Abstract
Estrogens mediate profound effects throughout the body, and regulate physiological and pathological processes in both women and men. The decreased incidence of many diseases in premenopausal women is attributed to the presence of 17β-estradiol, the predominant and most potent endogenous estrogen. In addition to endogenous estrogens, however, several manmade and plant-derived molecules also exhibit estrogenic activity. Traditionally, the actions of 17β-estradiol are ascribed to two nuclear estrogen receptors (ERs), ERα and ERβ, which function as ligand-activated transcription factors. However, 17β-estradiol also mediates rapid signaling events via pathways that involve transmembrane ERs, such as G-protein-coupled ER 1, (GPER, formerly known as GPR30). In the past 10 years, GPER has been implicated in both rapid signaling and transcriptional regulation. With the discovery of GPER-selective ligands that can selectively modulate GPER function in cell experiments and preclinical studies, and the use of GPER-knockout mice, many more potential roles for GPER are currently being elucidated. This Review highlights the physiological roles of GPER in the reproductive, nervous, endocrine, immune and cardiovascular systems, as well as its pathological roles in a diverse array of disorders including cancer. GPER is emerging as a novel therapeutic target and prognostic indicator for these diseases.
Introduction
17β-Estradiol is commonly recognized as the female sex hormone with a critical role in the development of the female reproductive organs and secondary sex characteristics. However, this hormone is also essential to the development and function of the male reproductive tract.1 In addition to the reproductive system, 17β-estradiol has important physiological roles in almost every other arena of the body, including the nervous, immune, vascular, muscular, skeletal and endocrine systems. As expected, therefore, 17β-estradiol and its receptors contribute to multiple disorders, including cancer, cardiovascular diseases, hypertension, osteoporosis, cognitive and behavioral alterations, neurodegenerative diseases, metabolic disorders (such as obesity and diabetes) and immune disorders.2 Our understanding of the widespread physiological effects of 17β-estradiol is complicated by the existence of multiple types of estrogen receptors (ERs) and multiple modes of cellular signaling mechanisms that can span time frames from seconds to hours, or even days.3,4 The pathophysiological mechanisms involving ERs are further complicated by a diverse array of 17β-estradiol-mimicking compounds, both synthetic and plant-derived, to which humans are increasingly exposed.5
In this Review, we provide a brief overview of estrogen signaling and describe the discovery and characterization of its receptors, with particular emphasis on G-protein-coupled estrogen receptor 1 (GPER). We will also discuss studies that have elucidated the functions and importance of GPER in health and disease and those that have revealed the therapeutic potential of small-molecule regulators of GPER activity.
Estrogen receptors
ERα and ERβ
The first and best-described 17β-estradiol receptor (now called ERα) was identified in the rat uterus in the 1960s.6,7 The second, less well-characterized receptor, ERβ, was identified in the rat prostate in 1996.8 These highly homologous receptors function as ligand-activated nuclear transcription factors that bind cis-acting estrogen response elements in the promoter and enhancer regions of hormonally regulated genes.9 Both ERα and ERβ are soluble receptors that can shuttle between the cytoplasm and the nucleus, but are found predominantly in the nucleus (only ~5% of these receptors are present in the cytoplasm).4 Highly divergent and sometimes opposing functions for the two receptors have been reported in studies of ERα-knockout and ERβ-knockout mice.10 In addition to their effects on gene expression (that is, their genomic effects), these ERs are also associated with rapid cellular signaling (termed non-genomic effects) that are thought to be mediated primarily by membrane-associated forms of these receptors.11
Although multiple modes of action were suggested for these two ERs as early as the 1960s,12,14 not all effects of 17β-estradiol, particularly the rapid and membrane-associated signaling events, could be attributed to ERα and ERβ.15 In some cases, antagonists of these receptors could not block certain rapid signaling events, which led to the prediction that alternate membrane-bound ERs also existed.16 Interestingly, most of the 17β-estradiol-mediated rapid signaling events are associated with G protein signaling or growth factor-mediated pathways.
GPER
In 2000, it was reported that rapid 17β-estradiol-mediated activation of extracellular signal-regulated kinases (ERKs) was dependent on the expression of an orphan, G protein-coupled receptor with seven transmembrane domains.17 This receptor, which was then known as GPR30, was cloned by many groups in the late 1990s.18–23 Following this initial report, other papers described 17β-estradiol-mediated, GPR30-dependent, generation of cyclic AMP (cAMP)25 and expression of Bcl-2,26 nerve growth factor27 and cyclin D2.28 Furthermore, other researchers described GPR30-mediated expression of c-Fos29 and an interaction between the effects of progestin and GPR30 expression.30–32 Two studies published in 2005 described binding of 17β-estradiol to GPR30 in GPR30-transfected COS7 and HEK293 cells as well as various breast cancer cell lines.33,34 Together, these results suggested that GPR30 was a 17β-estradiol-binding receptor, which led to its designation as G protein-coupled estrogen receptor 1 (GPER) in 2007. GPER is now known to be expressed in numerous tissues,24 and research into its functions has substantially increased.
Estrogen receptor ligands
Natural endogenous estrogens, predominantly 17β-estradiol, are the primary ligands of ERs. 17β-estradiol is synthesized predominantly in the ovaries, although it is also produced at many sites throughout the body, including the breast, brain, adipose tissue and the arterial wall, where it might have specialized local effects.35 The 17β-estradiol-based steroids estriol (a GPER antagonist at high concentrations36), estrone and estrone sulfate can also modulate biological functions, although their specific actions are less clear than those of 17β-estradiol.37 Plasma concentrations of 17β-estradiol in premenopausal women are ~0.2–1.0 nmol/l, although it increases by many hundredfold during pregnancy. Local concentrations in specific tissues can be much higher than the plasma values, for example in breast tissue (10–20-fold)38 or in the placenta at term (~12 μmol/l).39 The hydrophobic nature of these steroids allows them to diffuse passively through cell membranes and reach their intracellular targets, the ERs.40
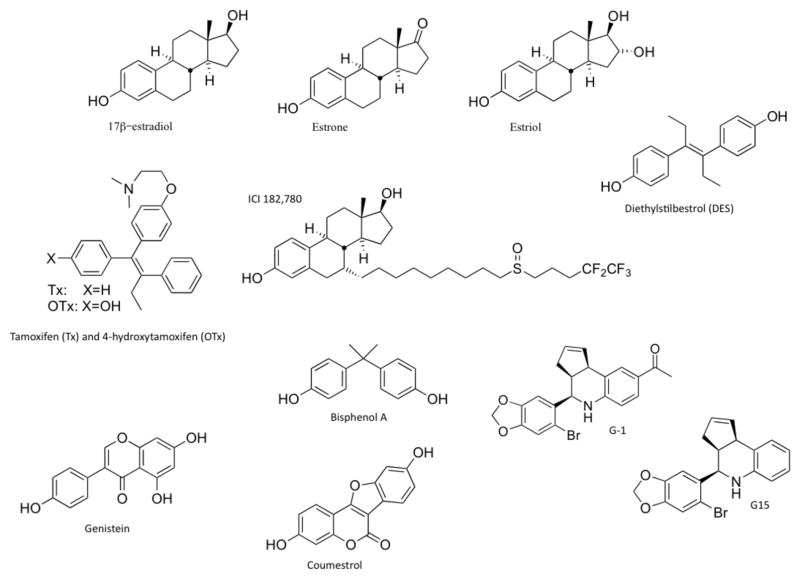
A large variety of natural and man-made chemicals also have estrogenic activity (Figure 1).5 Estrogenic compounds synthesized by plants (phytoestrogens) include flavonoids such as coumestans and isoflavones.41 Synthetic estrogenic compounds (known as xenoestrogens, environmental estrogens, or endocrine disruptors) include many pesticides, herbicides and plastic monomers.5 Their widespread use results in chronic low-level exposure to these compounds in humans.42 Although the majority of phytoestrogens and xenoestrogens are believed to exert their physiological effects through modulation of ERα and ERβ,43 many of these compounds also activate GPER, including the soy isoflavone genistein (for which serum concentrations up to 500 nmol/l have been measured44), nonylphenol, the pesticides DDT and DDE (dichlorodiphenyltrichloroethane and dichlorodiphenyldichloroethylene, respectively), bisphenols45 (such as bisphenol A, which promotes testicular seminoma cell proliferation46), the herbicide atrazine47 and possibly equol—a nonsteroidal equine estrogen found in premarin48 that is formed by human gut bacteria as a metabolite of the isoflavone, daidzein.49
Figure 1.
Structures of selective and nonselective estrogen receptor ligands. Compounds shown include the three major physiological forms of estrogen (17β-estradiol, estrone and estriol); the anticancer agent tamoxifen and its active metabolite 4-hydroxytamoxifen (which is both a selective estrogen receptor modulator and an agonist for GPER); fulvestrant, a selective estrogen receptor downregulator and agonist for GPER; diethylstilbestrol, a nonselective GPER agonist; the phytoestrogens genistein and coumestrol; and the xenoestrogen bisphenol A. Also shown are G-1 (a selective GPER agonist) and G15 (a selective GPER antagonist). Abbreviation: GPER, G-protein-coupled estrogen receptor 1.
17β-estradiol mimetics are also used extensively in clinical and therapeutic applications. For example, 17α-ethynylestradiol is the predominant estrogen used in female contraceptives. Drugs, such as tamoxifen and raloxifene, which are used in treatments for breast cancer and osteoporosis, act as ER agonists in some tissues and ER antagonists in others, which led to their designation as selective estrogen receptor modulators (SERMs).50 By contrast, fulvestrant is a ‘pure’ ER antagonist that leads to ER degradation and/or downregulation, which led to its designation as a selective estrogen receptor downregulator (SERD).51 However, some members of each of these classes of compounds can also act as GPER agonists,17,34 which complicates the interpretation of their mechanisms of action and the receptors involved under both physiological and disease conditions.52
GPER-selective ligands
Research into the specific activities of GPER has been aided by the discovery of GPER-selective agents. Since the identification of the first GPER-selective agonist G-1 in 2006, a number of reports have examined the disease-related or health-promoting effects associated with GPER activation. Importantly, studies using G-1 at concentrations as high as 1–10 μmol/l showed no notable activity of this agent towards ERα in terms of activating or inhibiting rapid signaling events,34 estrogen response element-mediated transcription,53 or ERα downregulation.53 Furthermore, G-1 had no activity on 25 other important G-protein-coupled receptors54 or in GPER-knockout mice,55–57 which provided evidence that G-1 is a specific ligand for GPER.
In 2009, a GPER-selective antagonist G15 was identified.58 G15 has a similar structure to G-1,58 and is effective in inhibiting all G-1-mediated effects tested to date as well as many 17β-estradiol-mediated effects.58–62 The core structures of G-1 and G15 have been used to generate several radiolabeled agents that can be used for imaging and potentially treatment of GPER-expressing tumors in viv.63,64
GPER signaling
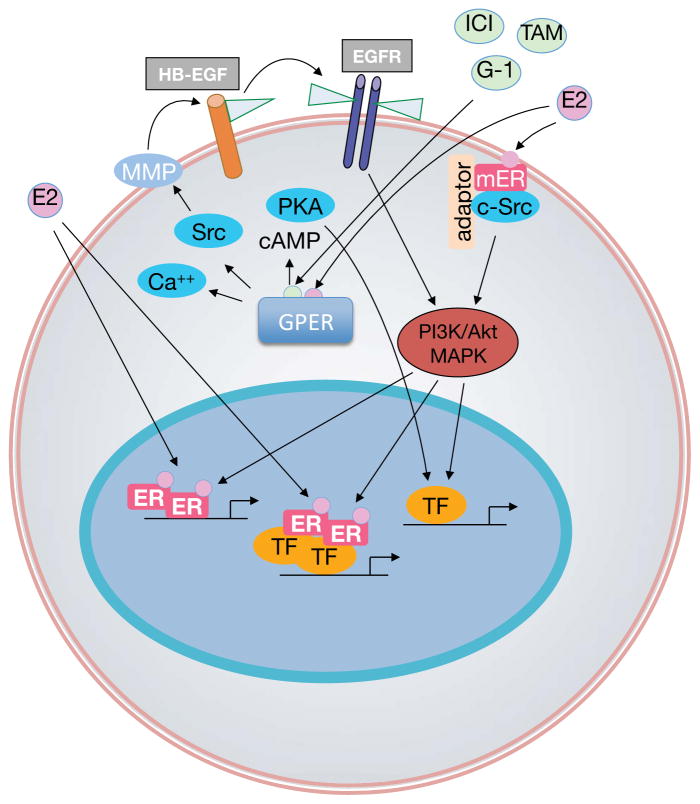
Although ERα and ERβ are accepted as the predominant nuclear receptors involved in the genomic effects of estrogen, evidence also indicates that rapid modulation of cell-signaling pathways occurs via a subpopulation of ERs located at the plasma membrane (Figure 2),4 which has led to speculation about the role of GPER.65 The localization of GPER, however, seems to be predominantly intracellular,34,203 consistent with reports that describe the constitutive internalization of plasma membrane GPER.135,204
Figure 2.
Nongenomic and genomic estrogen signaling pathways. Endogenous estrogens including 17β-estradiol (E2) are nonselective activators of the three known estrogen receptors (ERs), ERα, ERβ and GPER. E2 activates nuclear ERs, inducing receptor dimerization, and binding of receptor dimers to the promoters of target genes. Alternatively, activated ERs modulate the function of other classes of transcription factors (TF) through protein–protein interactions. Subpopulations of ERs at the plasma membrane (mER) activated by E2 interact with adaptor proteins (adaptor) and signaling molecules such as c-Src, which mediates rapid signaling via PI3K/Akt and MAPK pathways. E2, or selective agonists such as G-1, or SERDs such as fulvestrant, or SERMs such as tamoxifen, also activate GPER, which is predominantly localized intracellularly. GPER activation stimulates cAMP production, calcium mobilization and c-Src, which activates matrix metalloproteinases (MMP). MMPs cleave pro-heparin-binding-epidermal growth factor (HB-EGF), releasing free HB-EGF that transactivates EGF receptors (EGFR). EGFR in turn activates MAPK and PI3K/Akt pathway, which can induce rapid (nongenomic) effects (X), or genomic effects regulating gene transcription. E2-mediated transcriptional regulation may involve phosphorylation (P) of ER or other TFs that may directly interact with ER, or bind independently of ER within the promoters of target genes.
Signaling through GPER occurs via transactivation of the epidermal growth factor receptor (EGFR) and involves nonreceptor tyrosine kinases of the Src family.17 In this mechanism, which is now also accepted for other G-protein-coupled receptors,66 stimulation of GPER activates metalloproteinases and induces the release of heparin-binding EGF, which binds and activates EGFR67 leading to downstream activation of signaling molecules, such as ERK1 and ERK2.68 Moreover, 17β-estradiol-mediated activation of GPER stimulates production of cAMP,25,33 intracellular calcium mobilization34,69,70 and PI3K activation.34 Further research in human breast cancer cells suggested that sphingosine kinase71 and activation of integrin α5β172 were intermediates in 17β-estradiol-mediated EGFR transactivation. The latter suggests a role for GPER in fibronectin assembly.72
In addition to the above-mentioned rapid signaling events, GPER also regulates transcriptional activity, albeit indirectly, by activating signaling mechanisms that involve cAMP, ERK and PI3K.{Meyer, 2009 #1462} The genes regulated by GPER include FOS that encodes c-Fos,29 which forms a heterodimer with various other proteins to form the transcription factor AP-1. In turn, these signaling pathways also activate other transcription factors, such as steroidogenic factor 1,73 which induce expression of additional genes.74,75
GPER in Physiology and Disease
Reproductive system
The role of 17β-estradiol is best-defined in the reproductive system, where this hormone regulates uterine and mammary development and function. Although roles for GPER are implicated in almost every system of the body (Figure 3), conflicting observations have been published.24 No clear developmental or functional defects occur in the reproductive organs of GPER-knockout mice,76–79 whereas ERα-knockout mice displayed multiple reproductive defects.80 Furthermore, in wild-type mice treated with G-1, no changes in ductal growth or end bud formation were detected in mammary glands, and no uterine imbibition of water or proliferative response in the mammary gland or endometrium was observed.78 However, in another study, G-1 treatment of mice stimulated uterine epithelial proliferation by approximately threefold, as compared to the ~15-fold increase in proliferation observed with 17β-estradiol.58 Importantly, blocking GPER with G15 reduced the 17β-estradiol-mediated proliferative response by ~50%,58 which suggests that GPER contributes to this response. Surprisingly, high concentrations of G-1 (1,000-fold greater than those needed to observe a proliferative effect) reduce both 17β-estradiol-mediated uterine imbibition of water and proliferation, through inhibition of ERK1 and/or ERK2 in the stroma and phosphorylation of serine 118 in ERα. 81 These data suggest that GPER regulates uterine proliferation, independently of ERα, in a process that may involve crosstalk with the 17β-estradiol–ERα pathway.
Figure 3.
Involvement of GPER action in regulation of physiological responses, including neuroendocrine and cerebral functions, immune cell function, endocrine regulation and metabolism, cardiovascular and kidney function, and reproductive functions. In addition, studies using experimental models of disease and/or human tissue suggest roles for GPER in diseases (such as diabetes, arterial hypertension, proteinuric renal disease, and immune diseases such as multiple sclerosis and cancer;) shown in red. Collectively, such studies suggest the therapeutic potential of regulating GPER activity as a novel approach for the treatment of these conditions.
In addition to mammalian uterine effects, GPER is also involved in the regulation of meiotic arrest in oocytes of the Atlantic croaker and zebra fish. In vitro, 17β-estradiol and G-1 reduced both spontaneous and progestin-induced oocyte maturation, whereas knockdown of GPER or blockade of GPER with G15 prevented the inhibitory effects of 17β-estradiol, which occur via an EGFR-dependent pathway.61,82 Furthermore, GPER expression in granulosa and theca cells of the hamster ovary is regulated by gonadotropins and the estrous cycle84 and, in this location, GPER regulates the 17β-estradiol-mediated stimulation of primordial follicle formation.83 In humans, GPER enhances contractile responses to oxytocin in the myometrium, which suggests a role for GPER in uterine contractility during labor.85 Moreover, ERα, ERβ and GPER regulate the proliferative and apoptotic pathways involved in spermatogenesis86–88 during male reproductive development. Overall, the roles of GPER in the reproductive system are complex and require further investigation, particularly with regard to human physiology.
Nervous system and neurohormonal pathways
The effects of 17β-estradiol in the central and peripheral nervous system include maintenance of homeostasis, regulation of synaptic plasticity and cognition, neuroprotection and modulation of pain sensation. Although many of these effects might involve ERα and ERβ, increasing evidence indicates that GPER has multiple roles in 17β-estradiol-mediated neurological functions. GPER mRNA and protein expression have been found throughout the central and peripheral nervous system of male and female rodents, including in the hippocampus, hypothalamus and midbrain, as well as the spinal cord and dorsal root ganglia.70,89,90 However, conflicting results reporting expression in small arterial surface vessels and pericytes in the brain also exist.76 Both ERα and GPER activate the ERK1/2 pathway in trigeminal ganglion neurons and increase allodynia, indicating a role for these two ERs in temporomandibular disorder and migraine.91 Furthermore, in the rat, G-1 depolarizes spinal cord neurons,89 stimulates mechanical hyperalgesia via protein kinase C ξ92 and mediates visceral hypersensitivity in the absence of inflammation.93
17β-Estradiol has many beneficial effects on the brain, including reducing neuronal loss following stroke, increasing neuronal connectivity and improving cognitive performance.94 GPER has been implicated in 17β-estradiol-mediated effects on cholinergic neurons in the basal forebrain, which suggests that this ER might be an important regulator of cognitive function, particularly in women following menopause.95 In studies that used immortalized hippocampal cell lines, GPER (along with ERα) was implicated in the protective effects of 17β-estradiol against glutamate-induced injury,62 although in cortical neurons G-1 did not have any effect.96 However, in vivo studies showed that G-1 treatment replicates the effects of 17β-estradiol in promoting neuronal survival following global ischemia in the brain.97,98 Altogether, these results suggest that GPER agonists might represent a new therapeutic approach for stroke and chronic neurodegenerative diseases.99
In the brain, G-1 (like 17β-estradiol) attenuates serotonin receptor signaling in the paraventricular nucleus of the hypothalamus and reduces responses to oxytocin and adrenocorticotropic hormone, which suggests that GPER might have a role in mood disorders.100 Furthermore, G-1 exhibited antidepressant properties in a mouse model of depression, where it reproduced the effects of 17β-estradiol, which were inhibited by the GPER-selective antagonist G15.58 In primates, GPER contributes to 17β-estradiol-mediated regulation of luteinizing-hormone-releasing hormone neurons, which maintain gonadal function and fertility.101 This effect probably also involved additional mechanisms.102 However, whereas GPER activation promoted short latency prolactin secretion, G-1 did not affectthe 17β-estradiol-mediated negative feedback inhibition of either luteinizing hormone secretion or lordosis behavior in rats.103 Studies with ERα-knockout mice showed that ERα is required for 17β-estradiol regulated positive feedback control of hypothalamic gonadotropin release,104 which suggests that the actions of GPER are complex and possibly also require the presence of ERα.
Immune system
17β-estradiol displays multiple effects in the regulation of immune responses, including the development of T cells,105 autoimmune disease106,107 and inhibition of inflammation.106 Studies in ER-knockout and GPER-knockout mice have shown that GPER, along with ERα, contributes to 17β-estradiol-induced thymic atrophy;79 ERα mediated the early blockage of thymocyte development whereas GPER mediated thymocyte apoptosis. Furthermore, in GPER-knockout mice engineered to express LacZ from the GPER promoter, numbers of L-selectin-expressing T cells decreased, consistent with an altered production of these T cells in the thymus.76 By contrast, other studies using GPER-knockout mice could not find any difference in either 17β-estradiol-induced thymic atrophy108 or in 17β-estradiol-induced ameliorative effects on arthritis or bone loss in a model of postmenopausal rheumatoid arthritis.109 These findings suggest complex roles for 17β-estradiol and GPER in the immune system.
Estrogens are increasingly receiving attention as potential anti-inflammatory agents for the treatment of autoimmune diseases, particularly multiple sclerosis.107 In a mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), knocking out GPER impaired the protective role of 17β-estradiol.55 In two other studies, treatment with G-1 reproduced the ability of 17β-estradiol to protect against the clinical and histological manifestations of EAE through enhancing the immunosuppressive activity of CD4+Foxp3+ T cells, resulting in upregulation of programmed cell death55 and inhibition of inflammatory cytokine production by macrophages.54 These findings suggest a protective role of GPER in multiple sclerosis.
Although the protective effects of G-1 against EAE were absent in GPER-knockout mice, 17β-estradiol-mediated effects were partially retained, showing that ERα and GPER could activate independent, yet overlapping, mechanisms. Further research showed that the therapeutic effect of ethynyl estradiol in established EAE was mediated via GPER, but not via ERα, and possibly involved production of the anti-inflammatory cytokine IL-10.110 Recent studies showed that G-1 treatment elicits de novo production of IL-10 in T helper type 17 polarized cells, in vitro as well as in vivo, via an ERK1/2-dependent pathway.111 Thus, the immunomodulatory effects of G-1 mediated by activation of GPER indicate that GPER agonists might have novel clinical applications in chronic inflammatory diseases.
Cardiovascular system
Endogenous 17β-estradiol is implicated in sex-specific differences observed in arterial hypertension and cardiovascular disease,112,114 as the loss of 17β-estradiol production following menopause accelerates these conditions.112,149 However, the cellular mechanisms and signaling pathways conferring this protective effect of 17β-estradiol are only partially understood.115 Although ERα and ERβ are implicated in the cardiovascular protective effects of 17β-estradiol, a protective effect of 17β-estradiol is also seen in the absence of both ERα and ERβ.116–118 These observations provided the initial evidence for the existence of alternative receptors, such as GPER, and signaling pathways involved in 17β-estradiol-mediated regulation of cardiovascular function.
GPER is expressed in mouse77 and human137 myocardium, as well as in cultured cardiomyocytes.138 17β-Estradiol-mediated inhibition of calcium influx and contraction in mouse cardiomyocytes is independent of ERα and ERβ,116 and deletion of GPER from these cells leads to left ventricular dilatation and elevation of end-diastolic pressure in male, but not female, mice.139 In patients with myocardial infarction, ischemia–reperfusion injury after reopening of the occluded coronary artery is a critical determinant of outcome and complications, such as arrythmia and heart failure.140,141 Myocardial hypoxia owing to infarction142 is an important stimulus that upregulates GPER expression in cardiomyocytes.138 Several groups have independently demonstrated that G-1 treatment after myocardial infarction led to reduced reperfusion-related injury and infarct size, and improved contractile function in structurally normal hearts from rodents and humans of both sexes.137,140,143–145 Similar benefits were also obtained for G-1 treatment in cerebrovascular occlusion-related reperfusion injury, in animal models of stroke.97,146 Under these conditions, activation of GPER by G-1 resulted in reduced myocardial expression of proinflammatory cytokines (IL-1β, IL-6 and tumor necrosis factor),145 increased activation of Akt,132 ERK1/2,132,143 increased phosphorylation of eNOS132 and decreased mitochondrial permeability .144 These cardioprotective effects were blocked by an inhibitor of PI3 kinase.132
GPER is expressed in human endothelial23,119 and smooth muscle cells,57,120 as well as in intact arteries (Table 1).120 Expression of GPER in macrophages,128 which contribute to atherogenesis, also suggests a functional role for GPER in atherosclerosis and the associated inflammation (Table 1). In human endothelial cells, activation of GPER (but not of ERα) 121 inhibits cell proliferation,119 indicating an antiangiogenic role for this ER. In human and rat vascular smooth muscle cells, activation of GPER by either G-157,122 or raloxifene123 stimulates the ERK1/2 pathway and inhibits growth, similar to the effect of ERα activation in these cells.124 These findings are in keeping with the antiproliferative effects of 17β-estradiol on vascular smooth muscle cells in ERα and ERβ double-knockout mice.117 Moreover, the GPER agonists G-1,57,60,125,126 genistein127 and fulvestrant125 cause vasodilatation in human, porcine and rodent arteries, whereas this effect is blocked by the GPER antagonist G1560 and is absent in GPER-deficient mice.57
Elevated vascular resistance is a key feature of arterial hypertension.114 Although GPER-deficient mice exhibit a normal mean arterial blood pressure that does not change with age,77 infusion of the GPER agonist G-1 markedly lowers blood pressure in normotensive57 and hypertensive rats.60,129,130 In rats with hypertensive cardiomyopathy, G-1 treatment ameliorates diastolic dysfunction, reduces cardiac hypertrophy and decreases the size of myocytes.129 This effect is probably mediated through direct vasodilatory actions of G-157,126,131 or 17β-estradiol, as this hormone also has vasodilatory effects (which are derived at least in part from GPER, as they are blocked by the GPER antagonist G15).60 Vasodilatory actions of G-1 involve both nitric-oxide-dependent and nitric-oxide-independent pathways and have been observed in human, pig and rat arteries.57,60,126,130 Phosphorylation of eNOS as a result of GPER activation might contribute to this response.49,132 At least some of the vasoprotective effects mediated by GPER are probably the result of interference with endothelial cell dysfunction—a vascular abnormality common to hypertension and coronary artery disease.113,133
Altogether, these data indicate a central regulatory role for GPER in cardiovascular function and suggest that GPER agonists have potential roles in the treatment of vascular and myocardial disease in both men and women.
Renal system
Endogenous 17β-estradiol is also implicated in the sex-specific differences in renal disease,113 and GPER is implicated as it is expressed at high levels in renal tubules,90 as well as in renal epithelial cells.135 In humans, the GPER locus is associated with low-renin hypertension,134 which leads to kidney injury and vascular dysfunction (the latter abnormality is ameliorated by G-1 treatment).130 Endothelial cell dysfunction is also present in animals with glomerulosclerosis, which leads to proteinuria due to loss of the glomerular filter function. In hypertensive rats, GPER activation reduces proteinuria and improves creatinine clearance despite continued hypertension.136 These findings suggest renoprotective potential for GPER agonists in hypertensive nephropathy.
Pancreatic function and glucose metabolism
The increased prevalence of obesity, insulin resistance and diabetes after menopause indicates a protective role for endogenous 17β-estradiol in premenopausal women.147,148 These protective effects are largely attributed to signaling via nuclear ERα, 150,151 as its deletion results in obesity and insulin resistance.147,148 However, other forms of ERα signaling are also involved in metabolic diseases;151,152 for example, insulin secretion mediated by 17β-estradiol occurs through rapid signaling via membrane-bound ERs.160–162 Although ERα and ERb individually affect insulin action,147,148 mice deficient in GPER develop insulin resistance and obesity in a sex-dependent manner.57,77,153 GPER activation also has anti-inflammatory properties in pancreatic islets through attenuating the effects of proinflammatory cytokines154 that are important for maintenance of metabolic function.155 The protective, antidiabetic effects of 17β-estradiol in islet cells seem to involve activation of both membrane-bound ERα and GPER,56,156 and might also be induced by GPER agonists, such as genistein.158
GPER is expressed in whole adipose tissue in humans and rodents,57,159 as well as in the human liver,18–20,22,23 key target organs of insulin resistance.155 However, the role of GPER in 17β-estradiol-mediated metabolic protection is not clearly defined. GPER is expressed in the pancreatic islets of mice56,77,154,156,157 and humans,156 and in female mice it maintains normal metabolic function.77 GPER deficiency results in a reduction in insulin secretion (stimulated by 17β-estradiol, G-1 and glucose) from the pancreas, but does not affect the morphology of pancreatic β-cells, which suggests that GPER has a key role in maintaining the metabolic functions of insulin in mice77,163 and humans.164 Furthermore, the protective effect of 17β-estradiol on survival of pancreatic β-cells in a mouse model of type 1 diabetes mellitus is absent in GPER-deficient animals.56 Whether GPER contributes to peripheral insulin resistance is currently not known. However, expression of GPER has been reported in human skeletal muscle,56,77,154,156 and is unaffected by menopause.165
Bone growth and chondrocyte metabolism [heading level 2]
Bone and articular cartilage are hormone-sensitive tissues,166 and serum 17β-estradiol levels inversely correlate with the risk of hip fracture in both women and men.167 Perhaps the best evidence of a role for endogenous 17β-estradiol in overall bone health and formation of trabecular bone in particular is the postmenopausal onset of osteoporosis. The bone-preserving effects of estrogen therapy, especially with SERMs and SERDs,168 which act as GPER agonists, indirectly suggest a role for GPER in bone metabolism. Endogenous 17β-estradiol also has an important role in bone metabolism in men, since lack of 17β-estradiol owing to aromatase deficiency169 or mutations in ESR1 (which encodes ERα)170 in men leads to osteopenia, enhanced bone remodeling through increased bone resorption and osteoclast activity and suppression of bone growth-plate closure.171 Although part of this effect is mediated through ERα and ERβ,168 several avenues of research now suggest a role for GPER in bone and cartilage metabolism. In bone, GPER is expressed in osteocytes, osteoclasts and osteoblasts,172,173 and is also detected in chondrocytes,172,174 differentiation of which is regulated by GPER.174 In addition, GPER expression also regulates bone growth, as illustrated by several models of GPER-deficiency, albeit in a sex-dependent manner. GPER deficieny inhibits bone growth in female mice;77 similar results were reported in ovariectomized, estrogen-treated animals,108 suggesting a role for GPER in estrogen-induced bone growth and development. By contrast, GPER-deficient male mice had increased femur size, bone mineral density, trabecularization and cortical bone thickness.153 Tamoxifen, a GPER agonist, decreases tibia length independently from ERα or ERβ.52 Although in vitro studies and clinical trials with SERMs show beneficial effects on bone structure in postmenopausal women,168 the role of GPER in bone and chondrocyte metabolism in humans is still not clear and warrants further study.
GPER in cancer growth and metastasis
17β-Estradiol is a critical mediator of breast carcinogenesis and is involved in a number of other hormone-sensitive cancers. Normal breast tissue is highly sensitive to 17β-estradiol, which stimulates proliferation of this tissue during puberty and pregnancy; thus, the majority of breast cancers are highly responsive to 17β-estradiol and utilize 17β-estradiol signaling pathways in cancer initiation, progression and metastasis.175 This understanding has led to development of various cancer therapies that target 17β-estradiol signaling, the most widely used of which is tamoxifen.176 Antiestrogen therapy has been extended to include SERDs (such as fulvestrant), aromatase inhibitors (for postmenopausal women) and other SERMs (such as raloxifene).50 Many of these agents, particularly tamoxifen and fulvestrant, are also GPER agonists and have complex physiological and therapeutic actions. For example, long-term 17β-estradiol deprivation in the weakly metastatic human breast cancer cell line MCF-7 increased expression of GPER,197 whereas tamoxifen treatment of these cells stimulated proliferation via GPER-mediated transactivation of EGFR.198
GPER is expressed in ~50% of all breast cancers, regardless of their ER status,186 although conflicting results have been reported regarding coexpression of GPER and human epidermal growth factor receptor 2 (Her2).184,186,188 Nevertheless, in general, GPER expression in breast cancers correlates with clinical and pathological biomarkers of poor outcome. High levels of GPER protein expression in samples of human breast cancers also correlate with increased tumor size and metastasis.186 Importantly, in patients treated only with tamoxifen, GPER protein expression increased and survival was significantly lower in patients with initial GPER-positive tumors, suggesting breast cancer patients with high GPER expression should not be treated with tamoxifen alone.{Ignatov, 2011 #2254} In addition, GPER is widely expressed in cancer cell lines and primary tumors of the breast,17,18,34,177,184–188 endometrium,178–180,189,190 ovaries,47,53,181,191,192 thyroid,180 lung,182 prostate,183 testicular germ cells193 and the brain (unpublished work). In cell lines of thyroid, ovarian, endometrial and breast cancers, stimulation of GPER with 17β-estradiol53,180,194 or other estrogenic compounds, such as atrazine,47 genistein,180 bisphenol A46,195 or tamoxifen194 activates a signaling mechanism that typically promotes proliferation, although inhibition of proliferation has also been reported.69 In particular, genistein can stimulate MCF-7 cell growth via induction of acid ceramidase, which occurs through a GPER-dependent mechanism.196 In endometrial cancer190 and ovarian cancer,191 high levels of GPER expression also predicted poor survival, whereas among post-pubertal testicular germ cell tumors, GPER was highly expressed in intratubular germ cell tumors, seminomas and embryonal carcinomas, with little expression in teratomas.193
Importantly, treatment of the ERα-negative human breast cancer cell line SKBr3 with 17β-estradiol or tamoxifen increased the expression of several transcription regulators (including c-Fos) and cytokines (particularly connective tissue growth factor, which promotes cancer cell proliferation and migration).199 These data indicate that tamoxifen treatment might have a cancer-promoting effect through GPER as discussed above. In support of this view, endometrial GPER expression also correlated with tamoxifen-induced uterine pathology, including bleeding and abnormal endometrial thickening,189 which correlates with an increased incidence of endometrial cancer.201,202
The overall role of GPER in breast cancer progression is complex. Genistein stimulates the proliferation of MCF-7 cells through a GPER-dependent mechanism.196 Moreover, GPER is implicated in 17β-estradiol-mediated activation of cancer-associated fibroblasts, which promote tumor cell proliferation and metastasis through direct association of GPER with chromatin.200 GPER expression was induced in breast cancer cells under hypoxic conditions, which also suggests a cancer-promoting role for this ER, including a role in hypoxia-induced angiogenesis.138 However, G-1 inhibits endothelial cell proliferation, which indirectly suggests that GPER activity also inhibits angiogenesis.119 Despite these conflicting data on the role of GPER in cancer, targeting its activity represents an important new approach for cancer therapy.
Conclusions
The salutary effects of estrogens are well established in many diseases, and selective activation of GPER by G-1, phytoestrogens, SERDS, or SERMS can reproduce the beneficial effects of 17β-estradiol. The pace of research into the physiological and pathological functions of GPER has been accelerating over the past 5 years, and potential roles for GPER have now been identified in almost every system of the body. Thus, GPER-selective agents that mimic the beneficial effects of 17β-estradiol without its associated feminizing or other adverse effects could represent an important new family of drugs.
In addition, GPER-specific antagonists could be developed as important additions to the armamentarium of drugs used to treat estrogen-sensitive cancers and other diseases in which estrogen signaling is important. In this regard, the potential contribution of GPER-mediated signaling to the effects of existing clinically approved drugs, such as tamoxifen and fulvestrant, must be considered. GPER-mediated effects should also be taken into account in the future development of SERMs and SERDs. In addition, further research is required to determine to what extent the physiological effects of 17β-estradiol involve GPER signaling and the precise roles of nonselective estrogen receptor ligands in health and disease. The co-dependent, redundant and independent aspects of 17β-estradiol signaling through ERα, ERβ and GPER are likely to be very complex and specific to particular cell types, tissues, ligands and diseases. The data available to date nevertheless pose interesting questions about the therapeutic potential of specifically targeting GPER in disease.
Review criteria.
A search for original articles was performed in PubMed. The search terms used included “GPER”, “GPR30”, “estrogen”, “rapid signaling”, “SERM”, “reproduction”, “immune”, “vascular”, “nervous”, “metabolism”, “bone” and “cancer” with no restriction on the publication year, language or article type. Additional abstracts were also identified by searching Google Scholar using similar keywords. Reference lists within identified papers were also searched.
Key points.
Estrogen has critical nonreproductive roles in health and disease, including in the skeletal, nervous, endocrine, immune and cardiovascular systems, as well as in many diseases and cancers
The estrogen receptors (ERs) include ERα, ERβ and G-protein-coupled estrogen receptor 1 (GPER); their expression and signaling mechanisms are complex, and potentially exhibit redundant, independent, synergistic and/or antagonistic actions
Estrogenic compounds (selective ER modulators, ER antagonists, selective ER downregulators, phytoestrogens and xenoestrogens) have multifaceted effects on all types of ERs, as well as receptor-specific pharmacological profiles
GPER -selective agonists, such as G-1, mediate many salutary effects of estrogen in various tissues and organs without having any reproductive effects
GPER represents an important diagnostic, prognostic and therapeutic target; development of GPER-selective agonists and antagonists could contribute to the diagnosis and treatment of many diseases
Acknowledgments
We apologize to all colleagues who have been cited only cursorily, or have not been cited due to space constraints E. R. Prossnitz’s research is supported by grants CA116662, CA118743 and CA127731 from the NIH. M. Barton’s work is supported by grants 3200-108528/1 and K-33KO-122504/1 from the Swiss National Science Foundation.
Biographies
Dr Eric R. Prossnitz received his PhD from the University of California at Berkeley, CA, USA, and carried out his postdoctoral studies at the Scripps Research Institute, La Jolla, CA, USA, where he became Assistant Professor in 1994. He relocated to the University of New Mexico, Albuquerque, NM, USA, in 1997. He has studied mechanisms of G-protein-coupled receptor function for over 20 years, with particular focus on the role of receptor regulation through phosphorylation and binding of arrestin. His recent research has centered on estrogen signaling through GPER, the identification of highly selective ligands for this receptor and the determination of its cellular and physiological functions.
Dr Matthias Barton obtained his MD from Hannover Medical School in Germany .He received a SCORE Career Development Award from the Swiss National Science Foundation in 1999 and is currently Professor of Cardiology at the University of Zürich in Switzerland. Dr Barton’s research relates to the molecular mechanisms involved in coronary artery disease and atherosclerosis, with a special interest in novel disease-modifying factors, obesity, and gender differences. Since 1990, Dr. Barton has studied vascular effects of estrogen. His laboratory was the first to report a regulatory role of GPER/GPR30 for vascular function.
Footnotes
Competing interests:
E. R. Prossnitz declares US patent number 7,875,721. M. Barton declares no competing interests.
Author contributions
E. R. Prossnitz and M. Barton contributed equally to all aspects of this manuscript.
Contributor Information
Eric R. Prossnitz, Department of Cell Biology and Physiology, University of New Mexico Health Sciences Center, Albuquerque, NM 87131, USA
Matthias Barton, Molecular Internal Medicine, University of Zürich, LTK Y44 G22 Winterthurerstrasse 190, 8057 Zürich, Switzerland.
References
- 1.Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prossnitz ER, et al. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 4.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–76. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 5.Lorand T, Vigh E, Garai J. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens. Curr Med Chem. 2010;17:3542–74. doi: 10.2174/092986710792927813. [DOI] [PubMed] [Google Scholar]
- 6.Talwar GP, Segal SJ, Evans A, Davidson OW. The binding of estradiol in the uterus: a mechanism for depression of RNA synthesis. Proc Natl Acad Sci USA. 1964;52:1059–1066. doi: 10.1073/pnas.52.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soloff MS, Szego CM. Purification of estradiol receptor from rat uterus and blockade of its estrogen-binding function by specific antibody. Biochem Biophys Res Commun. 1969;34:141–7. doi: 10.1016/0006-291x(69)90540-3. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–14. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125:143–9. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- 11.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–41. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 12.Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature. 1975;253:357–9. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- 13.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 14.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58:1711–8. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehling M. Looking beyond the dogma of genomic steroid action: insights and facts of the 1990s. Journal of Molecular Medicine. 1995;73:439–47. doi: 10.1007/BF00202262. [DOI] [PubMed] [Google Scholar]
- 16.Wehling M. Specific, nongenomic actions of steroid hormones. Annual Review of Physiology. 1997;59:365–93. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- 17.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 18.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–17. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Gregor P. Cloning of a novel member of the G protein-coupled receptor family related to peptide receptors. Biochem Biophys Res Commun. 1997;231:651–4. doi: 10.1006/bbrc.1997.6161. [DOI] [PubMed] [Google Scholar]
- 20.Kvingedal AM, Smeland EB. A novel putative G-protein-coupled receptor expressed in lung, heart and lymphoid tissue. FEBS Lett. 1997;407:59–62. doi: 10.1016/s0014-5793(97)00278-0. [DOI] [PubMed] [Google Scholar]
- 21.O’Dowd BF, et al. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47:310–3. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- 22.Owman C, Blay P, Nilsson C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228:285–92. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- 23.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–41. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 24.Olde B, Leeb-Lundberg LM. GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol Metab. 2009;20:409–16. doi: 10.1016/j.tem.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 26.Kanda N, Watanabe S. 17beta-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol. 2003;121:1500–9. doi: 10.1111/j.1523-1747.2003.12617.x. [DOI] [PubMed] [Google Scholar]
- 27.Kanda N, Watanabe S. 17Beta-estradiol enhances the production of nerve growth factor in THP-1-derived macrophages or peripheral blood monocyte-derived macrophages. J Invest Dermatol. 2003;121:771–80. doi: 10.1046/j.1523-1747.2003.12487.x. [DOI] [PubMed] [Google Scholar]
- 28.Kanda N, Watanabe S. 17beta-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J Invest Dermatol. 2004;123:319–28. doi: 10.1111/j.0022-202X.2004.12645.x. [DOI] [PubMed] [Google Scholar]
- 29.Maggiolini M, et al. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 30.Ahola TM, Alkio N, Manninen T, Ylikomi T. Progestin and G protein-coupled receptor 30 inhibit mitogen-activated protein kinase activity in MCF-7 breast cancer cells. Endocrinology. 2002;143:4620–6. doi: 10.1210/en.2002-220492. [DOI] [PubMed] [Google Scholar]
- 31.Ahola TM, Manninen T, Alkio N, Ylikomi T. G protein-coupled receptor 30 is critical for a progestin-induced growth inhibition in MCF-7 breast cancer cells. Endocrinology. 2002;143:3376–84. doi: 10.1210/en.2001-211445. [DOI] [PubMed] [Google Scholar]
- 32.Ahola TM, et al. Progestin upregulates G-protein-coupled receptor 30 in breast cancer cells. Eur J Biochem. 2002;269:2485–90. doi: 10.1046/j.1432-1033.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 33.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 34.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 35.Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Progress in Brain Research. 2010;181:209–32. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 36.Lappano R, et al. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Molecular and Cellular Endocrinology. 2010;320:162–70. doi: 10.1016/j.mce.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualini JR, Gelly C, Nguyen BL, Vella C. Importance of estrogen sulfates in breast cancer. J Steroid Biochem. 1989;34:155–63. doi: 10.1016/0022-4731(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 38.Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003;86:245–53. doi: 10.1016/s0960-0760(03)00364-9. [DOI] [PubMed] [Google Scholar]
- 39.Diczfalusky E, Mancuso S. In: Foetus and Placenta. Klopper A, Diczfalusky E, editors. Blackwell; Oxford: 1969. [Google Scholar]
- 40.Muller RE, Johnston TC, Traish AM, Wotiz HH. Studies on the mechanism of estradiol uptake by rat uterine cells and on estradiol binding to uterine plasma membranes. Adv Exp Med Biol. 1979;117:401–21. doi: 10.1007/978-1-4757-6589-2_22. [DOI] [PubMed] [Google Scholar]
- 41.Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res. 2003;17:845–69. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- 42.Starek A. Estrogens and organochlorine xenoestrogens and breast cancer risk. Int J Occup Med Environ Health. 2003;16:113–24. [PubMed] [Google Scholar]
- 43.Singleton DW, Khan SA. Xenoestrogen exposure and mechanisms of endocrine disruption. Front Biosci. 2003;8:s110–8. doi: 10.2741/1010. [DOI] [PubMed] [Google Scholar]
- 44.Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. Journal of Nutrition. 2002;132:3168–71. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 45.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–9. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Chevalier N, Bouskine A, Fenichel P. Bisphenol A promotes testicular seminoma cell proliferation through GPER, the G-protein-coupled estrogen receptor. International Journal of Cancer. 2011 doi: 10.1002/ijc.25972. [DOI] [PubMed] [Google Scholar]
- 47.Albanito L, et al. G-protein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ Health Perspect. 2008;116:1648–55. doi: 10.1289/ehp.11297. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Setchell KD, Clerici C. Equol: history, chemistry, and formation. Journal of Nutrition. 2010;140:1355S–62S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowlands DJ, Chapple S, Siow RC, Mann GE. Equol-Stimulated Mitochondrial Reactive Oxygen Species Activate Endothelial Nitric Oxide Synthase and Redox Signaling in Endothelial Cells: Roles for F-Actin and GPR30. Hypertension. 2011;57:833–840. doi: 10.1161/HYPERTENSIONAHA.110.162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordan VC. SERMs: meeting the promise of multifunctional medicines. J Natl Cancer Inst. 2007;99:350–6. doi: 10.1093/jnci/djk062. [DOI] [PubMed] [Google Scholar]
- 51.Orlando L, et al. Molecularly targeted endocrine therapies for breast cancer. Cancer Treatment Reviews. 2011;36 (Suppl 3):S67–71. doi: 10.1016/S0305-7372(10)70023-2. [DOI] [PubMed] [Google Scholar]
- 52.Fitts JM, Klein RM, Powers CA. Tamoxifen regulation of bone growth and endocrine function in the ovariectomized rat: Discrinimation of responses involving ERalpha/ERbeta, GPER or ERRgamma using fulvestrant (ICI 182,780) Journal of Pharmacology and Experimental Therapeutics. 2011 doi: 10.1124/jpet.110.173955. [DOI] [PubMed] [Google Scholar]
- 53.Albanito L, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–66. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 54.Blasko E, et al. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol. 2009;214:67–77. doi: 10.1016/j.jneuroim.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, et al. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol. 2009;182:3294–303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas E, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circulation Research. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennis MK, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–7. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenei-Lanzl Z, et al. Estradiol inhibits chondrogenic differentiation of mesenchymal stem cells via nonclassic signaling. Arthritis and Rheumatism. 2011;62:1088–96. doi: 10.1002/art.27328. [DOI] [PubMed] [Google Scholar]
- 60.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. Journal of Cardiovascular Pharmacology. 2011 doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peyton C, Thomas P. Involvement of Epidermal Growth Factor Receptor Signaling in Estrogen Inhibition of Oocyte Maturation Mediated Through the G Protein-Coupled Estrogen Receptor (GPER) in Zebrafish (Danio rerio) Biology of Reproduction. 2011 doi: 10.1095/biolreprod.110.088765. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gingerich S, et al. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170:54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- 63.Nayak T, et al. Influence of charge on cell permeability and tumor imaging of GPR30-targeted 111In-labeled non-steroidal imaging agents. ACS Chem Biol. 2010;5:681–690. doi: 10.1021/cb1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramesh C, et al. Synthesis and characterization of iodinated tetrahydroquinolines targeting the G protein-coupled estrogen receptor GPR30. J Med Chem. 2010;53:1004–14. doi: 10.1021/jm9011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levin ER. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Molecular Endocrinology. 2011;25:377–84. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhola NE, Grandis JR. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci. 2008;13:1857–65. doi: 10.2741/2805. [DOI] [PubMed] [Google Scholar]
- 67.Prenzel N, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 68.Edwin F, et al. A historical perspective of the EGF receptor and related systems. Methods in Molecular Biology. 2006;327:1–24. doi: 10.1385/1-59745-012-x:1. [DOI] [PubMed] [Google Scholar]
- 69.Ariazi EA, et al. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res. 2010;70:1184–94. doi: 10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brailoiu E, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 71.Sukocheva O, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–10. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quinn JA, et al. Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G-protein-coupled receptor, GPR30. Mol Endocrinol. 2009 doi: 10.1210/me.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin BC, et al. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Research. 2009;69:5415–23. doi: 10.1158/0008-5472.CAN-08-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. Journal of Endocrinology. 2010;204:105–14. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 75.Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32–8. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isensee J, et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–30. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 77.Martensson UE, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–98. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 78.Otto C, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 79.Wang C, et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–48. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annual Review of Physiology. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 81.Gao F, Ma X, Ostmann AB, Das SK. GPR30 Activation Opposes Estrogen-Dependent Uterine Growth via Inhibition of Stromal ERK1/2 and Estrogen Receptor Alpha (ER{alpha}) Phosphorylation Signals. Endocrinology. 2011 doi: 10.1210/en.2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–26. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang C, Prossnitz ER, Roy SK. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology. 2008;149:4452–61. doi: 10.1210/en.2008-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C, Prossnitz ER, Roy SK. Expression of GPR30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148:4853–4864. doi: 10.1210/en.2007-0727. [DOI] [PubMed] [Google Scholar]
- 85.Maiti K, et al. G-1-Activated Membrane Estrogen Receptors Mediate Increased Contractility of the Human Myometrium. Endocrinology. 2011 doi: 10.1210/en.2010-0979. [DOI] [PubMed] [Google Scholar]
- 86.Chimento A, et al. GPER and ESRs are expressed in rat round spermatids and mediate oestrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax. International Journal of Andrology. 2011 doi: 10.1111/j.1365-2605.2010.01100.x. [DOI] [PubMed] [Google Scholar]
- 87.Chimento A, et al. 17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha. Molecular and Cellular Endocrinology. 2010;320:136–44. doi: 10.1016/j.mce.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 88.Sirianni R, et al. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149:5043–51. doi: 10.1210/en.2007-1593. [DOI] [PubMed] [Google Scholar]
- 89.Dun SL, et al. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res. 2009;87:1610–1619. doi: 10.1002/jnr.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hazell GG, et al. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. Journal of Endocrinology. 2009;202:223–36. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liverman CS, Brown JW, Sandhir R, McCarson KE, Berman NE. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009 doi: 10.1111/j.1468-2982.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuhn J, et al. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–9. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 93.Lu CL, et al. Estrogen rapidly modulates 5-hydroxytrytophan-induced visceral hypersensitivity via GPR30 in rats. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 94.Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Research. 2011 doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–92. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bryant DN, Dorsa DM. Roles of estrogen receptors alpha and beta in sexually dimorphic neuroprotection against glutamate toxicity. Neuroscience. 2010;170:1261–9. doi: 10.1016/j.neuroscience.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lebesgue D, et al. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–61. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Etgen AM, Jover-Mengual T, Suzanne Zukin R. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: Translational implications. Frontiers in Neuroendocrinology. 2011 doi: 10.1016/j.yfrne.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu H, et al. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-Protein Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol. 2009;21:316–21. doi: 10.1111/j.1365-2826.2009.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lebesgue D, Reyna-Neyra A, Huang X, Etgen AM. GPR30 differentially regulates short latency responses of luteinising hormone and prolactin secretion to oestradiol. Journal of Neuroendocrinology. 2009;21:743–52. doi: 10.1111/j.1365-2826.2009.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wintermantel TM, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pernis AB. Estrogen and CD4+ T cells. Current Opinion in Rheumatology. 2007;19:414–20. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- 106.Straub RH. The complex role of estrogens in inflammation. Endocrine Reviews. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 107.Niino M, Hirotani M, Fukazawa T, Kikuchi S, Sasaki H. Estrogens as potential therapeutic agents in multiple sclerosis. Cent Nerv Syst Agents Med Chem. 2009;9:87–94. doi: 10.2174/187152409788452054. [DOI] [PubMed] [Google Scholar]
- 108.Windahl SH, et al. The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab. 2009;296:E490–6. doi: 10.1152/ajpendo.90691.2008. [DOI] [PubMed] [Google Scholar]
- 109.Engdahl C, et al. Amelioration of collagen-induced arthritis and immune-associated bone loss through signaling via estrogen receptor alpha, and not estrogen receptor beta or G protein-coupled receptor 30. Arthritis and Rheumatism. 2010;62:524–33. doi: 10.1002/art.25055. [DOI] [PubMed] [Google Scholar]
- 110.Yates MA, Li Y, Chlebeck PJ, Offner H. GPR30, but not estrogen receptor-alpha, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 2010;11:20. doi: 10.1186/1471-2172-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brunsing RL, Prossnitz ER. Induction of IL10 in CD4+ T cells by the GPER agonist G-1. Immunol. 2011 doi: 10.1111/j.1365-2567.2011.03471.x. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–26. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 113.Sandberg K. Mechanisms underlying sex differences in progressive renal disease. Gend Med. 2008;5:10–23. doi: 10.1016/s1550-8579(08)80004-6. [DOI] [PubMed] [Google Scholar]
- 114.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–8. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 115.Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med. 2008;5 (Suppl A):S19–33. doi: 10.1016/j.genm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 116.Ullrich ND, Krust A, Collins P, MacLeod KT. Genomic deletion of estrogen receptors ERalpha and ERbeta does not alter estrogen-mediated inhibition of Ca2+ influx and contraction in murine cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2421–7. doi: 10.1152/ajpheart.01225.2007. [DOI] [PubMed] [Google Scholar]
- 117.Karas RH, et al. Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circulation Research. 2001;89:534–9. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- 118.Villablanca AC, et al. 17beta-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J Cardiovasc Transl Res. 2009;2:289–99. doi: 10.1007/s12265-009-9103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holm A, Baldetorp B, Olde B, Leeb-Lundberg LM, Nilsson BO. The GPER1 Agonist G-1 Attenuates Endothelial Cell Proliferation by Inhibiting DNA Synthesis and Accumulating Cells in the S and G2 Phases of the Cell Cycle. Journal of Vascular Research. 2011;48:327–335. doi: 10.1159/000322578. [DOI] [PubMed] [Google Scholar]
- 120.Haas E, et al. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–63. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- 121.Morales DE, et al. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91:755–63. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- 122.Ding Q, Gros R, Limbird LE, Chorazyczewski J, Feldman RD. Estradiol-mediated ERK phosphorylation and apoptosis in vascular smooth muscle cells requires GPR30. Am J Physiol Cell Physiol. 2009;297:C1178–87. doi: 10.1152/ajpcell.00185.2009. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi K, et al. Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. Journal of Endocrinology. 2003;178:319–29. doi: 10.1677/joe.0.1780319. [DOI] [PubMed] [Google Scholar]
- 124.Pare G, et al. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circulation Research. 2002;90:1087–92. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 125.Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the g protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology. 2010;86:58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol. 2010;298:H1055–61. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- 127.Mishra SK, et al. Endothelium-dependent relaxation of rat aorta and main pulmonary artery by the phytoestrogens genistein and daidzein. Cardiovascular Research. 2000;46:539–46. doi: 10.1016/s0008-6363(00)00049-3. [DOI] [PubMed] [Google Scholar]
- 128.Rettew JA, McCall SHt, Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;328:87–92. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 129.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2011;5:e15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–8. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lindsey SH, Chappell MC. GPR30 activation in salt-sensitive mRen2.Lewis females induces beneficial effects independent of alterations in blood pressure. FASEB J. 2009 [Google Scholar]
- 132.Filice E, et al. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol. 2009;60:3–10. [PubMed] [Google Scholar]
- 133.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 134.Lafferty AR, et al. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22) J Med Genet. 2000;37:831–5. doi: 10.1136/jmg.37.11.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sanden C, et al. G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Molecular Pharmacology. 2011;79:400–10. doi: 10.1124/mol.110.069500. [DOI] [PubMed] [Google Scholar]
- 136.Gilliam-Davis S, Cohen JA, Lindsey SH, Yamaleyeva L, Chappell MC. The Estrogen Receptor GPR30is Renoprotective in Diabetic Hypertensive Female Rats. Hypertension. 2010;56:e50–e166. [Google Scholar]
- 137.Patel VH, et al. G-protein coupled estrogen receptor 1 expression in rat and human heart: Protective role during ischaemic stress. International Journal of Molecular Medicine. 2010;26:193–9. doi: 10.3892/ijmm_00000452. [DOI] [PubMed] [Google Scholar]
- 138.Recchia AG, et al. The G protein-coupled receptor 30 is up-regulated by hypoxia inducible factor-1a (HIF-1a) in breast cancer cells and cardiomyocytes. J Biol Chem. 2011 doi: 10.1074/jbc.M110.172247. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Delbeck M, et al. Impaired left-ventricular cardiac function in male GPR30-deficient mice. Molecular Redicine Reports. 2010;4:37–40. doi: 10.3892/mmr.2010.402. [DOI] [PubMed] [Google Scholar]
- 140.Deschamps AM, Murphy E, Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends in Cardiovascular Medicine. 2011;20:73–8. doi: 10.1016/j.tcm.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buja LM, Weerasinghe P. Unresolved issues in myocardial reperfusion injury. Cardiovasc Pathol. 2010;19:29–35. doi: 10.1016/j.carpath.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 142.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–7. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- 143.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–13. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H16–23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weil BR, et al. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery. 2010;148:436–43. doi: 10.1016/j.surg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 146.Zhang B, et al. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. Journal of Immunology. 2010;184:4087–94. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. Regulation of metabolism by estrogen signaling. Journal of Endocrinology. 2011 doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 149.Meyer MR, Barton M. ERa, ERb, and gpER: Novel aspects of estrogen receptor signaling in atherosclerosis. Cardiovasc Res. 2009;83:605–610. doi: 10.1093/cvr/cvp187. [DOI] [PubMed] [Google Scholar]
- 150.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–7. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 151.Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear Estrogen Receptor Signaling in Endothelium. Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.R110.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chambliss KL, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. Journal of Clinical Investigation. 2010;120:2319–30. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ford J, et al. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. Journal of Bone and Mineral Research. 2011;26:298–307. doi: 10.1002/jbmr.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol. 2010;320:16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 155.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. Journal of Clinical Investigation. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu S, Mauvais-Jarvis F. Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets. 2009;1:273–275. doi: 10.4161/isl.1.3.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu S, Mauvais-Jarvis F. Minireview: Estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology. 2010;151:859–64. doi: 10.1210/en.2009-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fu Z, et al. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151:3026–37. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hugo ER, et al. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–7. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009;587:5031–7. doi: 10.1113/jphysiol.2009.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nadal A, et al. Rapid insulinotropic effect of 17beta-estradiol via a plasma membrane receptor. FASEB J. 1998;12:1341–8. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- 162.Ropero AB, Soria B, Nadal A. A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Molecular Endocrinology. 2002;16:497–505. doi: 10.1210/mend.16.3.0794. [DOI] [PubMed] [Google Scholar]
- 163.Sharma G, Prossnitz ER. Mechanisms of estrogen-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β cells. Endocrinology. 2011 doi: 10.1210/en.2011-0091. resubmitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumar R, Balhuizen A, Amisten S, Lundquist I, Salehi A. Insulinotropic and antidiabetic effects of 17β-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology. 2011 doi: 10.1210/en.2010-1361. in press. [DOI] [PubMed] [Google Scholar]
- 165.Pollanen E, et al. Differential Influence of Peripheral and Systemic Sex Steroids on Skeletal Muscle Quality in Pre- and Postmenopausal Women. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00701.x. [DOI] [PubMed] [Google Scholar]
- 166.Weise M, et al. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6871–6. doi: 10.1073/pnas.121180498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ohlsson C, Vandenput L. The role of estrogens for male bone health. European Journal of Endocrinology/European Federation of Endocrine Societies. 2009;160:883–9. doi: 10.1530/EJE-09-0118. [DOI] [PubMed] [Google Scholar]
- 168.Khosla S. Update on estrogens and the skeleton. Journal of Clinical Endocrinology and Metabolism. 2010;95:3569–77. doi: 10.1210/jc.2010-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carani C, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. New England Journal of Medicine. 1997;337:91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 170.Smith EP, et al. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. Journal of Clinical Endocrinology and Metabolism. 2008;93:3088–96. doi: 10.1210/jc.2007-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smith EP, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New England Journal of Medicine. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 172.Chagin AS, Savendahl L. GPR30 Estrogen Receptor Expression in the Growth Plate Declines as Puberty Progresses. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2007-0814. [DOI] [PubMed] [Google Scholar]
- 173.Heino TJ, Chagin AS, Savendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. Journal of Endocrinology. 2008;197:R1–6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- 174.Jenei-Lanzl Z, et al. Estradiol inhibits chondrogenic differentiation of mesenchymal stem cells via nonclassic signaling. Arthritis and Rheumatism. 2010;62:1088–96. doi: 10.1002/art.27328. [DOI] [PubMed] [Google Scholar]
- 175.Cordera F, Jordan VC. Steroid receptors and their role in the biology and control of breast cancer growth. Seminars in Oncology. 2006;33:631–41. doi: 10.1053/j.seminoncol.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 176.Sengupta S, Jordan VC. Selective estrogen modulators as an anticancer tool: mechanisms of efficiency and resistance. Advances in Experimental Medicine and Biology. 2008;630:206–19. doi: 10.1007/978-0-387-78818-0_13. [DOI] [PubMed] [Google Scholar]
- 177.Albanito L, et al. Epidermal growth factor induces G protein-coupled receptor 30 expression in estrogen receptor-negative breast cancer cells. Endocrinology. 2008;149:3799–808. doi: 10.1210/en.2008-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.He YY, Cai B, Yang YX, Liu XL, Wan XP. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci. 2009;100:1051–61. doi: 10.1111/j.1349-7006.2009.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Leblanc K, et al. Effects of 4-hydroxytamoxifen, raloxifene and ICI 182 780 on survival of uterine cancer cell lines in the presence and absence of exogenous estrogens. Int J Oncol. 2007;30:477–87. [PubMed] [Google Scholar]
- 180.Vivacqua A, et al. 17b-estradiol, genistein and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein-coupled receptor GPR30. Mol Pharmacol. 2006;70:1414–23. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- 181.Henic E, Noskova V, Hoyer-Hansen G, Hansson S, Casslen B. Estradiol attenuates EGF-induced rapid uPAR mobilization and cell migration via the G-protein-coupled receptor 30 in ovarian cancer cells. Int J Gynecol Cancer. 2009;19:214–22. doi: 10.1111/IGC.0b013e31819bcb75. [DOI] [PubMed] [Google Scholar]
- 182.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Seminars in Oncology. 2009;36:524–31. doi: 10.1053/j.seminoncol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chan QK, et al. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G(2) cell-cycle arrest. Cell Death and Differentiation. 2010;17:1511–23. doi: 10.1038/cdd.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tu G, Hu D, Yang G, Yu T. The correlation between GPR30 and clinicopathologic variables in breast carcinomas. Technol Cancer Res Treat. 2009;8:231–4. doi: 10.1177/153303460900800308. [DOI] [PubMed] [Google Scholar]
- 185.Arias-Pulido H, et al. GPR30 and estrogen receptor expression: new insights into hormone dependence of inflammatory breast cancer. Breast Cancer Research and Treatment. 2010;123:51–8. doi: 10.1007/s10549-009-0631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Filardo EJ, et al. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res. 2006;12:6359–66. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 187.Kuo WH, et al. The interactions between GPR30 and the major biomarkers in infiltrating ductal carcinoma of the breast in an Asian population. Taiwan J Obstet Gynecol. 2007;46:135–45. doi: 10.1016/S1028-4559(07)60007-2. [DOI] [PubMed] [Google Scholar]
- 188.Liu Q, Li JG, Zheng XY, Jin F, Dong HT. Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast carcinomas. Chinese Medical Journal. 2009;122:2763–9. [PubMed] [Google Scholar]
- 189.Ignatov T, et al. Role of GPR30 in endometrial pathology after tamoxifen for breast cancer. American Journal of Obstetrics and Gynecology. 2011;203:595, e9–16. doi: 10.1016/j.ajog.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 190.Smith HO, et al. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007;196:386.e1–11. doi: 10.1016/j.ajog.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 191.Smith HO, et al. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol. 2009;114:465–471. doi: 10.1016/j.ygyno.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu HD, et al. The expression of a novel estrogen receptor, GPR30, in epithelial ovarian carcinoma and its correlation with MMP-9. Acta Physiologica Sinica. 2011;62:524–528. [PubMed] [Google Scholar]
- 193.Franco R, et al. GPR30 is over-expressed in post puberal testicular germ cell tumors. Cancer Biol Ther. 2011;11 doi: 10.4161/cbt.11.6.14672. [DOI] [PubMed] [Google Scholar]
- 194.Vivacqua A, et al. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17b-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–46. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- 195.Dong S, Terasaka S, Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut. 2011;159:212–8. doi: 10.1016/j.envpol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 196.Lucki NC, Sewer MB. Genistein stimulates MCF-7 breast cancer cell growth by inducing acid ceramidase (ASAH1) gene expression. Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.M110.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jordan CV, et al. Exploiting the apoptotic actions of oestrogen to reverse antihormonal drug resistance in oestrogen receptor positive breast cancer patients. Breast. 2007;(Suppl 2):S105–113. doi: 10.1016/j.breast.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ignatov A, Ignatov T, Roessner A, Costa SD, Kalinski T. Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Research and Treatment. 2010;123:87–96. doi: 10.1007/s10549-009-0624-6. [DOI] [PubMed] [Google Scholar]
- 199.Pandey DP, et al. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO Journal. 2009;28:523–532. doi: 10.1038/emboj.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Madeo A, Maggiolini M. Nuclear alternate estrogen receptor GPR30 mediates 17beta-estradiol-induced gene expression and migration in breast cancer-associated fibroblasts. Cancer Research. 2010;70:6036–46. doi: 10.1158/0008-5472.CAN-10-0408. [DOI] [PubMed] [Google Scholar]
- 201.van Leeuwen FE, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343:448–52. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- 202.Diel P. Tissue-specific estrogenic response and molecular mechanisms. Toxicology Letters. 2002;127:217–24. doi: 10.1016/s0378-4274(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 203.Otto C, et al. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 204.Cheng SB, Graeber CT, Quinn JA, Filardo EJ. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011 doi: 10.1016/j.steroids.2011.02.018. [DOI] [PubMed] [Google Scholar]