Abstract
Objective:
Up to 90% of the flora of an infected root canal consists of obligate anaerobic bacteria. However, most studies have centred on microaerophiles. This quantitative assay investigated the antibacterial effects against Fusobacterium nucleatum and Parvimonas micra of gutta-percha (GP) points containing either calcium hydroxide (Ca(OH)2) or chlorhexidine (CHX) compared to those of conventional GP points.
Methods:
Standardized root canals were prepared in 192 bovine incisors. The canals were sterilized and then inoculated with one of two bacterial species (n=96 teeth per bacterium). After anaerobic incubation at 37°C, the inoculum was removed and the canals were filled with one of the three points. Control teeth were filled with a saline/serum mixture. After 0, 1, 4 and 8 days of anaerobic incubation, the numbers of viable bacteria (CFU/ml) were determined on both the points and the canal wall dentin. Six replicates were carried out for each experiment.
Results:
None of the points completely eradicated the bacteria in the canal. GP+CHX was significantly more effective than GP+Ca(OH)2 and GP (P<.05 in each case). The inhibitory effect of GP+Ca(OH)2 did not significantly differ from that of GP (P>.05). Compared to the controls, the antibacterial effect of the medicated points was reached initially.
Conclusion:
Within the limitations of the in vitro model, the incorporation of commonly used medicaments in GP does not offer a long-lasting antibacterial advantage over non-medicated GP.
Keywords: Gutta-percha, calcium hydroxide, chlorhexidine diacetate, Fusobacterium nucleatum, Parvimonas micra, root canal
INTRODUCTION
In microbiologic examinations of teeth with periapical inflammation, evidence of viable bacteria within the infected root canal and adjacent dentinal tubules is frequently found.1,2 One primary objective in endodontic treatment is the elimination of the bacterial flora in infected teeth. This is usually accomplished by chemo-mechanical preparation of the root canal along with the use of a temporary root-filling between the appointments. Calcium hydroxide (Ca(OH)2) is considered to be the endodontic antimicrobial agent of choice for reducing bacteria.2,3 Chlorhexidine (CHX) seems to be effective in controlling some microorganisms which are not sensitive to Ca(OH)2.4,5 Due to its broad biocidal spectrum, CHX is effective against aerobic and anaerobic bacteria even if it is administered in low concentrations.6 However, the poor handling properties of the traditional application forms of Ca(OH)2 and CHX, i.e. liquid suspension, paste or gel, might be one reason why such medicaments often fail to completely disinfect infected root canals.7 To minimize this problem the use of medicated points that contain the medicaments in a gutta-percha (GP) matrix has recently been recommended.8 The therapeutic ingredient is released on contact with moisture in the root canal8 and should therefore be active for a prolonged time.
Dentin and other substances present in the root canal in vivo can inhibit the antibacterial efficacies of medicaments.4,9 For example, Ca(OH)2 has been reported to completely disinfect Enterococcus faecalis in an in vitro model.10 Nevertheless, a review of clinical trials has suggested its limited effectiveness against root canal infections.3 To date, most of the studies using root canal inoculation models have tested the antibacterial activity of Ca(OH)2 and/or CHX against aerotolerant or microaerophilic species such as E. faecalis.8,10–13 Little is known about obligate anaerobes, although there are several cultivable species known which may serve as antimicrobial target cells. Furthermore, obligate anaerobic bacteria, such as the Gram-negative Fusobacterium nucleatum and the Gram-positive Parvimonas micra (formerly Micromonas micros or Peptostreptococcus micros), account for up to 90% of the endodontic flora in the apical part of infected root canals.14 These species have been shown to survive even after the root canal has been permanently filled with GP points and a sealer.1,14
The aim of this in vitro study was to evaluate the antibacterial effects of GP points containing either additions of Ca(OH)2 or CHX in comparison to conventional GP points in a modified root canal model inoculated with monocultures of F. nucleatum and P. micra.
MATERIALS AND METHODS
Preparation of Specimens
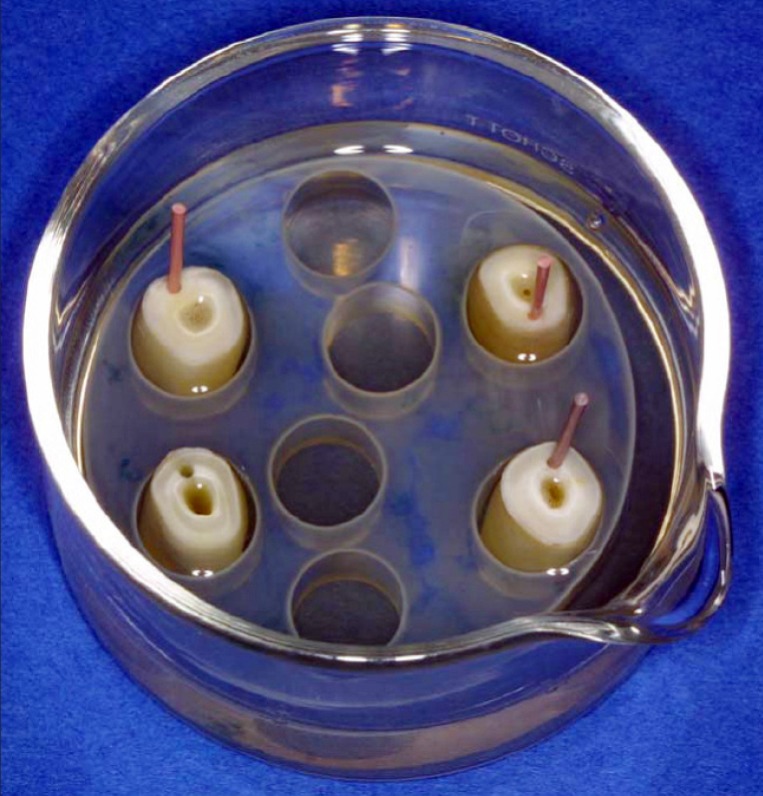
Bovine, lower central incisors of the same age (n=192) were cut at the level of the cementoenamel junction using a water-cooled diamond separating disk (Woco 50/Med, Conrad, Clausthal-Zellerfeld, Germany). Standardized root canals measuring 15 mm in length and 0.8 mm in diameter were drilled (Endoseal, Straumann, Basel, Switzerland) in the root dentin, parallel to the axis and the natural root canal of each incisor. The root canals were enlarged with ISO size 80 files (Micro-Mega, Besancon, France) and irrigated with 3 ml of sterile anaerobic 0.9% NaCl. The apex of the roots was shortened with a water-cooled polishing machine (Woco SF 20, Conrad) until the root canal floor was thinned to 0.5 mm. The prepared root specimens were autoclaved at 121°C for 20 minutes and placed in glass vessels containing a freshly prepared mixture of equal amounts of sterile anaerobic 0.9% NaCl solution and bovine serum (B-2771, Sigma, Steinheim, Germany). The cervical part of the root specimens protruded from the medium (Figure 1).
Figure 1.
Experiments were carried out in glas jars filled with sterile anaerobic NaCl/serum medium and containing the medicated and non-medicated root specimens as well as the bacterial growth controls.
Inoculation and Medication
The strains F. nucleatum American Type Culture Collection (ATCC) 10953 and P. micra ATCC 33270 were anaerobically incubated at 37°C in 10 ml of a thioglycolate medium (Oxoid Ltd, Basingstoke, Hampshire, UK) supplemented with 0.5 mg/l vitamin K and 5 mg/l hemin. After incubation over night, the suspensions were diluted 1:10 with fresh medium and further incubated up to an optical density at 600 nm (OD600) of 0.2. With 2 × 107 colony-forming units (CFU) per milliliter, this corresponded to an early logarithmic growth phase. The sterile root canals were inoculated with 5 μl of the bacterial suspension corresponding to 1 × 105 CFU (n=96 teeth per bacterium). The anaerobic environment was produced by activating BBL GasPak Plus envelopes (Becton-Dickinson Microbiology Systems, Sparks, USA) in anaerobic jars (GasPak Jars, Becton-Dickinson Microbiology Systems), which were subsequently placed in an incubator at 37°C for 2 days. The inoculum was removed with a sterile micropipette. No additional washing step was performed. The root canals were immediately medicated either with ISO size 80 GP points containing ca 52% Ca(OH)2 (Calcium Hydroxide Points, Lot number 489808, Roeko Coltène/Whaledent, Langenau, Germany) or ca 5% CHX diacetate (Activ Point, Lot number 399946, Roeko Coltène/Whaledent) or filled with conventional ISO size 80 GP points (Lot number 049808, Roeko Coltène/Whaledent). The production sterility of the points had been verified in earlier studies.15 Control teeth were filled with the sterile anaerobic NaCl/serum mixture.
Sampling and Statistical Analysis
After anaerobic incubation at 37°C for 0, 1, 4 and 8 days, each root canal was enlarged with a sterile ISO size 90 file (Micro-Mega). Each file was placed in a sterile tube containing 1.5 ml of sterile anaerobic 0.9% NaCl solution. The capped tube was ultrasonicated for 1 minute and then placed in a centrifuge for 10 minutes at 8000 x g. The sediment of dentinal shavings and bacteria was resuspended in 150 μl NaCl. Following serial dilutions of 10−1 to 10−3 in NaCl, 100 μl aliquots were plated onto agar plates and the numbers of CFU/ml were determined as previously described.16 At the selected time intervals, the points that had been removed were also transferred to sterile tubes containing 1.5 ml NaCl each. Again the numbers of CFU/ml in subsequent dilutions were calculated. Six replicates were carried out for each experiment including the control group. Precautions were taken to ensure asepsis throughout the experiments and to keep the bacteria's exposition to oxygen as low as possible. Preliminary results confirmed that opening the incubation vials frequently and/or for long periods had an unacceptable influence on bacterial growth.17 Statistical analysis was performed with the nonparametric Kruskal-Wallis test, followed by the Mann-Whitney test. The resulting P values were corrected by using the Holm procedure.
RESULTS
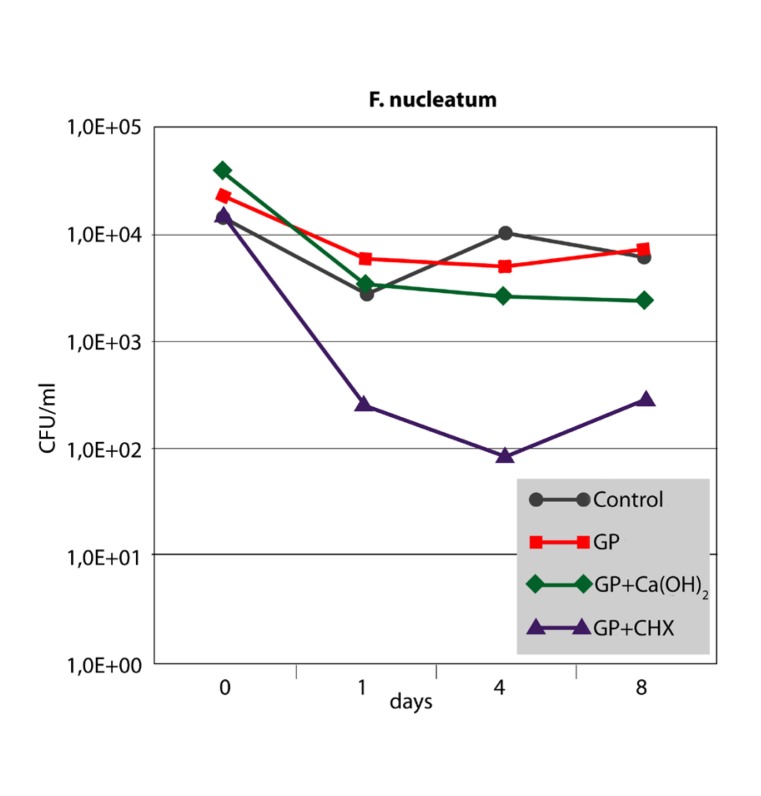
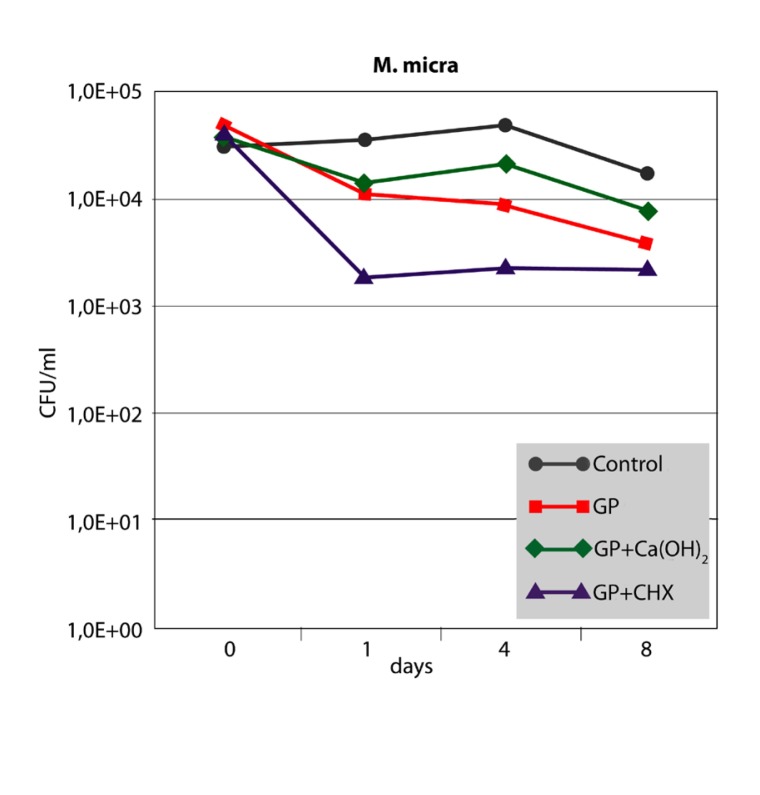
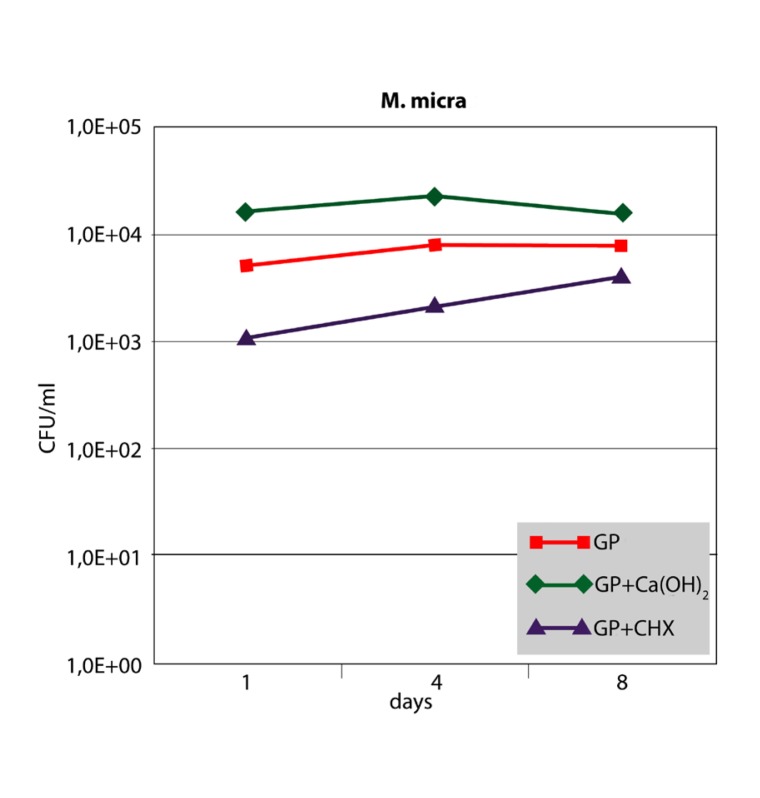
The antibacterial effect of the three different points against F. nucleatum and P. micra in the root canal is shown in Figures 2 and 3. For both strains, growth controls showed no significant differences up to 8 days (P>.05). After 1 day, bacterial cell counts in the root canals filled with GP+CHX were significantly lower than in the other groups (P<.05). However, the difference was no longer significant at day 8 for P. micra (P>.05). The inhibitory effect of GP+Ca(OH)2 did not significantly differ from that of GP alone or the growth control at any time interval (P>.05).
Figure 2.
Median CFU/ml counts (n=6) of F. nucleatum sampled from inoculated root canals filled with three different points at different time intervals in comparison with the growth control (root canals without points).
Figure 3.
Median CFU/ml counts (n=6) of P. micra sampled from inoculated root canals filled with three different points at different time intervals in comparison with the growth control (root canals without points).
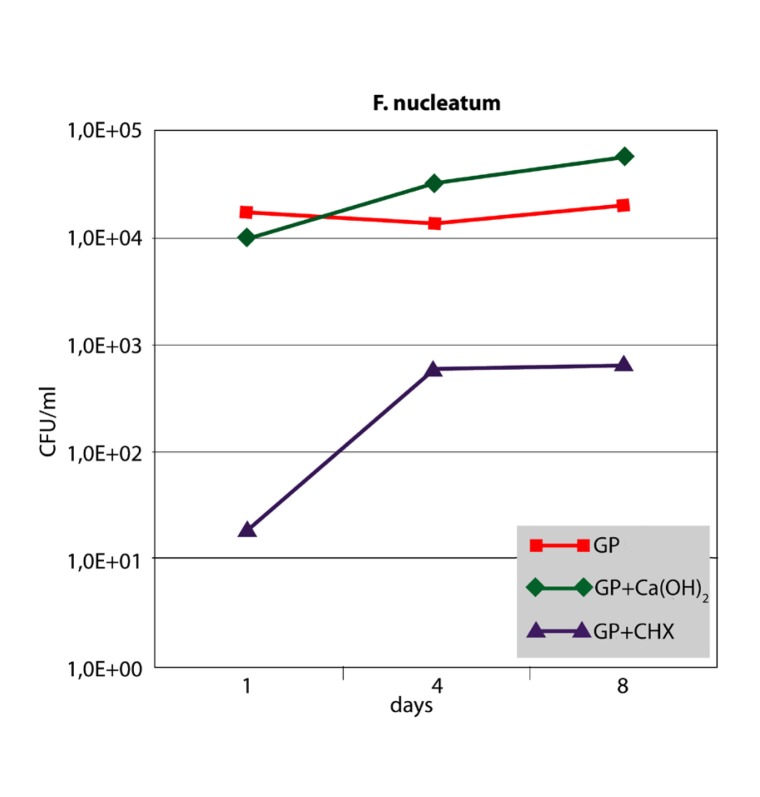
When F. nucleatum was sampled from the points, GP and GP+Ca(OH)2 showed constant and high bacterial cell counts over the 8-day period. No significant differences could be observed (P<.05). In contrast, GP+CHX showed lower cell counts after one day with bacterial growth increasing on the subsequent days (Figure 4). However, GP+CHX was significantly more effective than GP+Ca(OH)2 or GP at any time (P<.01). Sampling of P. micra from the points resulted in similar data (Figure 5). Significant differences were seen between GP+CHX and GP+Ca(OH)2 (P<.05). No significant differences were found between GP+Ca(OH)2 and GP (P>.05).
Figure 4.
Median CFU/ml counts (n=6) of F. nucleatum sampled from three different points at different time intervals.
Figure 5.
Median CFU/ml counts (n=6) of P. micra sampled from three different points at different time intervals.
DISCUSSION
In this study, the root canal inoculation test originally described by Haapasalo and Ørstavik was modified to include a quantitative assay and to involve obligate anaerobes as the antimicrobial target cells.11 Due to the thick dentin layer of bovine incisors, the root canal could be standardized. The smear layer produced during root canal preparation was not removed by irrigation, as this could have distorted the effects of the medicaments.10 In clinical situations, the antibacterial effect of the medicated GP points may be higher when the root canals are irrigated. In this study, however, the root canals were seeded only for a short period of time. The resulting early phase biofilms are known to be rather susceptible to medicaments compared to a maturated biofilm involving complex microbial interactions. When the points tested were removed from the root canals, some of the bacteria were inevitably brushed off on the canal walls. The progressive reaming of the standardized root canal from ISO size 80 to 90 aimed to sample residual bacteria up to dentin depths of 100 μm around the canal lumen. This approach of removing the canal wall dentin with sterile endodontic files and quantitatively determining the viable bacteria by plating the recovered dentin shavings has been described in previous studies.12, 13 The fact that it was difficult to standardize the amount of dentin removed may have contributed to the data variance. However, when the validity was considered, the bacterial samples taken from the root canal and the points showed comparable results. The numbers of CFU/ml from the control teeth were almost constant over the 8-day period with a bacteria load similar to that which is found in a clinically infected root canal.2,18 The results from the sampling of the growth control at 0, 1, 4 and 8 days after baseline showed that the viable counts of F. nucleatum and P. micra were not significantly affected by the short-term exposition of the bacteria to oxygen when the incubation vials were opened.17 Diluted serum was used to simulate the mixture of interstitial liquid and blood filtrate found in clinical situations5 and to allow any possible influences of plasma proteins on the medicaments (eg, buffering) to be taken into account.9 This NaCl/serum solution mixture also served as the growth control in earlier studies.5,16,17
Although the bacterial strains used are type strains, they were originally isolated from sick humans. According to the information provided by the ATCC product sheet, F. nucleatum was isolated from the inflamed gingiva of an adult male and P. micra from a patient with purulent pleurisy. Therefore, they should be suitable for early assays for determining the antimicrobial potency of medicated GP points. However, these strains have been used in laboratories over a long period of time and might therefore have lost some of their pathogenic features. Therefore, freshly isolated clinical strains could enhance further tests and might lead to results differing from the present observations.
According to the present results, CHX medicated points were significantly more effective against F. nucleatum and P. micra than Ca(OH)2 containing points. This finding supports studies suggesting that CHX has a strong antibacterial effect in concentrations ranging from 0.12% to 5%4,12 but contradicts the findings of others reporting similar efficacies for CHX and Ca(OH)2.13,18 The discrepancies in the results may be due to variations in the study design, the concentration and/or the delivery form of the antimicrobial agent. It has been shown that gels with 5% CHX are significantly more effective than CHX containing points.18 It was surmised that the active ingredients were released from the GP matrix very rapidly. This short-term release may constitute one of the reasons why in the present study, the maximum of the antibacterial effect of both CHX and Ca(OH)2 containing points was reached initially and decreased over the 8-day period.
The minor antibacterial effect of the Ca(OH)2 containing points in this study coincides with other findings.19,20 Measurements as a function of time revealed that within 1 day the major amount of Ca(OH)2 was released from the matrix and the concentration reached a saturation point.19 A significantly higher pH was only established in the immediate proximity of the Ca(OH)2 containing points after 1 day and could no longer be detected after 3 days.20 As a consequence, the alkalinity may not have been high enough to result in the eradication of bacteria. However, the delivery system does not seem to be the only factor that is responsible for the limited effectiveness. Although studies have found Ca(OH)2 to be more effective when applied as a paste,18,19 concern has grown about the limited effectiveness of Ca(OH)2 in general against some species frequently found in clinically infected canals.3 Despite the fact that there was no significant difference, the Ca(OH)2 containing points showed a less pronounced effect on P. micra than GP in this study. The ZnO of the GP matrix seems to have some antibacterial activity caused by the release of cytotoxic zinc ions.21 Nevertheless, the fact that chronic apical periodontitis often occurs in conjunction with inadequately filled root canals2 indicates that GP cannot be expected to lower the bacteria counts significantly in clinically treated root canals.
CONCLUSION
Under the limitations of the in vitro setting, our results suggest that GP loaded with ca 52% Ca(OH)2 do not possess a long-lasting antibacterial advantage over the non-medicated GP. The incorporation of ca 5% CHX in GP leads to an enhanced antibacterial effect against F. nucleatum and P. micra, at least in the short-term. However, factors not present in the root canal inoculation model may play an important role in the antimicrobial activity in vivo. Particularly, the multispecies communities of biofilms may promote increased resistance levels against antimicrobial agents.
Acknowledgments
The authors deny any financial affiliations related to this study. The authors thank Henry Frehse (Department of Operative Dentistry and Periodontology, University of Ulm) for the preparation of the root specimens and Prof. Dr. Rainer Muche (Institute of Biometry, University of Ulm) for the statistical support.
REFERENCES
- 1.Gomes BP, Pinheiro ET, Gade-Neto C, et al. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19:71–76. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 2.Blome B, Braun A, Sobarzo V, Jepsen S. Molecular identification and quantification of bacteria from endodontic infections using real-time polyermase chain reaction. Oral Microbiol Immunol. 2008;23:384–390. doi: 10.1111/j.1399-302X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 3.Sathorn C, Parashos P, Messer H. Antibacterial efficacy of calcium hydroxide intracanal dressing: a systematic review and meta-analysis. Int Endod J. 2007;40:2–10. doi: 10.1111/j.1365-2591.2006.01197.x. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadi Z, Abbott PV. Antimicrobial substantivity of root canal irrigants and medicament: a review. Aust Endod J. 2009;35:131–139. doi: 10.1111/j.1747-4477.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 5.Podbielski A, Spahr A, Haller B. Additive antimicrobial activity of calcium hydroxide and chlorhexidine on common endodontic bacterial pathogens. J Endod. 2003;29:340–345. doi: 10.1097/00004770-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Yesilsoy C, Whitaker E, Cleveland D, Phillips E, Trope M. Antimicrobial and toxic effects of established and potential root canal irrigants. J Endod. 1995;21:513–515. doi: 10.1016/s0099-2399(06)80524-8. [DOI] [PubMed] [Google Scholar]
- 7.Staehle HJ, Thomä C, Müller HP. Comparative in vitro investigation of different methods for temporary root canal filling with aqueous suspensions of calcium hydroxide. Endod Dent Traumatol. 1997;13:106–112. doi: 10.1111/j.1600-9657.1997.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 8.Ebert J, Roggendorf MJ, Frank K, Petschelt A. Antimicrobial activity of various 'active' gutta-percha points against Enterococcus faecalis in simulated root canals. Int Endod J. 2008;41:249–257. doi: 10.1111/j.1365-2591.2007.01349.x. [DOI] [PubMed] [Google Scholar]
- 9.Portenier I, Waltimo T, Ørstavik D, Haapasalo M. Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. J Endod. 2006;32:138–141. doi: 10.1016/j.joen.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Zehnder M, Luder H, Schatzle M, Kerosuo E, Waltimo T. A comparative study on the disinfection potentials of bioactive glass S53P4 and calcium hydroxide in contra-lateral human premolars ex vivo. Int Endod J. 2006;39:952–958. doi: 10.1111/j.1365-2591.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- 11.Haapasalo M, Ørstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 12.Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod. 2005;31:53–56. doi: 10.1097/01.don.0000134209.28874.1c. [DOI] [PubMed] [Google Scholar]
- 13.Lui JN, Sae-Lim V, Song KP, Chen NN. In vitro antimicrobial effect of chlorhexidine-impregnated gutta percha points on Enterococcus faecalis. Int Endod J. 2004;37:105–113. doi: 10.1111/j.0143-2885.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- 14.Sundqvist G. Taxonomy, ecology, and pathogenicity of the root canal flora. Oral Surg Oral Med Oral Pathol. 1994;78:522–530. doi: 10.1016/0030-4220(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 15.Seabra Pereira OL, Siqueira JF., Jr Contamination of gutta-percha and Resilon cones taken directly from the manufacturer. Clin Oral Investig. 2010;14:327–330. doi: 10.1007/s00784-009-0295-z. [DOI] [PubMed] [Google Scholar]
- 16.Rathke A, Staude R, Muche R, Haller B. Antibacterial activity of a triclosan-containing resin composite matrix against three common oral bacteria. J Mater Sci Mater Med. 2010;21:2971–2977. doi: 10.1007/s10856-010-4126-1. [DOI] [PubMed] [Google Scholar]
- 17.Spahr A, Lyngstadaas SP, Boeckh C, Andersson C, Podbielski A, Haller B. Effect of enamel matrix derivate Emdogain on the growth of periodontal pathogens in vitro. J Clin Periodontol. 2002;29:62–72. doi: 10.1034/j.1600-051x.2002.290110.x. [DOI] [PubMed] [Google Scholar]
- 18.Barthel CR, Zimmer S, Zilliges S, Schiller R, Göbel UB, Roulet JF. In situ antimicrobial effectiveness of chlorhexidine and calcium hydroxide: gel and paste versus gutta-percha points. J Endod. 2002;28:427–430. doi: 10.1097/00004770-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Economides N, Koulaouzidou EA, Beltes P, Kortsaris AH. In vitro release of hydroxyl ions from calcium hydroxide gutta-percha points. J Endod. 1999;25:481–482. doi: 10.1016/S0099-2399(99)80285-4. [DOI] [PubMed] [Google Scholar]
- 20.Schäfer E, Al Behaissi A. ph changes in root dentin after root canal dressing with gutta-percha points containing calcium hydroxide. J Endod. 2000;26:665–667. doi: 10.1097/00004770-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Silver S. Bacterial resistance to toxic metal ions - a review. Gene. 1996;179:9–19. doi: 10.1016/s0378-1119(96)00323-x. [DOI] [PubMed] [Google Scholar]