Abstract
Purpose.
To investigate the association between myopia progression and time spent outdoors and in various visual activities.
Methods.
Subjects were 835 myopes (both principal meridians −0.75 diopters [D] or more myopia by cycloplegic autorefraction) in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study with both progression data and at least one measure of activity associated with a progression interval. Activity data were collected by parental survey. Average activity level (mean of the activity at the beginning and the end of a 1-year progression interval) was the primary predictor in a repeated-measures mixed model. The model controlled for age, sex, ethnicity, refractive error at the beginning of the progression interval, clinic site, and type of autorefractor used. Effects were scaled based on performing an additional 10 hours per week of an activity.
Results.
In the multivariate model, the number of hours of reading for pleasure per week was not significantly associated with annual myopia progression at an a priori level of P ≤ 0.01, nor were the other near activities, the near-work composite variable diopter-hours, or outdoor/sports activity. The magnitude of effects was clinically small. For example, the largest multivariate effect was that each additional 10 hours of reading for pleasure per week at the end of a progression interval was associated with an increase in average annual progression by −0.08 D.
Conclusions.
Despite protective associations previously reported for time outdoors reducing the risk of myopia onset, outdoor/sports activity was not associated with less myopia progression following onset. Near work also had little meaningful effect on the rate of myopia progression.
In this study, no association was found between outdoor/sports activity and myopia progression, in contrast to previous findings linking outdoor/sports activity with a decreased risk of the onset of juvenile myopia.
Introduction
Longitudinal studies have found an association between more time spent in outdoor/sports activity and a reduction in the risk of the onset of juvenile myopia.1–2 These results are consistent with several cross-sectional studies that have also reported that myopes spend fewer hours in outdoor/sport activity than other refractive error groups,3–7 though one study conducted in rural China has reported no association between myopia and time spent outdoors.8 In contrast, studies of the associations between myopia and near-work activities have produced less consistent results. Two longitudinal studies have not found an association between increased near work and increased risk of myopia onset, while one found a positive association between incident myopia and a child liking to read.1,9–10 Cross-sectional studies, on the other hand, have found positive associations more consistently for various measures of near work where children who are already myopic spend more time in near-work activities than nonmyopes,5,6,11,12 though the association is not always found to be statistically significant.13,14
Many studies have also investigated the relationship between outdoor and near activity and myopia progression after the onset of myopia. One study of myopia progression considered close work, sports, and time outdoors.15 The overall group analysis did not show an effect of time spent in sports and outdoor activities on myopia progression, but there was a small protective effect when the analyses considered boys and girls separately with 0.23 diopters (D) less myopia progression over 3 years in boys for every hour per day spent in outdoor activity. Increased myopia progression was also found for more time spent reading and performing other close work among girls. In university students, Jacobsen et al. found that increased physical activity was associated with less myopia progression, and hours of study were associated with more myopia progression.16 A similar association between more time spent reading the scientific literature and faster myopia progression was reported for Norwegian engineering students.17 A study that examined the relationship between various activities (total near work, total reading and writing, or hours spent outdoors) and myopia progression in Singaporean children aged 6 to 12 years used a nested case-control analysis within a randomized clinical trial, looking only at the spectacle treatment arm (n = 153).18 None of the activity variables assessed was significantly associated with myopia progression. Tan et al.19 followed 168 Singaporean children (aged 7, 9, and 12 years) over 1 year. Parents completed entries of their children's activities for every half-hour period in a 24-hour near-work diary at study entry. There were no statistically significant correlations between progression and near work (reading and writing hours, computer hours, reading, writing, and computer hours, and the number of hours for each of these categories within 3 hours of bedtime) in the myopic children. Saw et al. found no statistically significant association in 543 myopic Singaporean children between axial elongation and reading, whether expressed as books read per week or hours per day of near work.20
The literature, therefore, shows limited support for more time in near work increasing the rate of progression of myopia. The small number of studies conducted in children addressing the relationship between time outdoors and myopia progression suggest either no effect or a small reduction in the rate of progression in boys only.15,18 The purpose of the current study was to explore the relationship between time spent in various activities and the rate of myopia progression in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study cohort. Of particular interest was whether the protective association seen between outdoor/sports activity and reduced risk of myopia onset also applied to myopia progression.
Methods
Subjects were myopic children (−0.75 D or more myopic in each principal meridian on cycloplegic autorefraction) who participated in the CLEERE Study between 1989 and 2009. The CLEERE Study is a multicenter, observational cohort study evaluating ocular component development and risk factors for juvenile-onset myopia in children of different ethnicities. The original Orinda Longitudinal Study of Myopia (Orinda, CA) became the CLEERE Study in 1997 with the addition of sites enrolling African-American (Eutaw, AL); Asian (Irvine, CA); and Hispanic (Houston, TX) children. In 2000, a site to enroll Native-American children (Tucson, AZ) was added. Each affiliated university's institutional review board approved the protocol and informed consent documents in accordance with the tenets of the Declaration of Helsinki. In addition to written parental consent, children provided assent. Only myopic children were included in this analysis of the CLEERE data; these children had at least two consecutive annual myopic study visits (so that an annual progression rate could be calculated) and at least one measure of near work or outdoor/sports activity with a temporally associated myopia progression rate.
Activity data were gathered annually at the same time each year using a questionnaire that asked the parent: “During the school year, how many hours per week (outside of regular school hours) would you estimate this child: 1) studies or reads for school assignments; 2) reads for fun (pleasure); 3) watches TV; 4) uses a computer/plays video games; and 5) engages in outdoor/sports activities?”
Reported hours per week across all five activities that exceeded 82 hours were deleted (n = 19), because it was assumed that 82 hours per week outside of school were not reasonably available to a child. This maximum value was calculated as follows: 168 (24 hours × 7 days) possible hours per week, minus an assumed 30 hours per week spent in school (6 hours × 5 days), and 56 hours were spent sleeping (7 days × 8 hours), leaving 82 hours for other activities. The range of the excluded responses was 84 hours to 347 hours per week. Over half of the excluded responses were between 90 and 100 hours per week. Diopter-hours were calculated to include the estimated accommodative effort as a result of performing near work at varying distances. This was calculated as a comprehensive near-work exposure, defined as: 3 × hours of reading + 3 × hours of studying + 2 × video/computer hours + hours watching television.6 Parents also provided information on their own myopia on the baseline medical history form consisting of both parents' year of birth, whether they wore spectacles or contact lenses, and, if so, the age when they were first prescribed spectacles and how they primarily used the spectacles at the time of the survey (distance, near, or both). A parent was considered myopic if he or she used the spectacles primarily for distance or for both distance and near with the spectacles first prescribed before age 17 years.21 Cycloplegic autorefraction was conducted by certified study personnel with an autorefractor (Canon R-1; Canon, Lake Success, NY; no longer manufactured) from 1989 to 2000 and with a different autorefractor model (Grand Seiko WR 5100-K; Grand Seiko Co., Hiroshima, Japan) from 2001 to 2009. For cycloplegic autorefraction, subjects fixated on a reduced Snellen target through a +4.00 D Badal lens in primary gaze. Immediately following measurement in primary gaze, the track holding the Snellen target was rotated 30° and placed before a front-surface mirror on the patient's right. Five autorefraction measurements were then taken in peripheral gaze. Relative peripheral refractive error was calculated as the spherical equivalent of the average refraction in primary gaze subtracted from the spherical equivalent of the average refractive error in 30° temporal gaze. For subjects with grade 1 or 2 iris color (in general, a blue or a gray iris, along with a green iris with a lesser amount of brown pigment),22 testing was performed 30 minutes after one drop of proparacaine 0.5% and two drops of tropicamide 1.0%. When subjects had dark iris color greater than grade 2, testing was performed 30 minutes after one drop of proparacaine 0.5% and one drop each of tropicamide 1.0% and cyclopentolate 1.0%.23 Ten autorefractor measurements were made according to a standard protocol.24
These analyses measured refractive error progression by calculating the change in spherical equivalent over consecutive years. Myopia progression was considered to be any increase in the myopic spherical equivalent. For each progression interval (i.e., a year), two sources of activity data were potentially available. Baseline data were the “before” values of the very first progression interval. Data available at the beginning of the progression interval were identified as “before” data. The activity data from the end of the progression interval were identified as “after” data. This means that a given activity measurement could be defined as both “before” and “after” data for different intervals. For example, if we were to examine progression between year 2 and year 3, then activity data for year 2 would provide the “before” measurement and data from year 3 would be the “after” measurement. In investigating the progression from year 3 to year 4, year-3 data would now be the “before” activity data.
The choice of the most appropriate time point at which to select the activity data is somewhat arbitrary. In terms of potential causality, activity before the progression period would be the most logical choice, while activity at the end of the progression period may better represent more recent exposure or the effect rather than the cause of progression. We chose to use the average of the before and after data during the interval of progression as the primary variable in analyses of progression. If both observations were not available, the subject was not included in the average analysis but still appeared in the before or after analyses, as appropriate. A repeated-measures mixed model approach was the primary method used to analyze the progression data. The basic model included the following covariates: age, refractive error at the beginning of the progression interval, sex, site, an indicator for an autorefractor model change in the year 2001, and interaction terms for age × sex, age × ethnicity, and site × instrument. The predictor variables of average activity data were then added to this base model. Additional models included number of myopic parents and relative peripheral refraction evaluated along with interaction terms with the activity variables. Given that multiple comparisons were made, a P value of ≤ 0.01 was used as the criterion for statistical significance. To provide a reasonable estimate of the magnitude of an effect, the results have been rescaled to represent the increment in myopia progression with 10 hours of additional activity per week. All analyses were completed using statistical analysis software (SAS 9.2; SAS Institute, Cary, NC).
Results
There were 835 subjects with both myopia progression data and at least one measure of activity. Their ages at baseline ranged from 6 to 14 years old. Almost 60% of the sample was female (57.2%). Hispanics accounted for one-third of the subjects (31.4%). Asians and Whites were each roughly 20% of the sample, and Native Americans and African Americans were slightly more than 10% of the sample (10.4% and 13.7%, respectively). The average age (± SD) of myopia onset was 10.4 years (± 1.8 years), and the mean spherical equivalent refraction (± SD) at baseline was −1.82 D (± 1.06 D). The average annual rate of progression for the sample as a whole was −0.39 D (± 0.32 D). Table 1 shows the distribution of myopia progression between consecutive visits grouped into categories rounded to the nearest 0.25 D. Only approximately 2% of the data represent suspect levels of progression or regression.
Table 1. .
Distribution of Myopia Progression between Consecutive Visits Based on Groups Rounded to the Nearest 0.25 D
|
Refractive Error Progression Groups (D) |
n |
% |
| −1.75 to −2.00 | 20 | 0.9 |
| −1.25 to −1.50 | 116 | 5.2 |
| −0.75 to −1.00 | 488 | 21.9 |
| −0.25 to −0.50 | 1054 | 47.2 |
| 0 | 384 | 17.2 |
| 0.25 to 0.50 | 144 | 6.5 |
| 0.75 to 1.00 | 22 | 1.0 |
| 1.25 to 1.50 | 3 | 0.1 |
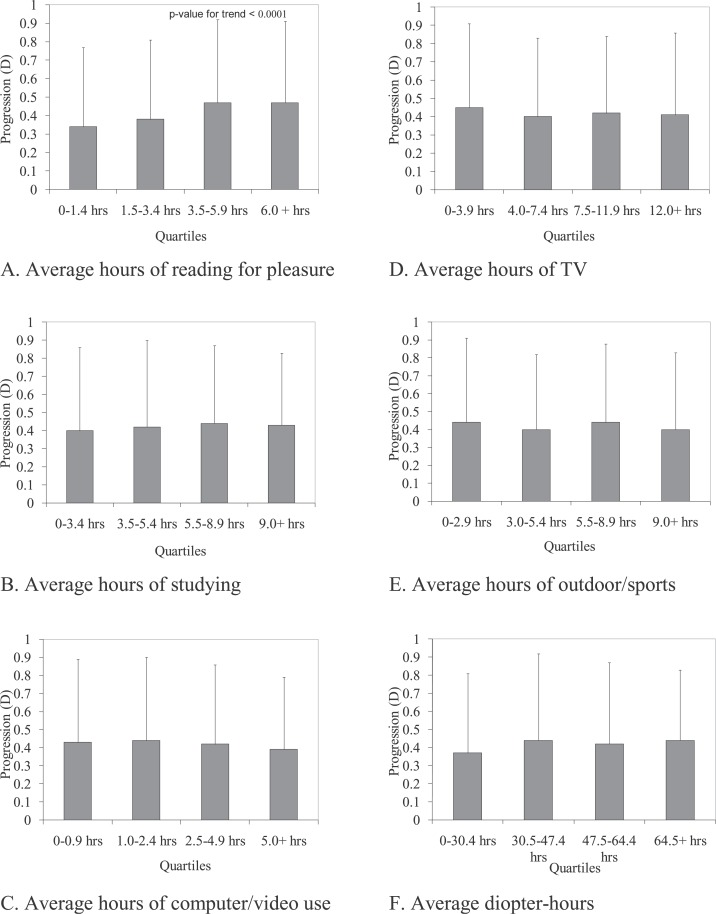
Table 2 presents the univariate regression coefficients relating activities and myopia progression. The only significant univariate coefficient was reading for pleasure measured as −0.13 D more progression per year for 10 hours of pleasure reading per week. Other forms of near work, the diopter-hours composite variable for near work, and outdoor/sports activity did not have significant univariate associations with progression. Quartile cut points were determined for the observed values of each activity and the average refractive error progression within each quartile was calculated. The means for the quartiles of each activity (±SD) are shown in the Figure (A–F). Consistent with the regression coefficient results in Table 2, hours of reading for pleasure (Fig. A) was the only activity that had a significant univariate association with myopia progression, showing greater progression with higher quartiles of reading (P < 0.0001); however, the magnitude of the difference in progression between the highest and lowest quartiles was small (0.13 D). Overall, the mean progression rate per year across the quartiles for the remaining activities and diopter-hours were quite similar, averaging between −0.40 D and −0.44 D.
Table 2. .
Activity Parameter Estimates from Univariate and Multivariate Analyses of Annual Progression
|
Activity Variable |
Univariate Estimate (D, 99% CI) |
Multivariate Estimate (99% CI) |
||
|
Average Over Progression (D) |
Before Progression (D) |
After Progression (D) |
||
| Reading for pleasure | −0.13* (−0.21, −0.05) | −0.07 (−0.14, 0.003) | −0.02 (−0.07, 0.04) | −0.08* (−0.14, −0.02) |
| Studying | −0.02 (−0.08, 0.05) | 0.004 (−0.06, 0.07) | 0.01 (−0.04, 0.06) | −0.01 (−0.06, 0.03) |
| Computer/video games | 0.06 (−0.02, 0.14) | −0.05 (−0.13, 0.04) | −0.02 (−0.08, 0.03) | −0.02 (−0.08, 0.03) |
| TV | 0.02 (−0.03, 0.06) | −0.006 (−0.05, 0.04) | −0.008 (−0.04, 0.03) | 0.01 (−0.02, 0.05) |
| Outdoor/sports activities | 0.01 (−0.05, 0.07) | 0.03 (−0.03, 0.08) | 0.02 (−0.02, 0.06) | 0.01 (−0.03, 0.06) |
| Diopter-hours | −0.008 (−0.02, 0.004) | −0.007 (−0.02, 0.004) | −0.001 (−0.01, 0.008) | −0.007 (−0.02, 0.002) |
The estimates are effects on annual progression associated with an additional 10 hours of weekly activity. The multivariate model controlled for age, spherical equivalent at the beginning of the progression interval, sex, race, site, sex × age, race × age, autorefractor transition indicator, and autorefractor transition indicator × site.
P ≤ 0.001
Figure .
Average myopia progression by quartiles for average activity hours in a progression interval (error bar represents 1 standard deviation).
Table 2 also presents the multivariate parameter estimates for the effect of an average of 10 additional hours of activity per week on myopia progression, controlling for all covariates. The estimates, including reading for pleasure, were all clinically small and not statistically significant at the 0.01 level in these multivariate models. For completeness, Table 2 also has the multivariate estimates (with 99% confidence intervals [CI]) for activities before and after the myopia progression interval. For each activity, the parameter estimates varied little with the choice of time point. The only noticeable difference was the change in the P value for reading for pleasure hours at the end of the progression interval, which was statistically significant though negligible in magnitude.
Interactions between each of the activity variables and age, ethnicity, and baseline refractive error were not statistically significant, nor were interactions with relative peripheral refraction and the number of myopic parents. There were, however, statistically significant interactions between sex and hours of studying (P = 0.003) and diopter-hours (P = 0.001). Ten hours of additional studying per week added −0.09 D progression per year to the myopia progression of boys, while girls had 0.06 D per year less myopia progression. Ten additional diopter-hours per week added −0.02 D per year to the progression of boys, while in girls 10 diopter-hours was associated with essentially no decrease in progression (0.004 D less progression per year).
Based on our sample size, we have over 80% power to find an association with children's activities for a regression slope of 0.01 D (i.e., a 0.1 D increase or decrease in annual progression in refractive error per 10 hours of activity per week) for each one of the activity variables examined. The nonsignificant coefficients in Table 2 are well estimated, typically around ± 0.05 D progression per 10 hours per week of an activity. Axial length changes were also analyzed using models similar to those used for refractive error progression. There were no statistically significant associations found for axial elongation with any of the activities, regardless of the time point chosen (data not shown).
Discussion
In this ethnically diverse cohort of school-aged children, none of the activities analyzed was consistently associated with any clinically meaningful myopia progression. The univariate significance for reading for pleasure did not remain after multivariate adjustment, nor was reading for pleasure consistently related to myopia progression at all time points (average, before, and after). Near work in the form of studying and as the composite variable diopter-hours had opposite effects depending on sex, with more hours increasing progression in boys but either decreasing progression or having little effect in girls. Unlike the association of outdoor/sports activities with the onset of myopia found in our earlier analysis1 and subsequently by Guggenheim et al.,2 the current analyses indicated no significant association between outdoor/sports activities and the rate of myopia progression. Axial length changes were not related to any of the activity variables. This negative result for axial length, even for reading for pleasure, underscores the lack of meaningful association between these activities and the rate of myopia progression. These differences indicate that factors that affect the risk of myopia onset may differ from those that affect the rate of myopia progression.
Only one study in children has found associations for either outdoor/sports activities or near work with myopia progression, though each was found only in one sex. Pärssinen and Lyyra15 reported a positive effect of outdoor/sports activities reducing myopia progression in boys. They also reported a detrimental effect of reading and near work increasing myopia progression in girls. They found that an average of −0.18 D progression over three years (roughly −0.06 D per year) was associated with 1 hour of daily reading for girls, but that there was no significant association in the boys. In the current study, there were statistically significant differences for hours of studying and diopter-hours depending on sex; however, both of these sex interactions with near work indicated only slightly more progression in boys compared with girls. These represent results in the opposite direction to those seen in Pärssinen and Lyyra.15 In addition, there was no interaction between time outdoors and sex.
The protective association found for outdoor/sports activities before the onset of myopia,1 in contrast to the lack of association after onset reported here, suggests that time outdoors may not exert a general inhibitory effect on ocular growth. It is unknown why this protective effect would not apply to both risk of onset and progression. One would have to propose a complex system for a different mechanism to control eye growth before compared with after the eye becomes myopic. Potential support for different processes driving the onset of myopia and the progression of myopia may be found in microarray analyses done in chicks. Studies have found that there was little evidence of common changes in retinal gene expression when comparing the expression seen at times close to the initiation of the study (perhaps analogous to myopia onset) and those further into the study (surrogates for myopic progression).25,26 This variability in gene expression merely raises the possibility of involvement of different processes for myopia onset and progression; further research would be required in order to determine their applicability to human myopia. The possible existence of multiple controllers for eye growth would have implications for therapies. What appears promising with respect to reducing the risk of myopia onset may not be as effective at slowing progression and vice versa.
Negative results always raise issues of whether the study was powered to find the potentially small effect sizes seen in previous intervention studies. Studies have found that alterations to near viewing conditions can affect the rate of progression of myopia by small amounts. For example, the Correction of Myopia Evaluation Trial (COMET) found a three-year decrease in progression of 0.20 D in the progressive addition lenses (PAL) group compared with the single vision lenses group, an average yearly difference in progression of 0.067 D though the majority of the effect was seen in the first year.27 A similar study completed in Japan found a difference in the 18-month progression rate between their PAL treatment group and single vision lens groups of 0.17 D (an average annual difference in progression of 0.11 D).28 Whether these effects are related to hyperopic defocus reduction of accommodative lag or to physical, mechanical effects of accommodation remains to be seen.29 Whatever the source of these changes in progression, the current study was powered to find effects of this magnitude, but it did not. The current study suggests that reductions in the time children spend in near work are unlikely to have any meaningful effect in slowing myopia progression.
Another potential limitation is the range of outdoor activity present in this myopic sample. The range is comparable with that of the nonmyopes from our paper on myopia onset1; however, the median, the 75th percentile, and the 95th percentile are 5 hours per week greater in the nonmyopes. The results for our myopes were similar to a report from rural China of 6.1 hours per week,8 but several other studies have reported more activity in samples of children with at least 12 hours of outdoor activity over all refractive groups.4,5,13
We explored whether the levels of outdoor activity reported in our sample were too low to expect an effect on progression. Our previous work indicated a decrease in the probability of onset even comparing the lowest quartile of activity (0–6 hours) with the next quartile of 6 to 9 hours of activity. Therefore, even small amounts of time outdoors seem to have measurable effects on the risk of onset. We did an additional evaluation of the association between performing at least 9 hours of outdoor activity (the lower bound of the upper quartile of activity in this paper) and onset of myopia and verified that this amount of activity was significantly associated with a decreased risk of onset in a model similar to that used by Jones et al. controlling for parental myopia (OR = 2.67, 95% CI = 1.75, 4.06).1
It also could be that there is a threshold effect, requiring a certain amount of time spent outdoors to affect eye growth that our myopes have not attained. Using the upper quartile of outdoor activity as a threshold (at least 9 hours) showed no evidence of an association with myopia progression. Likewise, the dose-dependent effect of time outdoors on the risk of onset argues against a threshold hypothesis. The possibility of a threshold requires further investigation beginning with myopia onset.
There are several potential limitations to our study. The survey instrument used to collect the activity information has several potential sources of bias.30 One relates to parents as the source of information on children's activities, as others have done.9,31 Given the age of the majority of children, this is likely to be a more reliable source of data than the children. Another issue is the potential recall bias of a questionnaire. Myopia research has employed other, more time-consuming methods,32,33 but comparisons of these more intensive methods to other questionnaires have shown reasonable consistency. Cross-sectional results from other studies have found estimates similar to ours using more complex questionnaires.4,13 The question we used to elicit information included sports as well as time spent outdoors. The phrase “outdoor activities” may not have encouraged parents to report all types of activity done outside. For example, combining outdoor activities with sports activities may have led parents to only report those outdoor activities that were active, as opposed to sedentary, such as sun-bathing. Guggenheim et al.2 recently reported that time spent outdoors rather than time spent in physical activity showed the stronger effect on decreasing myopia incidence, though physical activity had a smaller, independent protective effect as well. Rose et al.13 also found that it was a lack of outdoor activity rather than sports in general that was associated with being myopic in their cross-sectional analysis. As such, our combined question may affect the magnitude of effect that we find; however, our brief questionnaire has been successful in detecting differences in both cross-sectional and longitudinal analyses of myopia onset.1,6,30,34
While it would have been useful to be able to assess seasonal progression for differences by time of year, we were unable to do so because the questionnaire was administered once annually, at roughly the same time every year. Parents were specifically asked to estimate the amount of time their children spent in activities during the school year. Evidence indicating that myopia progression is slower in the summer than in winter has been reported (Gwiazda JE, et al. IOVS 2012;53:ARVO E-Abstract 2309).35–37 Myopes have been reported to spend more time outdoors in summer at similar levels to emmetropes.38 These findings suggest that assessment of visual activities across seasons may be important.
Proper assessment of visual activity is further complicated by temporal integration of visual signals within shorter time frames than seasons. Small amounts of time spent in unrestricted viewing have strong inhibitory effects when used to interrupt myopigenic stimuli, such as form deprivation or minus lens wear, in species such as tree shrews39–40 and monkeys.41 Additionally, altering the available fixation distance to greater than 1 m and removing the lenses that were creating the myopigenic stimuli has a similar inhibitory effect in tree shrews.39 Likewise, the introduction of levels of light higher than indoor ambient levels (either higher ambient light or sunlight) has reduced the amount of myopia induced in deprivation myopia studies.42–43 Higher levels of light slowed compensation to negative lenses in chicks,44 but did not prevent the compensation to negative lenses in either chicks44 or monkeys (Smith EL, et al. IOVS 2012;53:ARVO E-Abstract 4659). Some temporal integration effect may also be present in humans, and it is unknown whether different relationships would have been found if the activities and myopic progression were assessed with finer temporal resolution, either season by season or within the day or the week. Nevertheless, our results indicate that whatever the benefit that may have accrued in summer, more time outdoors during the school year did not affect the rate of myopic progression during the school year.
Conclusions
The performance of outdoor/sports activity was not associated with the annual progression of juvenile-onset myopia. The number of hours spent reading for pleasure was inconsistently significant with minimal clinical relevance and other forms of near work were not associated with myopia progression in any consistent or clinically relevant manner. Given the results of this study and suggestive evidence from animal studies, research delineating whether factors related to myopia onset are distinct from myopia progression may be warranted. Determining whether a threshold exists for an outdoor effect may also be required to progress towards developing a potential treatment using outdoor activity. Ultimately, a randomized clinical trial of outdoor activity will be needed to verify that sufficient duration of activity is being done to elicit an effect.
Appendix
The Cleere Study Group (as of January 2012)
Clinical Centers. Franklin Primary Health Center, Inc. Sandral Hullett, MD, MPH (Principal Investigator, 1997–2007); Robert N. Kleinstein, OD, MPH, PhD (Co-Investigator, 1997–2007); Janene Sims, OD (Optometrist, 1997–2001 and 2004–2007); Raphael Weeks, OD (Optometrist, 1999–2007); Sandra Williams (Study Coordinator, 1999–2007); LeeAndra Calvin (Study Coordinator, 1997–1999), Melvin D. Shipp, OD, MPH, DrPH (Co-Investigator, 1997–2004). Drs. Kleinstein and Sims are affiliated with the University of Alabama at Birmingham School of Optometry.
University of California, Berkeley School of Optometry, Berkeley, CA. Nina E. Friedman, OD, MS (Principal Investigator, 1999–2001); Pamela Qualley, MA (Study Coordinator, 1997–2001); Donald O. Mutti, OD, PhD (Principal Investigator, 1996–1999); Karla Zadnik, OD, PhD (Optometrist, 1996–2001).
University of Houston College of Optometry. Ruth E. Manny, OD, PhD (Principal Investigator, 1997–2007); Suzanne M. Wickum, OD (Optometrist, 1999–2007); Ailene Kim, OD (Optometrist, 2003–2007); Bronwen Mathis, OD (Optometrist, 2002–2007); Mamie Batres (Study Coordinator, 2004–2007). Sally Henry (Study Coordinator, 1997–1998); Janice M. Wensveen, OD, PhD (Optometrist, 1997–2001); Connie J. Crossnoe, OD (Optometrist, 1997–2003); Stephanie L. Tom, OD (Optometrist, 1999–2002); Jennifer A. McLeod (Study Coordinator, 1998–2004); Julio C. Quiralte (Study Coordinator, 1998–2005); Gaby Solis (Study Coordinator, 2005–2007).
Southern California College of Optometry, Fullerton, CA. Susan A. Cotter, OD, MS (Principal Investigator, 2004–2007, Optometrist, 1997–2004); Julie A. Yu, OD (Principal Investigator, 1997–2004; Optometrist 2005–2007); Raymond J. Chu, OD (Optometrist, 2001–2007); Carmen N. Barnhardt, OD, MS (Optometrist 2004–2007); Jessica Chang, OD (Optometrist, 2005–2007); Kristine Huang, OD (Optometrist, 2005–2007); Rebecca Bridgeford (Study Coordinator, 2005–2006); Connie Chu, OD (Optometrist, 2004–2005), Soonsi Kwon, OD (Optometrist, 1998–2004); Gen Lee (Study Coordinator, 1999–2003); John Lee, OD (Optometrist, 2000–2003); Robert J. Lee, OD (Optometrist, 1997–2001); Raymond Maeda, OD (Optometrist, 1999–2003); Rachael Emerson (Study Coordinator, 1997–1999); Tracy Leonhardt (Study Coordinator, 2003–2004).
University of Arizona, Department of Ophthalmology and Vision Science, Tucson, AZ. J. Daniel Twelker, OD, PhD (Principal Investigator, 2000–present); Dawn Messer, OD, MDH (Optometrist, 2000–present); Denise Flores (Study Coordinator, 2000–2007); Rita Bhakta, OD (Optometrist, 2000–2004); Katie Garvey, OD (Optometrist, 2006–present); Mabel Crescioni, DrPH (2009–present).
Resource Centers
Chairman's Office, The Ohio State University College of Optometry, Columbus, OH. Karla Zadnik, OD, PhD (Chairman, 1997-present); Jodi M. Malone, RN (Study Coordinator, 1997–present).
Videophakometry Reading Center, The Ohio State University College of Optometry, Columbus, OH. Donald O. Mutti, OD, PhD (Director, 1997–present); Vidya Subramanian, MS (Reader, 2006–2009); Huan Sheng, MD MS (Reader, 2000–2006); Holly Omlor (Reader, 2003–2006); Meliha Rahmani (Reader, 2004–2006); Jaclyn Brickman (Reader, 2002–2003); Amy Wang (Reader, 2002–2003); Philip Arner (Reader, 2002–2004); Samuel Taylor (Reader, 2002–2003); Myhanh T. Nguyen (Reader, 1998–2001); Terry W. Walker (Reader, 1997–2001).
Optometry Coordinating Center, The Ohio State University College of Optometry, Columbus, OH. Lisa A. Jones-Jordan, PhD (Director, 1997–present); Linda Barrett (Data Entry Operator, 1997–2008); John Hayes, PhD (Biostatistician, 2001–2007); G. Lynn Mitchell, MAS Biostatistician, 1998–present); Melvin L. Moeschberger, PhD (Consultant, 1997–present); Loraine Sinnott, PhD (Biostatistician, 2005–present); Pamela Wessel (Program Coordinator, 2000–present); Julie N. Swartzendruber, MA (Program Coordinator, 1998–2000); Austen Tanner (Data Entry 2008–2010).
Project Office, National Eye Institute, Rockville, MD. Donald F. Everett, MA.
Committees
Executive Committee. Karla Zadnik, OD, PhD (Chairman); Lisa A. Jones-Jordan, PhD; Robert N. Kleinstein, OD, MPH, PhD; Ruth E. Manny, OD, PhD; Donald O. Mutti, OD, PhD; J. Daniel Twelker, OD, PhD; Susan A. Cotter, OD, MS.
Footnotes
Supported by National Eye Institute/National Institutes of Health Grants U10-EY08893 and R24-EY014792, the Ohio Lions Eye Research Foundation, and the E.F. Wildermuth Foundation.
Disclosure: L.A. Jones-Jordan, None; L.T. Sinnott, None; S.A. Cotter, None; R.N. Kleinstein, None; R.E. Manny, None; D.O. Mutti, None; J.D. Twelker, None; K. Zadnik, None
6 See the Appendix for the members of the CLEERE Study Group.
References
- 1.Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012;53:2856–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng L, Gwiazda J, Thorn F. Children's refractions and visual activities in the school year and summer. Optom Vis Sci. 2010;87:406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93:997–1000 [DOI] [PubMed] [Google Scholar]
- 5.Ip JM, Saw SM, Rose KA, et al. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49:2903–2910 [DOI] [PubMed] [Google Scholar]
- 6.Mutti DO, Mitchell GL, Moeschberger ML, et al. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–3640 [PubMed] [Google Scholar]
- 7.Rose KA, Morgan IG, Smith W, et al. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008;126:527–530 [DOI] [PubMed] [Google Scholar]
- 8.Lu B, Congdon N, Liu X, et al. Associations between near work, outdoor activity, and myopia among adolescent students in rural China: the Xichang Pediatric Refractive Error Study report no. 2. Arch Ophthalmol. 2009;127:769–775 [DOI] [PubMed] [Google Scholar]
- 9.Saw SM, Shankar A, Tan SB, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:1839–1844 [DOI] [PubMed] [Google Scholar]
- 10.Williams C, Miller LL, Gazzard G, Saw SM. A comparison of measures of reading and intelligence as risk factors for the development of myopia in a UK cohort of children. Br J Ophthalmol. 2008;92:1117–1121 [DOI] [PubMed] [Google Scholar]
- 11.Saw SM, Hong CY, Chia KS, et al. Nearwork and myopia in young children. Lancet. 2001;357:390. [DOI] [PubMed] [Google Scholar]
- 12.Zylbermann R, Landau D, Berson D. The influence of study habits on myopia in Jewish teenagers. J Pediatr Ophthalmol Strabismus. 1993;30:319–322 [DOI] [PubMed] [Google Scholar]
- 13.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285 [DOI] [PubMed] [Google Scholar]
- 14.Saw SM, Tan SB, Fung D, et al. IQ and the association with myopia in children. Invest Ophthalmol Vis Sci. 2004;45:2943–2948 [DOI] [PubMed] [Google Scholar]
- 15.Parssinen O, Lyyra AL. Myopia and myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci. 1993;34:2794–2802 [PubMed] [Google Scholar]
- 16.Jacobsen N, Jensen H, Goldschmidt E. Does the level of physical activity in university students influence development and progression of myopia?--a 2-year prospective cohort study. Invest Ophthalmol Vis Sci. 2008;49:1322–1327 [DOI] [PubMed] [Google Scholar]
- 17.Kinge B, Midelfart A, Jacobsen G, Rystad J. The influence of near-work on development of myopia among university students. A three-year longitudinal study among engineering students in Norway. Acta Ophthalmol Scand. 2000;78:26–29 [DOI] [PubMed] [Google Scholar]
- 18.Saw SM, Nieto FJ, Katz J, et al. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci. 2000;77:549–554 [DOI] [PubMed] [Google Scholar]
- 19.Tan NW, Saw SM, Lam DS, et al. Temporal variations in myopia progression in Singaporean children within an academic year. Optom Vis Sci. 2000;77:465–472 [DOI] [PubMed] [Google Scholar]
- 20.Saw SM, Chua WH, Gazzard G, et al. Eye growth changes in myopic children in Singapore. Br J Ophthalmol. 2005;89:1489–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walline JJ, Zadnik K, Mutti DO. Validity of surveys reporting myopia, astigmatism, and presbyopia. Optom Vis Sci. 1996;73:376–381 [DOI] [PubMed] [Google Scholar]
- 22.Seddon JM, Sahagian CR, Glynn RJ, et al. Evaluation of an iris color classification system. The Eye Disorders Case-Control Study Group. Invest Ophthalmol Vis Sci. 1990;31:1592–1598 [PubMed] [Google Scholar]
- 23.Kleinstein RN, Mutti DO, Manny RE, et al. Cycloplegia in African-American children. Optom Vis Sci. 1999;76:102–107 [DOI] [PubMed] [Google Scholar]
- 24.Zadnik K, Mutti DO, Friedman NE, Adams AJ. Initial cross-sectional results from the Orinda Longitudinal Study of Myopia. Optom Vis Sci. 1993;70:750–758 [DOI] [PubMed] [Google Scholar]
- 25.Stone RA, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vision Res. 2010;50:2322–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone RA, McGlinn AM, Baldwin DA, et al. Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropia. Invest Ophthalmol Vis Sci. 2011;52:5765–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500 [DOI] [PubMed] [Google Scholar]
- 28.Hasebe S, Ohtsuki H, Nonaka T, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49:2781–2789 [DOI] [PubMed] [Google Scholar]
- 29.Berntsen DA, Mutti DO, Zadnik K. Study of Theories about Myopia Progression (STAMP) design and baseline data. Optom Vis Sci. 2010;87:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones-Jordan LA, Mitchell GL, Cotter SA, et al. Visual activity prior to and following the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 2011;52:1841–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46:51–57 [DOI] [PubMed] [Google Scholar]
- 32.Rah MJ, Walline JJ. Lynn Mitchell G, Zadnik K. Comparison of the experience sampling method and questionnaires to assess visual activities in pre-teen and adolescent children. Ophthalmic Physiol Opt. 2006;26:483–489 [DOI] [PubMed] [Google Scholar]
- 33.Saw SM, Nieto FJ, Katz J, Chew SJ. Estimating the magnitude of close-up work in school-age children: a comparison of questionnaire and diary instruments. Ophthalmic Epidemiol. 1999;6:291–301 [DOI] [PubMed] [Google Scholar]
- 34.Jones-Jordan LA, Mitchell GL, Cotter SA, et al. Visual activity prior to and following the onset of juvenile myopia [published online ahead of print October 5, 2010]. Invest Ophthalmol Vis Sci . doi:10.1167/iovs.10-5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulk GW, Cyert LA, Parker DA. Seasonal variation in myopia progression and ocular elongation. Optom Vis Sci. 2002;79:46–51 [DOI] [PubMed] [Google Scholar]
- 36.Goss DA, Rainey BB. Relation of childhood myopia progression rates to time of year. J Am Optom Assoc. 1998;69:262–266 [PubMed] [Google Scholar]
- 37.Donovan L, Sankaridurg P, Ho A, et al. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci. 2012;89:1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng L, Gwiazda J, Thorn F. Children's refractions and visual activities in the school year and summer. Optom Vis Sci. 2010;87:406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norton TT, Siegwart JT Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaikh AW, Siegwart JT Jr, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–315 [DOI] [PubMed] [Google Scholar]
- 41.Smith EL III, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–299 [PubMed] [Google Scholar]
- 42.Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–5354 [DOI] [PubMed] [Google Scholar]
- 43.Smith EL III, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253 [DOI] [PubMed] [Google Scholar]