Abstract
Veterinarians contacted to identify cats diagnosed with osteoarthritis (OA) provided information on signalment, method of diagnosis, treatment and concurrent disease. Owners of 50 cats were interviewed to collect information on specific OA signs observed in the home, relating to mobility, self-maintenance, social and exploratory behavior, and activity and habits at diagnosis and after treatment. Mean age at diagnosis was 12 y; concurrent diseases were common (44%). Owner-reported abnormalities led to OA diagnosis in most cases; either as the primary finding (30%), or combined with abnormal physical examination or radiographic findings (64%). Owners frequently reported changes in mobility, particularly gait, jumping, and use of stairs. Oral or injectable disease-modifying osteoarthritis drugs were the most common treatments (71%). Feline OA diagnosis and therapeutic monitoring appear to rely heavily on owner-perceived signs; physical examination abnormalities may not be detected. Questioning of owners revealed various observable signs potentially useful in OA detection and monitoring.
Résumé
Signes d’arthrose que perçoivent les propriétaires de chats. Des vétérinaires furent contactés pour identifier des cas d’arthrose féline, et ils ont fourni les informations concernant le signalement, la méthode de diagnostic et les traitements administrés à ces chats. Les propriétaires de 50 chats arthrosiques furent sondés pour caractériser les signes d’arthrose liés à la mobilité et l’activité, les soins du corps, le comportement exploratoire, et les habitudes particulières du chat au moment du diagnostic et suite au traitement. L’âge moyen était de 12,0 ans, et plusieurs chats avaient des maladies concomitantes (44 %). Le diagnostic est fondé sur les observations des propriétaires rapportées au vétérinaire compatibles avec de l’arthrose (30 %), ou sur leur recoupement avec les découvertes de l’examen physique ou radiographique (64 %). Les changements au niveau de la mobilité (surtout la démarche, le saut, et la façon de prendre les escaliers) étaient fréquents. Les traitements les plus fréquents étaient les agents structuro-modulateurs (71 %). Actuellement, les observations de changements subtils à la maison de la part du propriétaire sont utilisées pour le diagnostic et le suivi de l’arthrose féline, car des anomalies ne sont pas toujours évidentes lors de l’examen physique. Le questionnement précis des propriétaires a révélé d’autres signes potentiels d’arthrose féline.
(Traduit par les auteurs)
Introduction
Osteoarthritis (OA) causes chronic pain and disability across mammalian species, but the severity of radiographic signs does not correlate well with expressed pain or functional impairment (1), thereby hindering diagnosis and therapy. Cats are notoriously difficult subjects when it comes to pain assessment; consequently, feline pain recognition and intervention have historically been deficient (2,3). Feline OA is particularly challenging for owners and veterinarians to identify, purportedly because signs such as overt lameness are rare (4,5). Unalleviated chronic pain is a welfare concern for cats, and functional limitations and pain may contribute to behavior problems (e.g., house-soiling, altered social interactions) (6). The latter may cause nuisance, property damage, injury (e.g., due to aggression), and loss of the human-animal bond with consequent euthanasia or surrender (7).
The radiographic prevalence of feline degenerative joint changes, including OA, is high and increases rapidly with age (4,6,8–17). The infrequent diagnosis of OA-related pain and impaired mobility has raised the question of whether cats lack significant OA pain despite having radiographic signs, or whether they have a species peculiarity making OA pain detection especially difficult (e.g., a lack of lameness as a prominent sign). Reported findings associated with the disease include thickened joints, crepitus, reduced range of motion, objection to manipulation or palpation of affected joints, abnormal gait, anorexia, weight loss, inappropriate elimination, seclusion, and grumpiness toward or avoidance of other household members (human or animal) (6,8,10–14,16,17). Overweight cats have an increased risk of non injury-related lameness, which may include OA (6,18). Recent studies show differences in demeanor, activity, mobility, self-grooming, and elimination habits between cats with and without OA, and in cats with OA before and after treatment, supporting both the presence of OA pain and disability affecting quality of life, and that signs are apparent to cat owners (6,10–12,16,17,19).
History-taking allows the veterinarian to place an emphasis on evaluation of body systems with a suspicion of disease. The gold standard for OA diagnosis is arguably a combination of radiographic and physical examination findings compatible with OA, but even pain detection on a thorough orthopedic examination does not necessarily relate to radiographic OA (67% of apparently painful joints had no radiographic signs of OA in 1 study, and only 36% of joints with radiographic OA were painful in another) (11,12). These considerations, combined with the variable nature of feline cooperation with orthopedic examination, highlight the importance of the anamnesis in veterinary detection of this disease. However, a better understanding of the feline OA signs observed by owners in the home is needed. The goal of this study was twofold: to use a population of cats diagnosed with OA to determine how veterinarians achieved their diagnosis, in particular with respect to the use of owner-reported signs, and to examine owner perceptions of signs that could reflect OA pain. With respect to the latter, we hypothesized that affected cats would demonstrate detectable changes in the categories of mobility and activity, and self-maintenance, social, play and exploratory behaviors, and that these changes could be observed by owners in the home and would contribute to disease detection. Specific objectives were: i) to determine the contribution of owners’ reports of signs perceived in the home, relative to the contribution of the physical and radiographic examinations, to OA diagnosis in veterinary practice; ii) to identify additional signs that might be of use in OA detection and monitoring, based on owners’ observation of specific behavior changes or other signs noted in the home around the time of OA diagnosis; and iii) to determine what treatments were commonly prescribed by the veterinarian and whether or not owners perceived an effect on these signs.
Materials and methods
Owners of pet cats with a veterinary clinical diagnosis of OA were selected to participate in a phone interview. Information collected included: signalment, method of diagnosis, concurrent medical conditions, treatments for OA, their observations of specific changes in their pet’s behavior at OA diagnosis and after treatment. All procedures were approved by the Institutional Animal Care and Use Committee (Rech-1482).
Owners were recruited via i) a search of the medical archives of the Université de Montréal’s Veterinary Teaching Hospital (VTH), and ii) solicitation of veterinarians at feline-only practices, and at high-volume companion animal practices, in the greater Montreal and Quebec city regions. A letter describing the study purpose and requesting participation was sent to veterinarians, and followed 7 to 10 d later by an initial phone contact, which was followed by further phone, e-mail, and/or in-person contacts as necessary. Veterinarians were asked to provide: contact information for owners of cats they had diagnosed with OA, patient signalment, and a brief explanation of how the diagnosis was achieved (i.e., whether physical examination abnormalities were identified, whether the diagnosis was confirmed radiographically, or by response to treatment, as well as any other treatment(s) prescribed).
All owner interviews were conducted between March and June 2010. A single interviewer (MK) telephoned each owner, briefly described the purpose of the study, and obtained consent for participation. Interviews were conducted in French or English, according to the respondent’s preference. However, the same sequence was followed in both languages, and a standardized script was used to minimize variation that could affect responses. The interview was pilot-tested on cat owners to determine length and comprehensibility of questions; it took approximately 20 min to complete and the only problem identified was that French- and English-speaking respondents interpreted the item for 1 specific behavior, “climbing,” differently; it was therefore removed from the survey. Questions on signalment, manner of diagnosis, and treatment were included in order to confirm the information obtained from the veterinarian. All responses were kept confidential, and identifying information was removed prior to data assessment. Interview questions are shown in Table 1. Descriptive statistics were used to summarize the general characteristics of the population.
Table 1.
Questions and response options for the cat owner interviews
| Question | Response options | ||
|---|---|---|---|
| Age at diagnosis | Open-ended | ||
| Sex | MI/FI/MN/FS | ||
| Breed | Open-ended | ||
| Still living in the home | Y/N | ||
| Manner of OA diagnosis | Owner history/Veterinary exam/Both | ||
| OA treatments and effect | Open-ended | ||
| Signs noted by owners at time of OA diagnosis | Open-ended | ||
| Presence of specific changes (see list below) at time of OA diagnosis. If yes, describe. | Y/N Open-ended |
||
| Responsiveness to treatment of noted changes | Resolved/Improved/No change | ||
| Categories of changes | |||
| Activity Daily schedule Jumping Stairs Speed Play/hunting Agility Gait Sound of footsteps Posture |
Areas of home used Resting spots Time spent resting Use of litter box Self-grooming Coat/claw condition Scratching/Claw sharpening Appetite Weight |
Vocalization Rubbing behavior Mood Interactions with family members Interactions with family pets New behaviors |
|
MI — intact male, FI — intact female, MN — neutered male, FS — spayed female.
Results
Sixteen of 25 veterinary clinics contacted (64%) contributed subjects (n = 53) to the study. Together with patients of the VTH (n = 4), this yielded a total of 56 owners of 57 cats. Of these, 51 owners (91%) were contacted successfully and agreed to participate. Two owners indicated that their cats were recently deceased; their interviews were excluded. One owner completed surveys for 2 cats, yielding a total of 50 surveys.
In the majority of cases (n = 42), the full medical record was not available for review. Veterinarians provided an exact date of diagnosis in 22 cases (44%); owner-reported duration since diagnosis was noted in the remaining cases. In 16 cases, the time from diagnosis to interview was ≤ 6 mo; in 12 cases, it was 6 mo to 1 y; in 9 cases, it was 1 to 2 y; in 13 cases, > 2 y. Signalment and concurrent disease information are presented in Table 2.
Table 2.
Age, gender, breed, and presence of concurrent abnormalities for the cats
| Age | 12.0 (3.6) y | |
| Sex | Female spayed | 26 (52%) |
| Male neutered | 24 (48%) | |
| Breed | Domestic | 42 (84%) |
| Himalayan | 2 (4%) | |
| Persian | 2 (4%) | |
| Cornish Rex | 1 (2%) | |
| Maine Coon | 1 (2%) | |
| Siamese | 1 (2%) | |
| Tonkinese | 1 (2%) | |
| Concurrent disease | Overall prevalence | 22 (44%) |
| Renal disease | 11 (22%) | |
| Diabetes mellitus | 6 (12%) | |
| Cardiac murmur/confirmed disease | 7 (14%) | |
| Hyperthyroidism | 5 (10%) | |
| Ocular disease/visual deficits | 3 (6%) | |
| Possible neurologic diseasea | 1 (2%) | |
| Allergic dermatitis | 2 (4%) | |
| Feline lower urinary tract disease | 1 (2%) | |
| Hearing loss | 1 (2%) | |
| Diarrhea | 1 (2%) | |
| Obesity | 5 (10%) |
Age is shown as mean (standard deviation). All other results are shown as number (percent).
The neurologic diagnosis that was considered but not confirmed for this cat was intervertebral disc disease.
Aspects of the veterinary clinical evaluation contributing to OA diagnosis are presented in Table 3. Initial diagnosis was based on a combination of changes in the home reported by the owner (i.e., the anamnesis), and findings on veterinary physical (e.g., pain or palpable joint abnormalities) or radiographic examination for 32 cats (64%). Twelve of these cats (38%) had both radiographic and physical examination abnormalities, 9 (28%) had radiographic signs of OA without physical examination abnormalities (2 were also confirmed by response to treatment), and 11 (34%) had physical examination abnormalities but were not evaluated radiographically (3 were also confirmed by response to treatment). For 15 cats (30%), initial diagnosis was based on owner-reported changes in the absence of physical examination abnormalities, and radiographic evaluation was not performed; in 5 of these (33%), response to treatment was used to confirm the diagnosis. Of 4 cases treated with an oral glucosamine supplement, 1 demonstrated resolution of primary signs (hiding and difficulty climbing stairs), and 3 owners reported general subjective improvements; the remaining cat was treated with meloxicam and the primary complaint resolved (urination and defecation outside the litter box, on beds and sofas). For 3 cats (6%), initial diagnosis was based solely on the veterinarian’s examination, in the absence of historical abnormalities observed by the owner; 1 of these cases (33%) was confirmed by both radiographic evaluation and therapeutic response, and 1 by therapeutic response alone. Treatment was administered for OA in 49 cats (98%), and usually was with a single agent (n = 29, 59% of treated cases), but in some cases (n = 21, 43%) was with more than 1 agent (either concurrently or sequentially); specific treatment use is indicated in Table 4.
Table 3.
Reported use and apparent contributions to OA diagnosis of the various aspects of the veterinary clinical evaluation
| Aspect of evaluation | Cases assessed | Abnormal finding(s) | Cases affected | |
|---|---|---|---|---|
| Physical examinationa | 50 (100%) | All | 26 (52%) | |
| Observable | Limping/stiffness | 5 (10%) | ||
| Postural abnormality | 3 (6%) | |||
| Palpable | Pain upon palpation | 9 (18%) | ||
| Palpable changes other than pain | 10 (20%) | |||
| Joint swelling | 6 (12%) | |||
| Joint laxity | 4 (8%) | |||
| Crepitus | 3 (6%) | |||
| Muscle atrophy | 2 (4%) | |||
| Decreased range of motion | 1 (2%) | |||
| Unspecified | 6 (12%) | |||
| Owner report | 50 (100%) | Suspicious for OA | History | 47 (94%) |
| Supporting diagnosis | Treatment response | 12 (24%) | ||
| Radiographs | 22 (44%) | Change(s) compatible with OA | 22 (44%) |
Results are presented as number (percent of cases diagnosed).
Details of how the physical examination was conducted were not collected from veterinarians. It is therefore not known whether all aspects of the orthopedic examination (e.g., gait/posture evaluation, joint palpation and manipulation) were performed in all cases.
Table 4.
OA treatments administered
| Treatment | Details | Number (%) | Monotherapy (%) |
|---|---|---|---|
| DMOADsa | 35 (71%) | 17 (59%) | |
| Injectable PSGAGsb | |||
| Injectable pentosan PSc | |||
| Oral glucosamine supplement | |||
| NSAIDsd | 16 (33%) | 8 (28%) | |
| Meloxicam | |||
| Tolfenamic acid | |||
| Dietary therapy | MediCal Mobility Support® | 12 (24%) | 4 (14%) |
| Other | Gabapentin | 3 (6%) | 0 |
| Glucocorticoids | 3 (6%) | 0 | |
| Laser | 1 (2%) | 0 | |
| Herbal supplement | 1 (2%) | 0 |
For specific treatments, percentages shown are of the total number treated (Number) or of the total number treated with a single agent (Monotherapy).
DMOADs — Disease-modifying osteoarthritis drugs
PSGAGs — Polysulfated glycosaminoglycans (Adequan®; Novartis Animal Health, Mississauga, Ontario).
Pentosan PS — Pentosan polysulfate (Cartrophen Vet; Arthropharm Services, Embrun, Ontario).
NSAID — Non-steroidal anti-inflammatory drug.
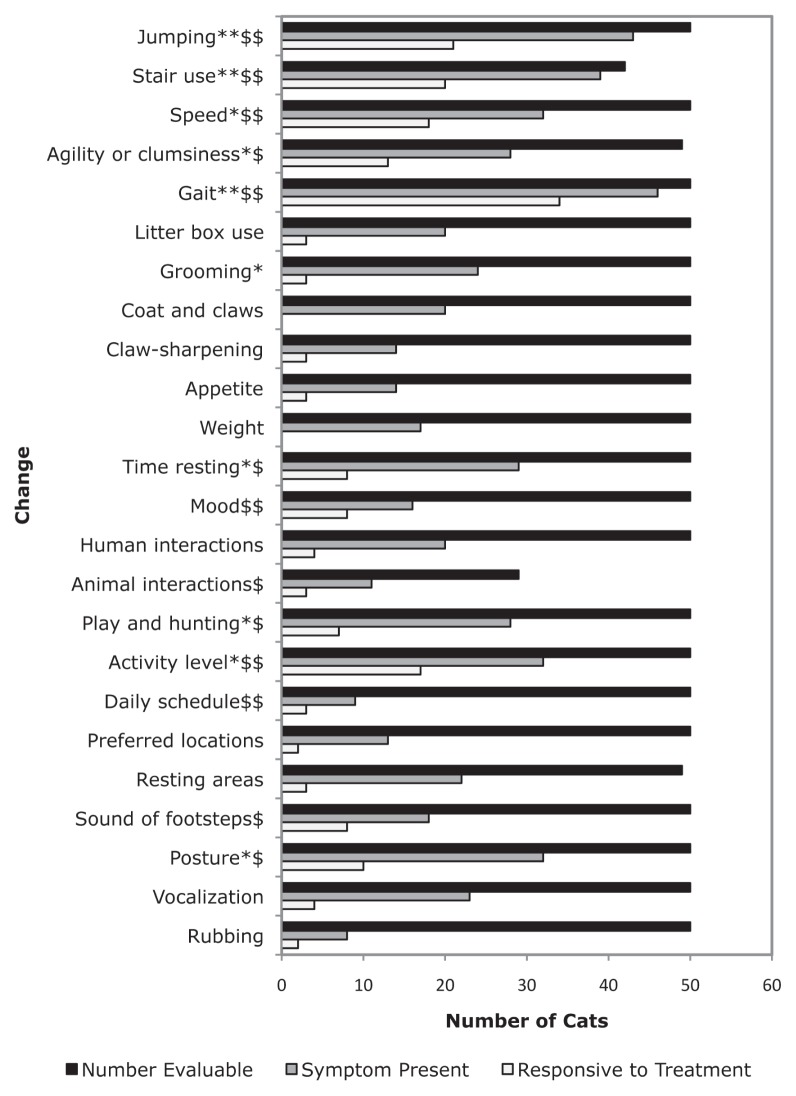
Figure 1 summarizes owner perceptions of prevalence of signs and treatment responsiveness. Gait changes included limping or stiffness (which was more severe after rest in 8 cases), and changes in limb carriage or appearance (n = 15). Owners also reported reductions or cessation in jumping (n = 22) and stair use (n = 8) as well as changes in the ways in which cats performed these activities (n = 27, n = 32, for jumping and stair use respectively), such as hesitation, stumbling or falling, or doing several small jumps instead of 1 large one, or a few stairs at a time instead of the entire flight. Some cats (n = 6) were also reported to have begun “asking” for help (by vocalizing, staring, or tapping with a paw). Changes in litter box use included elimination outside the box (n = 11), which was sometimes interpreted as being due to an inability to reach the litter box prior to elimination (due to urgency) or reluctance to negotiate stairs en route, as well as apparent difficulty maneuvering in the box (n = 9). General decreases in grooming were reported (n = 15), but also decreases (n = 7) and an increase (n = 1) in grooming of particular areas, and 1 cat started leaning against something while grooming. Owners noted various coat changes (n = 14), and claws that were longer, brittle, or more dull (n = 5), in addition to decreases in (n = 8) or changes in the manner of (n = 5) claw-sharpening (e.g., on horizontal instead of vertical surfaces). Appetite changes included reductions (n = 8; it increased in 2 of these after treatment), increases (n = 2), and increased variability (n = 2). Where time resting was affected, it was generally (n = 27) increased. Social behavior changes included a poorer mood (n = 9), occasional increased (n = 1) or decreased (n = 1) general fearfulness, increased (n = 2), or decreased (n = 1) friendliness to strangers, and increased (n = 9) or decreased (n = 7) interactions with family members (e.g., following them more, sleeping with them more or less). Several cats (n = 14) reacted to being picked up or to being touched in certain areas (e.g., near joints diagnosed with OA). Changes in interactions with household animals were reported to be decreased playfulness and tolerance, and increased frequency of being chased or picked on, rather than chasing or picking on the other animals. Changes to play and hunting (n = 24) were usually reductions or cessation; others were playing in a recumbent position (n = 2), or no longer following birds in the windows (n = 1). Changes in overall activity and habit changes were usually decreased activity level (n = 26), less time spent outside (n = 6), and seeking heat/sun (n = 4). Miscellaneous changes included altered overall character or frequency of vocalizations, increased vocalizing at the owner (n = 6), meowing from other parts of the house or when moving about (n = 6), or decreased meowing to be let out (n= 1). Changes of posture were described as a change in preferred positions (n = 7; e.g., sitting or lying more), or asymmetry or other abnormalities of posture (n = 18; e.g., hunched appearance, legs held loosely rather than tucked against the body, holding a paw up). Head- or body-rubbing behavior changes were general reductions (n = 3), an increase (n = 1), a change in targets (n = 1), or clumsiness (n = 1). Other changes volunteered by owners (n = 6) were: less adventurous, lying down/getting up slowly or with difficulty, hiding, and worsening of signs in damp weather.
Figure 1.
Owner-perceived changes associated with OA: Sign prevalence and perception of response to therapy.
** change present in ≥ 75% of OA-affected cats; * change present in ≥ 50% of OA-affected cats; $$ perceived sign responsiveness to treatment in ≥ 50% of treated cats; $ perceived sign responsiveness to treatment in ≥ 25% of treated cats.
Discussion
Our findings suggest that owners perceive particular signs linked to feline OA, in the home setting, and owner-perceived signs contribute substantially to the diagnosis of feline OA in veterinary practice in Quebec. This is consistent with other reports that have found owners to be capable of identifying signs of feline OA in the home and response to therapy (6,10–12,16).
Our relatively high response rates from veterinarians and owners, and the gender and breed distribution of the cats in our sample are consistent with reports in the literature and suggest a good representation of pet cats visiting veterinarians in our area (20,21). A high mean age is consistent with selection based on the presence of OA, which affects geriatric animals preferentially (4,8,9,11,17), and is compatible with the high prevalence of concurrent geriatric diseases. We found inconsistencies in appetite and weight changes, compatible with previous reports (6,18), and unsurprising in view of veterinarian recommendations for diet changes and the presence of concurrent geriatric diseases affecting weight and appetite.
Small numbers of cats were diagnosed with OA at each participating clinic, despite recent research reporting a high prevalence of feline degenerative joint disease including OA (4,6,8–16,17). This may have been due to a lower prevalence in our population; alternatively, it could indicate that feline OA continues to be challenging to diagnose in private veterinary practice. The diagnosis of most cases in this study was based at least in part on owner-reported abnormalities suggestive of OA (n = 47, 94%); 33 of these cases (70%), were confirmed by radiographic and/or physical examination findings, or a clear response to treatment. It was rare for veterinarians to detect OA based on their physical examination alone, in the absence of reported historical abnormalities; conversely, they were unable to identify physical examination abnormalities in several cats with both historical and radiographic findings supportive of OA. Although it is possible that radiographic findings were not the cause of the reported signs in some of these cases, it seems prudent to conclude that a lack of musculoskeletal abnormalities upon physical examination does not preclude the presence of clinical OA. Hence, the variable correlation of palpation with radiographic OA findings previously reported (11,12) may not simply reflect a weak relationship between structural joint changes and clinical (i.e., affecting pain and function) OA; physical examination abnormalities (e.g., pain) may also be particularly difficult to confirm in the cat.
The most common owner-reported changes in this study related to mobility (gait, jumping, and stair use), followed by changes in activity level, time spent resting, and self-grooming, and changes in social behavior such as interactions with humans and animals and mood, and litter box use, consistent with previous studies (6,10–12,17,19). Additionally, our findings support other proposed signs of OA such as vocalization and objections to handling, and changes in resting areas, play and hunting behavior, and posture (13). In general, the usefulness of subjective behavior parameters in addition to more objective measures, primarily of mobility such as gait, jumping, and use of stairs, is supported.
Interestingly, gait changes were the most common sign reported by owners, who also perceived them to be responsive to therapy. Our study design may have inadvertently selected for particularly attentive owners or limping cats (perhaps because this sign is more obviously suggestive of OA than are many others). Gait changes could be common at home but rarely observed during examination; however, one might then expect owners to report them more frequently. Veterinarians in our study were indeed far more likely to detect abnormalities on palpation than to observe stiffness or limping during their examination, despite many of the owners of examined cats having noted gait changes at home. Notwithstanding these possibilities, our findings strongly suggest that gait changes may be useful in the diagnosis of at least a subpopulation of OA-affected cats.
Some of the OA diagnoses based on owner reports and unconfirmed by abnormal radiographic or physical examination findings (or a clear response to treatment reported to the veterinarian) could have been incorrect; veterinarians were not asked to indicate their degree of certainty in these cases. The retrospective nature of the study may also have introduced recollection bias; questioning about specific behaviors was expected to reduce subjectivity. Despite these potential limitations, our findings correspond well with previous reports, including a recent study comparing groups of OA-affected and unaffected cats for possible OA signs in the home (16), and a cross-sectional study in 100 cats with OA (17). This supports their validity and makes additional signs uncovered in our study worthy of further investigation.
There are relatively few products licensed for the chronic treatment of feline OA. Unlike non-steroidal anti-inflammatory drugs (NSAIDs), the analgesic effects of glucosamine, pentosan polysulfate (PS), and polysulfated glycosaminoglycan (PSGAG) alone or in combination with other nutraceutical(s), have not been clearly established (19,22), and their effects on OA signs may be via other mechanisms. In our sample, frequent use of glucosamine and PSGAG may have reflected fear of adverse effects of long-term NSAID use in geriatric cats or in those with concurrent diseases (5). The infrequent use of therapeutic diets was likely due to their having become available only very recently. Although information was collected on owners’ perception of treatment effects on the OA signs they observed, the nature of the treatments used, the potential for recall bias, and the lack of veterinary confirmation in some cases make us unable to draw conclusions regarding treatment effects.
Although behavior changes in the home appear pertinent in the diagnosis of feline OA, the sensitivity and specificity (i.e., predictive validity) of these signs for use in the detection and monitoring of this disease have not been determined. Our study was performed to collect information on the methods of feline OA diagnosis in private practice, and on owner-perceived signs in the home that may be associated with OA. It did not include a control population of owners of age-matched, unaffected cats, and it included cats with concurrent diseases (e.g., cardiac disease, renal disease, hyperthyroidism, and ocular disease could cause weakness, diabetic neuropathy-associated pain, and visual deficits, affecting apparent mobility, activity level and social interactions) (23–26). Future research is therefore needed to determine whether the identified OA signs bear any confounding relationship to other age-related problems, such as geriatric diseases other than OA, cognitive decline, and sensory deficits. Prospective, placebo-controlled, blinded studies will also be needed to confirm the therapeutic responsiveness associated with these OA signs. This will help to improve the certainty of OA diagnosis by practitioners, when physical examination findings are negative or equivocal.
Feline OA diagnosis remains challenging for practitioners; it continues to be relatively infrequent, despite a growing body of research supporting the disease’s prevalence and importance in aged cats. Pending future studies confirming the specificity of these signs, owners of cats at risk for OA should be questioned carefully about subtle behavior changes in the home, particularly those relating to mobility (e.g., gait, stair use, and jumping changes) at the veterinary appointment. This is especially important because some owners may not volunteer this information without prompting, thinking it is due to “normal aging,” and because a thorough orthopedic examination may not adequately detect OA pain in many cats. Careful examination of gait and posture, as well as palpation and manipulation of the joints should also be performed in at-risk cats, particularly since some owners of cats with OA may not observe abnormalities in the home. Where physical examination abnormalities are found, or where they are lacking but owners report possible OA signs, further diagnostics and/or a therapeutic trial should be considered, in order to improve detection, diagnostic certainty, and treatment of feline OA.
Acknowledgments
The authors are grateful to Ms Dominique Gauvin, research agent, for her technical assistance. Funded in part by a Morris Animal Foundation Grant (#D09FE-803A) for the pilot study, “TOP-CAT: Tracking Osteoarthritis Pain in the CAT.” Dr. Klinck is the recipient of a Pfizer Animal Health — Morris Animal Foundation #D10-901 fellowship, “Creating Validated Pain Scales for Feline Osteoarthritis Pain Identification and Quantification.” CVJ
Footnotes
Veterinarians and survey respondents were residents of Quebec.
Presented in part at the Annual Scientific Symposium of Animal Behavior, American College of Veterinary Behaviorists and American Veterinary Society of Animal Behavior, Atlanta, Georgia, July 30, 2010.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Dieppe PA. Relationship between signs and structural change in osteoarthritis: What are the important targets for therapy? J Rheumatol. 2005;32:1147–1149. [PubMed] [Google Scholar]
- 2.Muir WW, Wiese AJ, Wittum TE. Prevalence and characteristics of pain in dogs and cats examined as outpatients at a veterinary teaching hospital. J Am Vet Med Assoc. 2004;224:1459–1463. doi: 10.2460/javma.2004.224.1459. [DOI] [PubMed] [Google Scholar]
- 3.Lascelles D, Waterman A. Analgesia in cats. In Practice. 1997;19:203–213. [Google Scholar]
- 4.Hardie EM, Roe SC, Martin FR. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997) J Am Vet Med Assoc. 2002;220:628–632. doi: 10.2460/javma.2002.220.628. [DOI] [PubMed] [Google Scholar]
- 5.Lascelles BDX. Feline degenerative joint disease. Vet Surg. 2010;39:2–13. doi: 10.1111/j.1532-950X.2009.00597.x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett D, Morton C. A study of owner observed behavioural and lifestyle changes in cats with musculoskeletal disease before and after analgesic therapy. J Feline Med Surg. 2009;11:997–1004. doi: 10.1016/j.jfms.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patronek GJ, Glickman LT, Beck AM, McCabe GP, Ecker C. Risk factors for relinquishment of cats to an animal shelter. J Am Vet Med Assoc. 1996;209:582–588. [PubMed] [Google Scholar]
- 8.Godfrey DR. Osteoarthritis in cats: A retrospective radiological study. J Small Anim Pract. 2005;46:425–429. doi: 10.1111/j.1748-5827.2005.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 9.Clarke SP, Mellor D, Clements DN, et al. Prevalence of radiographic signs of degenerative joint disease in a hospital population of cats. Vet Rec. 2005;157:793–799. doi: 10.1136/vr.157.25.793. [DOI] [PubMed] [Google Scholar]
- 10.Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg. 2008;10:235–241. doi: 10.1016/j.jfms.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke SP, Bennett D. Feline osteoarthritis: A prospective study of 28 cases. J Small Anim Pract. 2006;47:439–445. doi: 10.1111/j.1748-5827.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 12.Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client-specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Int Med. 2007;21:410–416. doi: 10.1892/0891-6640(2007)21[410:eocoma]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Hardie EM. Management of osteoarthritis in cats. Vet Clin North Am Small Anim Pract. 1997;27:945–953. doi: 10.1016/s0195-5616(97)50088-x. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey DR. Osteoarthritis in cats: A prospective series of 40 cases. BSAVA Congress Proceedings. J Small Anim Pract. 2003;44:418. [Google Scholar]
- 15.Lascelles BDX, Henry JB, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg. 2010;39:535–544. doi: 10.1111/j.1532-950X.2010.00708.x. [DOI] [PubMed] [Google Scholar]
- 16.Zamprogno H, Hansen BD, Bondell HD, et al. Item generation and design testing of a questionnaire to assess degenerative joint disease–associated pain in cats. Am J Vet Res. 2010;71:1417–1424. doi: 10.2460/ajvr.71.12.1417. [DOI] [PubMed] [Google Scholar]
- 17.Slingerland LI, Hazelwinkel HA, Meij BP, Picavet P, Voorhout G. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J. 2011;187:304–309. doi: 10.1016/j.tvjl.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc. 1998;212:1725–1731. [PubMed] [Google Scholar]
- 19.Lascelles BDX, DePuy V, Thompson A, et al. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Intern Med. 2010;24:487–495. doi: 10.1111/j.1939-1676.2010.0495.x. [DOI] [PubMed] [Google Scholar]
- 20.Toribio JLM, Norris JM, White JD, Dhand NK, Hamilton SA, Malik R. Demographics and husbandry of pet cats living in Sydney, Australia: Results of cross-sectional survey of pet ownership. J Feline Med Surg. 2009;11:449–61. doi: 10.1016/j.jfms.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu K, Anderson WM, Rieser MY. Population characteristics and neuter status of cats living in households in the United States. J Am Vet Med Assoc. 2009;234:1023–1030. doi: 10.2460/javma.234.8.1023. [DOI] [PubMed] [Google Scholar]
- 22.Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: Network meta-analysis. BMJ. 2010;16:341–350. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kittleson MD. Feline myocardial disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Vol. 2. St. Louis, Missouri: Elsevier; 2005. pp. 1082–1104. [Google Scholar]
- 24.Polzin DJ, Asborne CA, Ross S. Chronic kidney disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Vol. 2. St. Louis, Missouri: Elsevier; 2005. pp. 1751–1785. [Google Scholar]
- 25.Mooney CT. Hyperthyroidism. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Vol. 2. St. Louis, Missouri: Elsevier; 2005. pp. 1544–1560. [Google Scholar]
- 26.Nelson RW. Diabetes mellitus. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. Vol. 2. St. Louis, Missouri: Elsevier; 2005. pp. 1563–1591. [Google Scholar]