Abstract
A great Dane dog was presented with a small, superficial wound on the left tarsus that rapidly progressed to a large necrotic area. The dog had undergone radiation therapy in the left tarsal region 33 months previously. Necrotizing fasciitis was diagnosed on histopathological examination, and bacterial culture revealed methicillin-resistant Staphylococcus pseudintermedius.
Résumé
Fasciite nécrosante causée par Staphylococcus pseudintermedius résistant à la méthicilline à un site antérieurement irradié chez un chien. Un chien Grand danois a été présenté avec une petite blessure superficielle sur le tarse gauche qui a rapidement progressé pour devenir une grande région nécrotique. Le chien avait subi de la radiothérapie dans la région du tarse gauche 33 mois auparavant. La fasciite nécrosante a été diagnostiquée à l’examen histopathologique et la culture bactérienne a révélé Staphylococcus pseudintermedius résistant à la méthicilline.
(Traduit par Isabelle Vallières)
A 6-year-old 93.3-kg castrated male great Dane dog was admitted to a private 24-hour veterinary hospital with a 3-day history of progressive left hind limb lameness, pain in the left tarsal region and lethargy, and a 2-day history of swelling and warmth in the left tarsal region. The owners had noticed a possible small, superficial wound in the skin in the caudal tarsal region at the time the lameness started, after a day spent at a canine daycare. The left tarsal region had been irradiated at the Western College of Veterinary Medicine Veterinary Medical Centre 33 mo previously for an incompletely excised grade 2 soft tissue sarcoma. The radiation protocol consisted of 18 fractions of 300 cGy for a total dose of 5400 cGy, delivered with a cobalt therapy unit. Two days prior to admission the dog had started meloxicam [0.1 mg/kg body weight (BW), PO, q24h], prescribed by a veterinarian who did not examine the dog.
On initial examination, the dog was depressed and non-ambulatory, and the left tarsal region was markedly swollen, erythematous, painful, and warm to the touch, with sanguinous discharge from a 0.3-cm wound on the caudal- lateral aspect. Rectal temperature was 41.4°C, heart rate was 132 beats/min, respiratory rate was 34 breaths/min, mucous membranes were pink and moist with a normal capillary refill time, and thoracic and cardiac auscultation were unremarkable. Abdominal palpation was not performed as the dog was unable to stand. A complete blood (cell) count (CBC) revealed mild neutrophilia [12 900 neutrophils/mm3; reference interval (RI): 3500 to 12 000 neutrophils/mm3], and mild thrombocytopenia (177 000 platelets/mm3; RI: 200 000 to 500 000 platelets/mm3). Moderate elevation of alkaline phosphatase (546 U/L; RI: 20 to 150 U/L) and mild decrease of blood urea nitrogen (2.3 mmol/L; RI: 2.5 to 8.9 mmol/L) were found on serum biochemical analysis. Left tarsal region radiographs revealed soft tissue swelling overlying the cranial aspect of the left tibia and dorsal aspect of the tarsus, and mild degenerative changes associated with the tarsal joint. Differential diagnoses considered by the attending clinician were cellulitis, lymphedema secondary to radiation therapy, and tumor recurrence.
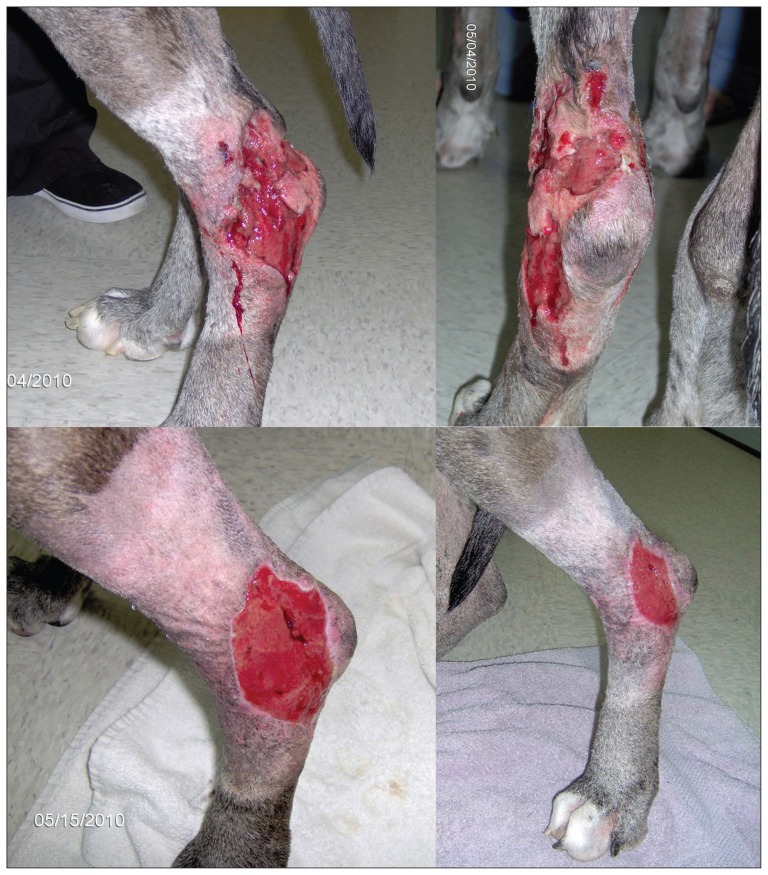
Isotonic crystalloid fluid (1.1 mL/kg BW per hour), enrofloxacin (Baytril; Bayer, Toronto, Ontario), 5.0 mg/kg BW, IV, q12h, cefazolin (Cefazolin; Sandoz Canada, Boucherville, Quebec), 22.0 mg/kg BW, IV, q8h, and hydromorphone (Hydromorphone; Sandoz Canada), 0.05 mg/kg BW, IV, as needed for pain were started, and meloxicam (Metacam; Boehringer Ingelheim, Burlington, Ontario), 0.1 mg/kg BW, SQ, q24h was continued. Over the first 24 h, the soft tissue inflammation progressed to involve a region 25 cm proximal and 20 cm distal to the left tarsus, and a 30 cm × 15 cm area of dehiscence developed (Figure 1 top). Incisional biopsies of skin in the left tarsal region and of the left popliteal lymph node were submitted, and tramadol (Ultram; Janssen, Toronto, Ontario), 1.1 mg/kg BW, PO, q8h was started 24 h after admission. The pyrexia resolved after 48 h. Vomiting developed 72 h after admission and sucralfate (Sulcrate; Axcan Pharma, Mont-Saint-Hilaire, Quebec), 0.02 g/kg BW, PO, q8h was started; the vomiting resolved after 24 h. Serum biochemical analysis 72 h after admission revealed hypoalbuminemia (17 g/L; RI: 25 to 44 g/L), increased alkaline phosphatase (397 U/L; RI: 20 to 150 U/L), decreased alanine aminotransferase (< 5 U/L; RI: 10 to 118 U/L), decreased blood urea nitrogen (1.2 mmol/L; RI: 2.5 to 8.9 mmol/L), and decreased total protein (52 g/L; RI: 54 to 82 g/L). Fresh frozen plasma (3.5 units) was transfused. From day 1 to day 5 the tarsal region dehiscence continued to progress, but at a slower rate, and by day 5 the swelling of the region had decreased.
Figure 1.
Necrosis and ulceration that developed within 24 h after admission for swelling, pain, and warmth in the left tarsal region of a 6-year-old castrated male great Dane dog (top image). Granulation tissue and wound contraction were present 5 d (bottom left image) and 13 d (bottom right image) after surgical debridement of the wound in the left tarsal region.
The dog was discharged on day 5 on enrofloxacin (Baytril; Bayer), 4.8 mg/kg BW, PO, q24h, amoxicillin (Amoxil; Pfizer Canada, Kirkland, Quebec), 21.4 mg/kg BW, PO, q12h, and tramadol (Ultram, Janssen), 1.1 mg/kg BW, PO, q8h, with biopsy results still pending. Histopathological results were available on day 7 and revealed marked, suppurative, interstitial dermatitis, cellulitis, hypodermal necrosis, intraepithelial neutrophilic micropustules, spongiosis and dermal edema, and marked suppurative lymphadenitis, consistent with a necrotizing fasciitis. A Gram stained smear revealed rare, gram-positive, coccoid bacteria that occurred as pairs, clusters, and chains. Surgical debridement was performed on day 7 and the wound was dressed with unpasteurized honey. Bacterial culture was performed on 2 subcutaneous swabs and on a tissue sample. Enrofloxacin was discontinued and clindamycin (Antirobe; Pharmacia Animal Health, Orangeville, Ontario), 9.7 mg/kg BW, PO, q12h was started. Open wound management with lavage and honey dressing was continued every 48 h. Granulation tissue and wound contraction were present on day 5 and day 13 after debridement (Figure 1 bottom). Staphylococcus pseudintermedius (recently recognized as distinct from S. intermedius) was grown in pure culture. This organism was reported as methicillin-resistant S. pseudintermedius (MRSP) by the diagnostic laboratory, and was resistant to β-lactams (amoxicillin + clavulanic acid), fluoroquinolones (enrofloxacin and marbofloxacin), chloramphenicol, tetracycline, trimethoprim + sulfamethoxazole, and clindamycin. Secondary intention wound healing continued and the wound was completely healed 52 d after initial presentation, at which time the clindamycin and amoxicillin were discontinued.
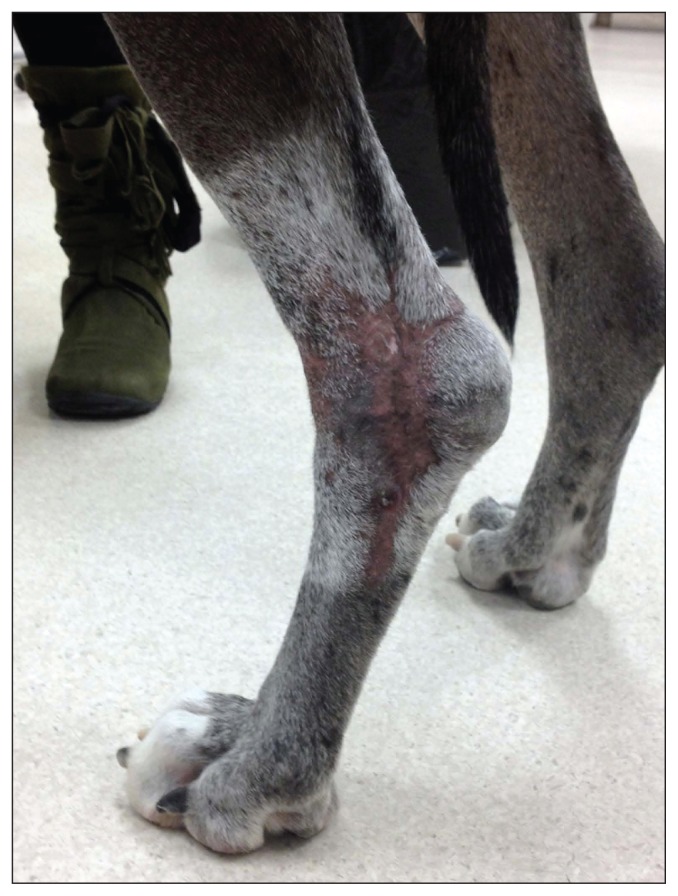
The dog was seen at the Western College of Veterinary Medicine Veterinary Medical Centre for a recheck examination with the radiation oncology service 18 mo later. Alopecia and leukotrichia were present in the previously irradiated region (Figure 2). There was no evidence of tumor recurrence. Cultures of nasal, pharyngeal, and rectal bacterial swabs were done to detect persistent MRSP colonization; no MRSP were detected. Culture of nasal and pharyngeal swabs collected from the dog’s owners was also done by an infectious disease physician at Royal University Hospital; MRSP was not grown from either source.
Figure 2.
Appearance of the infection site 18 mo after initial presentation for necrotizing fasciitis and 51 mo after radiation therapy in the left tarsal region. Alopecia and leukotrichia are present in the irradiated region and are common late effects of radiation therapy.
Discussion
Necrotizing fasciitis is a rare, sudden onset, rapidly progressive bacterial soft tissue infection (1). The infection can progress from a small skin lesion to a large region of necrosis and ulceration within several hours, often with systemic signs of shock. Prompt diagnosis and emergent, aggressive surgical debridement are critical to decrease morbidity and mortality (1). However, the lack of pathognomonic signs and low awareness of this disease among veterinarians may impede a prompt diagnosis, and the disease may be missed early in its presentation, as in the case presented in this report. Because diagnosis is based on histopathological examination and bacterial culture, which are not available in the time frame in which treatment should be initiated, treatment must be initiated based on a clinical diagnosis (2). Many early clinical signs of necrotizing fasciitis, including localized swelling, erythema, and warmth, do not differentiate this condition from other soft tissue infections. One consistent clinical sign in human patients with necrotizing fasciitis is severe pain out of proportion to the physical examination findings; this is also present in most dogs with necrotizing fasciitis, and was present in the dog in this report (1,3). The intense pain is caused by severe tissue damage in the subcutaneous region from bacterial toxins and tissue-damaging enzymes, which occurs with little change in the overlying skin (3).
Despite delayed diagnosis and surgical intervention, the dog in this report was successfully treated. Survival rates appear to be higher for canine patients with necrotizing fasciitis than human patients with the disease, despite the barriers that exist to early diagnosis in veterinary patients (1). It is of interest that 4 of the 17 dogs reported with necrotizing fasciitis have been great Danes, although the case numbers are too low to report significant predisposing factors (1,4–10).
Changes in vasculature, including progressive obliteration and thrombosis of capillaries and arterioles, persist for years after irradiation (11). Irradiated skin also has denser collagen content. Reduction in the microvascular network, secondary to obliteration of vessels, and impaired diffusion of oxygen, secondary to fibrosis, lead to decreased tissue oxygenation (12). Tissue hypoxia interrupts normal wound healing mechanisms, and predisposes a wound to bacterial invasion. As well, atrophy of the epidermis, common years after irradiation, may reduce the skin’s resistance to injury (12). For these reasons, prior irradiation has been suggested as a possible predisposing factor for necrotizing fasciitis in human patients (13–15). This possibility should also be considered in veterinary patients treated with radiation therapy.
In the present case MRSP was not isolated until later in the progression of disease, and after the initiation of emperic cefazolin and enrofloxacin therapy. Although previous reports have implicated S. pseudintermedius as the sole cause of necrotizing fasciitis in a dog, these infections are often polymicrobial including anaerobes, streptococci, and the Enterobacteriaceae; organisms which may have been susceptible to the antimicrobials used in this case (3,9,16). The MRSP mono-culture reported here may reflect eradication of susceptible organisms by antimicrobial therapy rather than a true single etiology infection. Additionally, the positive outcome suggests that the role of potential co-infectors in disease was greater than that of MRSP; once the infectious burden was lessened with cefazolin and enrofloxacin it was below the pathogenic threshold, allowing clinical resolution. Additional testing to assess virulence of this S. pseudintermedius would have been of interest; unfortunately at the time of the writing of this report the isolate was no longer available.
The recent emergence of MRSP in dogs is complicating the treatment of once familiar infections, and making therapy of complicated cases increasingly difficult (17). Methicillin resistance signals cross-resistance to all β-lactam antimicrobials: the penicillins (including amoxicillin and clavulanic acid), cephalosporins, and carbapenems. Additionally, these organisms are increasingly resistant to non-β-lactam antimicrobials and cases of infections with nearly pan-resistant organisms have been reported (18,19). Unlike methicillin-resistant S. aureus, MRSP infections are frequently community associated. In this case, the origin of the offending organism was not determined although the lack of recent hospital exposure prior to the onset of necrotizing fasciitis suggests community acquisition. The increasing incidence of multidrug resistant organisms in veterinary clinics and the community highlights the essential role that early antimicrobial susceptibility testing plays in guiding effective, evidence-based therapy.
Acknowledgments
The authors thank Drs. Maria Just, Puran Das, and Lois Herperger for providing photographs of the wound progression. The authors also thank Dr. Stephen Sanche, Infectious Diseases, Department of Medicine, University of Saskatchewan for performing the human cultures. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Naidoo SL, Campbell DL, Miller LM, et al. Necrotizing fasciitis: A review. J Am Anim Hosp Assoc. 2005;41:104–109. doi: 10.5326/0410104. [DOI] [PubMed] [Google Scholar]
- 2.Prescott JF, Miller CW, Mathews KA, et al. Update on canine streptococcal toxic shock syndrome and necrotizing fasciitis. Can Vet J. 1997;38:241–242. [PMC free article] [PubMed] [Google Scholar]
- 3.Jamal N, Teach SJ. Necrotizing fasciitis. Pediatr Emerg Care. 2011;27:1195–1199. doi: 10.1097/PEC.0b013e31823b583c. [DOI] [PubMed] [Google Scholar]
- 4.Betschel SD, Guru V, Miller CW, et al. Canine toxic-shock syndrome. An emerging disease? Adv Exp Med Biol. 1997;418:189–92. 189–192. doi: 10.1007/978-1-4899-1825-3_46. [DOI] [PubMed] [Google Scholar]
- 5.Prescott JF, DeWinter L. Canine streptococcal toxic shock syndrome and necrotising fasciitis. Vet Rec. 1997;140:263. [PubMed] [Google Scholar]
- 6.Jenkins CM, Winkler K, Rudloff E, et al. Necrotizing fasciitis in a doberman pinscher. J Vet Emerg Crit Care. 2001;11:299–305. [Google Scholar]
- 7.Worth AJ, Marshall N, Thompson KG. Necrotising fasciitis associated with Escherichia coli in a dog. N Z Vet J. 2005;53:257–260. doi: 10.1080/00480169.2005.36556. [DOI] [PubMed] [Google Scholar]
- 8.Kulendra E, Corr S. Necrotising fasciitis with sub-periosteal Streptococcus canis infection in two puppies. Vet Comp Orthop Traumatol. 2008;21:474–477. doi: 10.3415/vcot-07-05-0043. [DOI] [PubMed] [Google Scholar]
- 9.Weese JS, Poma R, James F, et al. Staphylococcus pseudintermedius necrotizing fasciitis in a dog. Can Vet J. 2009;50:655–656. [PMC free article] [PubMed] [Google Scholar]
- 10.Csiszer AB, Towle HA, Daly CM. Successful treatment of necrotizing fasciitis in the hind limb of a great dane. J Am Anim Hosp Assoc. 2010;46:433–438. doi: 10.5326/0460433. [DOI] [PubMed] [Google Scholar]
- 11.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. doi: 10.1080/02841860510007440. [DOI] [PubMed] [Google Scholar]
- 12.Tibbs MK. Wound healing following radiation therapy: A review. Radiother Oncol. 1997;42:99–106. doi: 10.1016/s0167-8140(96)01880-4. [DOI] [PubMed] [Google Scholar]
- 13.Maluf FC, William WN, Jr, Rigato O, et al. Necrotizing fasciitis as a late complication of multimodal treatment for locally advanced head and neck cancer: A case report. Head Neck. 2007;29:700–704. doi: 10.1002/hed.20558. [DOI] [PubMed] [Google Scholar]
- 14.Mortimore S, Thorp M. Cervical necrotizing fasciitis and radiotherapy: A report of two cases. J Laryngol Otol. 1998;112:298–300. doi: 10.1017/s0022215100158414. [DOI] [PubMed] [Google Scholar]
- 15.Miyagawa T, Kawai K, Onozawa M, et al. Unusual presentation of necrotizing fasciitis in a patient who had achieved long-term remission after irradiation for testicular cancer. Int J Urol. 2005;12:332–334. doi: 10.1111/j.1442-2042.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- 16.Weiss A, Nelson P, Movahed R, Clarkson E, Dym H. Necrotizing fasciitis: Review of the literature and case report. J Oral Maxillofac Surg. 2011;69:2786–94. doi: 10.1016/j.joms.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 17.van Duijkeren E, Catry B, Greko C, et al. Review on methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother. 2011;66:2705–2714. doi: 10.1093/jac/dkr367. [DOI] [PubMed] [Google Scholar]
- 18.Rubin JE, Gaunt MC. Urinary tract infection caused by methicillin- resistant Staphylococcus pseudintermedius in a dog. Can Vet J. 2011;52:162–164. [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin JE, Gard S, Aucoin D. The emergence of methicillin resistant Staphylococcus pseudintermedius in western Canada. 2nd American Society for Microbiology-European Society of Clinical Microbiology and Infectious Diseases Conference on Methicillin-resistant Staphylococci in Animals: Veterinary and Public Health Implications; 2011. p. 60. [Google Scholar]