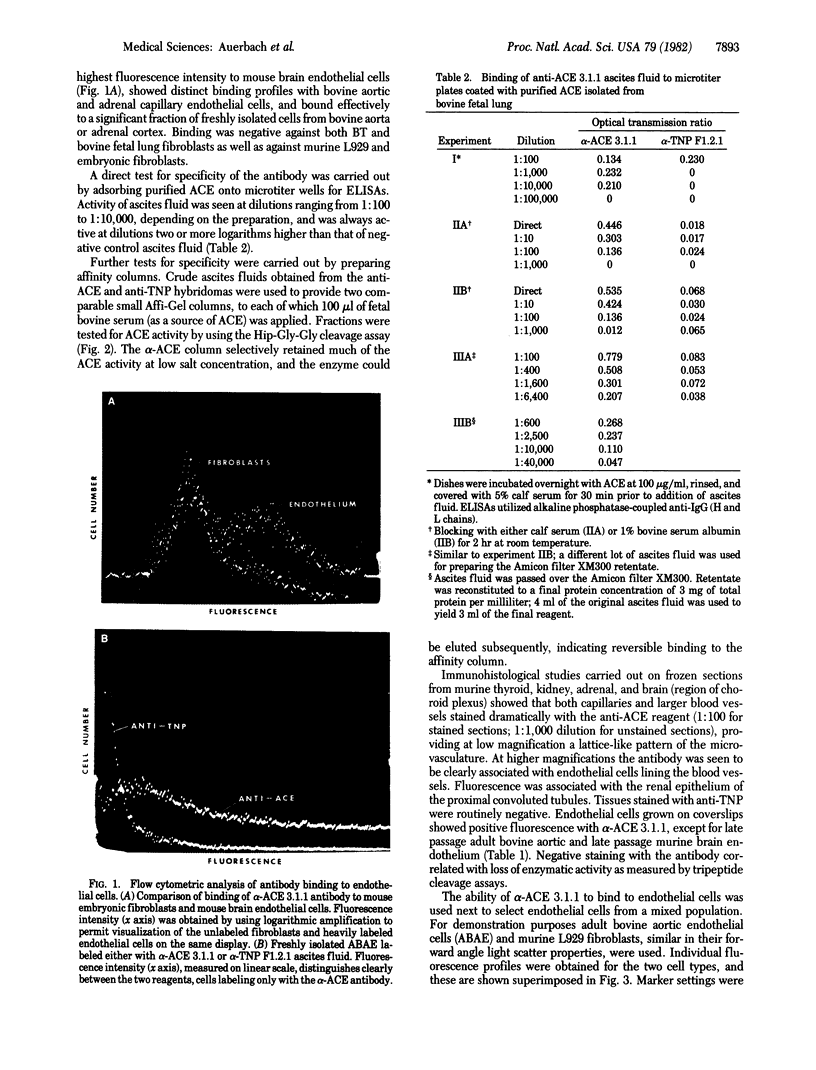
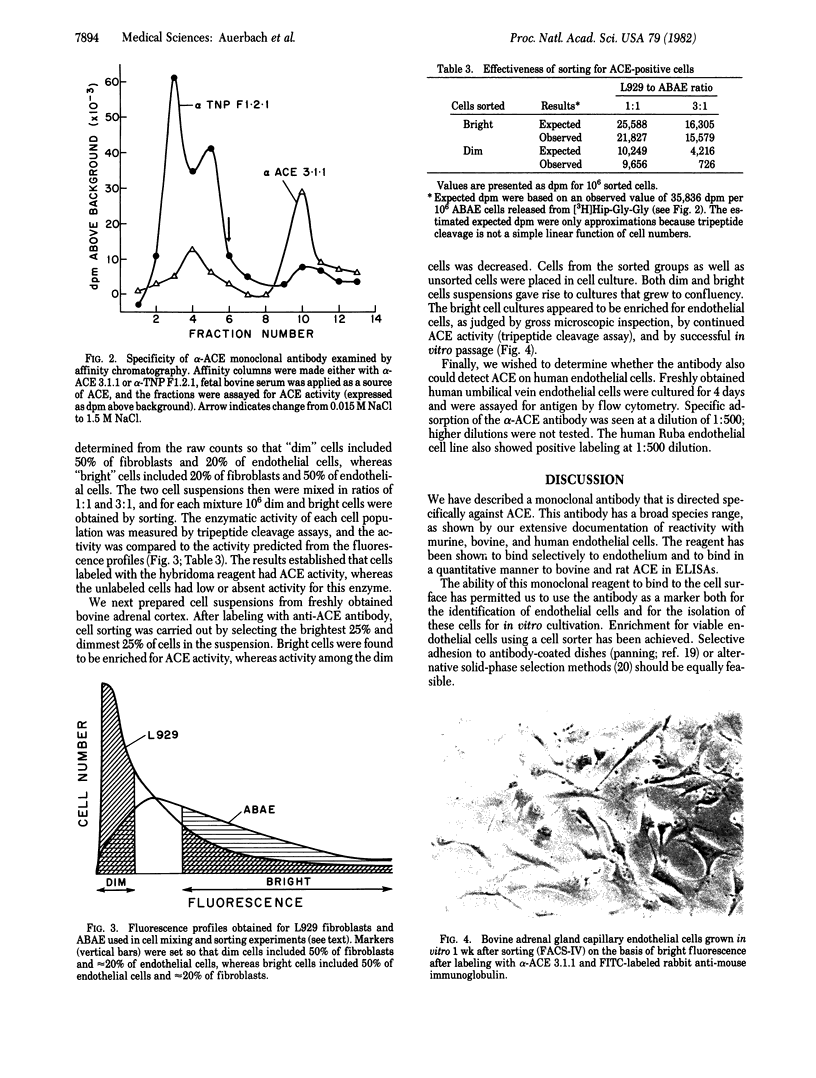
Abstract
A monoclonal antibody has been prepared against rat angiotensin-converting enzyme (ACE). By selection for antibody binding to endothelial cells of bovine rather than rat origin we have obtained a reagent that has broad cross-species binding properties and that can at the same time serve as a useful marker for the surface of endothelial cells. The IgM-producing clone that we have established, alpha-ACE 3.1.1, has been grown in ascites form to yield ascites fluid that binds selectively to immobilized ACE at a greater than 1:10,000 dilution. By use of enzyme-linked immunosorbent assays, immunofluorescence histology, and flow cytometry, we have demonstrated the presence of ACE on endothelial cells of murine, bovine, and human origin. By means of a fluorescence-activated cell sorter (FACS-IV) we have been able to selectively isolate viable endothelial cells from a mixture of endothelial cells and fibroblasts. We believe the antibody will be useful not only for the selection and in vitro cultivation of endothelial cells but also as a tool for the identification and pharmacological study of ACE.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Coffino P., Laskov R., Scharff M. D. Immunoglobulin production: method for quantitatively detecting variant myeloma cells. Science. 1970 Jan 9;167(3915):186–188. doi: 10.1126/science.167.3915.186. [DOI] [PubMed] [Google Scholar]
- DeBault L. E., Kahn L. E., Frommes S. P., Cancilla P. A. Cerebral microvessels and derived cells in tissue culture: isolation and preliminary characterization. In Vitro. 1979 Jul;15(7):473–487. doi: 10.1007/BF02618149. [DOI] [PubMed] [Google Scholar]
- Del Vecchio P. J., Ryan J. W., Chung A., Ryan U. S. Capillaries of the adrenal cortex possess aminopeptidase A and angiotensin-converting-enzyme activities. Biochem J. 1980 Feb 15;186(2):605–608. doi: 10.1042/bj1860605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Sariola H., Karkinen-Jäskeläinen M., Saxén L. The origin of the glomerular endothelium. Cell Differ. 1982 Jan;11(1):35–39. doi: 10.1016/0045-6039(82)90014-8. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong S., Fox R. I., Rose J. E., Liu J., Tsoukas C. D., Carson D. A., Vaughan J. H. Solid-phase selection of human T lymphocyte subpopulations using monoclonal antibodies. J Immunol Methods. 1981;46(2):153–163. doi: 10.1016/0022-1759(81)90132-0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest. 1980 Apr;65(4):841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Lanzillo J. J., Fanburg B. L. Angiotensin I converting enzyme from human plasma. Biochemistry. 1977 Dec 13;16(25):5491–5495. doi: 10.1021/bi00644a015. [DOI] [PubMed] [Google Scholar]
- Lanzillo J. J., Fanburg B. L. Membrane-bound angiotensin-converting enzyme from rat lung. J Biol Chem. 1974 Apr 10;249(7):2312–2318. [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., DeMars R. In vitro chemical mutagenesis and viral transformation of a human endothelial cell strain. Cancer Res. 1981 Mar;41(3):1114–1126. [PubMed] [Google Scholar]
- Ryan U. S., Clements E., Habliston D., Ryan J. W. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell. 1978;10(3):535–554. doi: 10.1016/s0040-8166(16)30347-0. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Ryan J. W., Whitaker C., Chiu A. Localization of angiotensin converting enzyme (kininase II). II. Immunocytochemistry and immunofluorescence. Tissue Cell. 1976;8(1):125–145. doi: 10.1016/0040-8166(76)90025-2. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Soffer R. L., Sonnenblick E. H. Physiologic, biochemical, and immunologic aspects of angiotensin-converting enzyme. Prog Cardiovasc Dis. 1978 Nov-Dec;21(3):167–175. doi: 10.1016/0033-0620(78)90022-1. [DOI] [PubMed] [Google Scholar]
- Wagner R. C., Matthews M. A. The isolation and culture of capillary endothelium from epididymal fat. Microvasc Res. 1975 Nov;10(3):286–297. doi: 10.1016/0026-2862(75)90033-3. [DOI] [PubMed] [Google Scholar]






