neurosci. 2009;16:302–306. doi: 10.1016/j.jocn.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Egashira K, Nakano K, Ohtani K, Kubo M, Koga J, Iwai M, Horiuchi M, Gang Z, Yamagishi S, Sunagawa K. Upregulation of the ligand-RAGE pathway via the angiotensin II type I receptor is essential in the pathogenesis of diabetic atherosclerosis. J Mol Cell Cardiol. 2007;43:455–464. doi: 10.1016/j.yjmcc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Kawaguchi M, Sugimoto K, Uekita H, Sakamoto N, Yokoyama K, Maruyama Y, Takeishi Y. Advanced glycation end product-mediated matrix metallo-proteinase-9 and apoptosis via renin–angiotensin system in type 2 diabetes. J Atheroscler Thromb. 2010;17:578–589. doi: 10.5551/jat.3590. [DOI] [PubMed] [Google Scholar]
- Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002;36:1331–1336. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Patel SD, Baskerville KA, Simpson IA. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem. 2011;119:1029–1040. doi: 10.1111/j.1471-4159.2011.07487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. discussion 1980–1971. [DOI] [PubMed] [Google Scholar]
- Lu M, Chen J, Lu D, Yi L, Mahmood A, Chopp M. Global test statistics for treatment effect of stroke and traumatic brain injury in rats with administration of bone marrow stromal cells. J Neurosci Methods. 2003;128:183–190. doi: 10.1016/s0165-0270(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Maillard-Lefebvre H, Boulanger E, Daroux M, Gaxatte C, Hudson BI, Lambert M. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inflammatory diseases. Rheumatology. 2009;48:1190–1196. doi: 10.1093/rheumatology/kep199. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Green EJ, Hurwitz BE, Morikawa E, Dietrich WD, McCabe PM, Ginsberg MD, Schneiderman N. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res. 1992;575:238–246. doi: 10.1016/0006-8993(92)90085-n. [DOI] [PubMed] [Google Scholar]
- Mast H, Thompson JL, Lee SH, Mohr JP, Sacco RL. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke. 1995;26:30–33. doi: 10.1161/01.str.26.1.30. [DOI] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, McKay RD. Matrix metalloproteinase-9 regulates survival of neurons in newborn hippocampus. J Biol Chem. 2012;287:12184–12194. doi: 10.1074/jbc.M111.297671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen F, Hilger T, Hoehn M, Hossmann KA. Thrombolytic treatment of clot embolism in rat: comparison of intra-arterial and intravenous application of recombinant tissue plasminogen activator. Stroke. 2002;33:2999–3005. doi: 10.1161/01.str.0000038096.60932.f4. [DOI] [PubMed] [Google Scholar]
- Origlia N, Capsoni S, Cattaneo A, Fang F, Arancio O, Yan SD, Domenici L. Abeta-dependent inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE. J Alzheimers Dis. 2009;17:59–68. doi: 10.3233/JAD-2009-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala J, Liebkind R, Gordin D, Thorn LM, Haapaniemi E, Forsblom C, Groop PH, Kaste M, Tatlisumak T. Diabetes mellitus and ischemic stroke in the young: clinical features and long-term prognosis. Neurology. 2011;76:1831–1837. doi: 10.1212/WNL.0b013e31821cccc2. [DOI] [PubMed] [Google Scholar]
- Qiu J, Xu J, Zheng Y, Wei Y, Zhu X, Lo EH, Moskowitz MA, Sims JR. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke. 2010;41:2077–2082. doi: 10.1161/STROKEAHA.110.590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, Vaughn D, Wilcox RE. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenger V, Zeier M, Henle T, Ritz E. Advanced glycation endproducts (AGEs) as uremic toxins. Die Nahrung. 2001;45:172–176. doi: 10.1002/1521-3803(20010601)45:3<172::AID-FOOD172>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Liang RS, Shi SS, Chen JP, Chen CM, Wang CH, Xie HS, Chen Y, Ouyang LQ. Effect of baicalin on matrix metalloproteinase-9 expression and blood–brain barrier permeability following focal cerebral ischemia in rats. Neurochem Res. 2011;36:2022–2028. doi: 10.1007/s11064-011-0526-y. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep. 2007;7:242–248. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by increasing superoxide production. Ann Neurol. 2011;70:583–590. doi: 10.1002/ana.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KK, Huan Y. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2008;Chapter 5(Unit5):47. doi: 10.1002/0471141755.ph0547s40. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, Okamoto H, Yamamoto H. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–268. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, Roberts C, Chen J. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]