Abstract
Juvenile neuronal ceroid lipofuscinosis (Batten disease) is a progressive and fatal autosomal-recessive inherited lysosomal storage disorder of childhood. Core symptoms include vision loss, seizures, and mental and motor decline. This article presents data from 2 studies of neuropsychological function in juvenile neuronal ceroid lipofuscinosis. In the first cross-sectional pilot study, 15 children with genetic or clinicopathologic confirmation of juvenile neuronal ceroid lipofuscinosis completed a brief test of attention (mean age = 14.3 ± 2.9 years, range = 8.75-18.74 years; 7 males, 8 females). Average attention performances were significantly below age-expected normative data. A second longitudinal study was then initiated to study neuropsychological function in greater depth, including change in function over time. The authors have enrolled 18 children to date (mean age = 12.88 ± 3.59 years, range = 6.26-18.65; 11 males, 7 females). Of these, 5 children have completed a second (annual) re-evaluation. Results thus far indicate significant impairment in domains of auditory attention, memory, estimated verbal intellectual function, and verbal fluency. Neuropsychological impairment was significantly correlated with disease duration and with motor function as assessed by a disease-specific clinical neurologic rating scale. There was no significant difference between males and females in neuropsychological test performance. Neuropsychological function was worse among children with a positive seizure history. Juvenile neuronal ceroid lipofuscinosis–affected children exhibited significant and pervasive impairments on tests of auditory attention, verbal memory and repetition, verbal fluency, and an estimate of verbal intellectual ability. Preliminary follow-up data from an annual reassessment showed progressive declines in cognitive function, in particular on a task of working memory. Neuropsychological deficits are pervasive and progressive. Future research will focus on clarifying the relationship among disease duration, motor function, and neuropsychological performances, including the relative sensitivity of neuropsychological testing at different stages of motor impairment or disease duration.
Keywords: Batten disease, neuronal ceroid lipofuscinoses, neuropsychological assessment
The neuronal ceroid lipofuscinoses are fatal, autosomal recessive, lysosomal storage diseases. Although rare, the major pediatric variants of the neuronal ceroid lipofuscinoses (infantile, late infantile, and juvenile) are collectively the most common neurodegenerative disorders of childhood, affecting approximately 8 per 100 000 worldwide.1 Juvenile-onset neuronal ceroid lipofuscinosis, also known as Batten disease, is the most prevalent childhood variant and is characterized by vision loss, neurobehavioral impairment and dementia, seizures, and motor decline.2,3 The underlying gene defect in juvenile neuronal ceroid lipofuscinosis is an autosomal recessive mutation in the ceroid-lipofuscinosis-3 gene located at p12.1 of chromosome 16. Ceroid-lipofuscinosis-3 encodes a protein of as yet unknown function.4 The most frequent mutation causing juvenile neuronal ceroid lipofuscinosis is a 1-kilo-base deletion in the ceroid-lipofuscinosis-3 gene, accounting for approximately 80% to 85% of known mutations.4,5
Neurocognitive deficits are prominent in juvenile neuronal ceroid lipofuscinosis but have not been well quantified. In the past 3 decades, there have been only a few formal studies of cognitive function in juvenile neuronal ceroid lipofuscinosis and none in a North American population. In European studies, children affected by juvenile neuronal ceroid lipofuscinosis exhibit significant impairments in overall verbal intellectual abilities and in selected neuropsychological domains.6-8 Collectively, these investigations have found significant impairments in verbal intelligence, attention and working memory, expressive language, motor speed and dexterity, and short-term memory. By contrast, receptive speech and comprehension abilities were relatively better preserved although still weak in relation to age-based normative data. In these studies, the duration and severity of disease among patients was variable, and the degree of motor dysfunction, seizure burden, and functional capacity was not well described or examined in relation to cognitive impairments. Consequently, the rate of cognitive decline, and the relationship of cognitive impairments to other clinical features, is not well understood. We are conducting a longitudinal study to prospectively characterize the natural history of neuropsychological and other symptoms in affected children in the United States and Canada. Here we describe preliminary results of the study of neuropsychological functioning in a group of North American children with juvenile neuronal ceroid lipofuscinosis.
Methods
We have administered neuropsychological tests in 2 investigations of cognitive function in juvenile neuronal ceroid lipofuscinosis. In both studies, eligible children were aged 5 to 18 with clinically probable or genetically confirmed juvenile neuronal ceroid lipofuscinosis. All participants, except 5 who were enrolled in the second investigation (study 2), were evaluated at mobile recruitment/research settings during annual meetings of the Batten Disease Support & Research Association; the remaining 5 children were enrolled at the Batten Disease Diagnostic and Clinical Research Center (University of Rochester, http://dbb.urmc.rochester.edu/labs/pearce/bddcrc/index.htm). Demographic and disease history information, motor neurologic function, and Clinical Global Impression of cognitive function were obtained from parents in a concurrent investigation of the Unified Batten Disease Rating Scale, a clinical rating scale for juvenile neuronal ceroid lipofuscinosis.9
The first investigation, conducted in 2003, was a pilot study of attention skills in 15 children with juvenile neuronal ceroid lipofuscinosis. Attention was defined by a digit span task (Test of Memory and Learning: Digits Forward, Digits Backward).10 Digits Forward, considered a test of simple attention, requires subjects to repeat number sequences verbatim. Digits Backward, considered a more demanding task of working memory, requires subjects to reverse the presented sequences. For each task, sequences become longer as the test progresses.
Pilot results from the first cross-sectional investigation led to a more in-depth investigation of cognitive abilities in juvenile neuronal ceroid lipofuscinosis, with a focus on describing the natural history of the disease over time. The second investigation, initiated in 2004, is an ongoing longitudinal investigation of neurobehavioral function in juvenile neuronal ceroid lipofuscinosis. Thus far, 18 genetically confirmed children with juvenile neuronal ceroid lipofuscinosis in the longitudinal study have completed their initial assessments; of these, 5 subjects have completed a second (annual) evaluation. In this longitudinal study, annual neuropsychological testing involves a brief (15-20 minutes) battery of 5 cognitive domains: estimated verbal cognitive abilities, verbal memory, auditory attention, verbal repetition, and verbal fluency (Table 1).11-16 Because there are few psychometrically sound tools for evaluating visually impaired children, the decision was made to use established, well-standardized neuropsychological measures. However, in consideration for vision loss in juvenile neuronal ceroid lipofuscinosis, the measures selected were originally developed to be presented verbally and require only a verbal response.
Table 1.
Neuropsychological Tests: Longitudinal Assessment of Cognitive Function in Juvenile Neuronal Ceroid Lipofuscinosis
| Vocabulary skills | |
| Ages 6-16 y | Wechsler Intelligence Scale for Children, fourth edition, Vocabulary11 |
| Ages 7-18 y | Wechsler Adult Intelligence Scale, third edition, Vocabulary12 |
| Verbal memory | |
| Ages 6-17 y | Wide Range Assessment of Memory and Learning, Story Memory13 |
| Age 18 y | Wechsler Memory Scale, third edition, Logical Memory14 |
| Auditory attention | |
| Ages 6-16 y | Wechsler Intelligence Scale for Children, fourth edition, Digit Span11 |
| Ages 7-18 y | Wechsler Adult Intelligence Scale, third edition, Digit Span12 |
| Verbal repetition | |
| All ages | Clinical Evaluation of Language Fundamentals, Recalling Sentences15 |
| Verbal fluency | |
| Ages 6-12 y | NEPSY, A Developmental Neuropsychological Assessment, Verbal Fluency16 |
| Ages 13-18 y | Controlled Oral Word Association; F-A-S |
All subjects were independently examined by 2 or 3 neurologists; motor neurologic function was based on the median motor score among all examiners (see Marshall et al9). We also examined the median Clinical Global Impression score of cognitive function among all raters performing neurologic examinations (0 = normal, 4 = severely impaired).
All statistical analyses were performed with the Statistical Package for the Social Sciences, version 13.0. Disease duration was calculated by subtracting subjects’ age of first symptom onset from their age at the time of neuropsychological assessment. For group analyses, neuropsychological test scores were converted to age-standardized scaled scores (mean = 10, SD = 3) or z scores (mean = 1, SD = 0). In examining data from each study, mean age-corrected scores were compared with established age-based normative data using 1-sample t tests. In the longitudinal study, the t test for independent samples was used to examine sex differences across the cognitive domains. Finally, Pearson correlation coefficients were used to examine the correlation between disease duration and each cognitive domain.
Results
Study 1: Pilot Study of Attention Skills
Fifteen children participated in the pilot study of attention skills (mean age = 14.3 ± 2.9 years, range = 8.75-18.74 years; 7 males, 8 females). Juvenile neuronal ceroid lipofuscinosis is most commonly found in Northern European populations, and in this study, all children were Caucasian. Fourteen children had a positive seizure history. All had clinical symptoms of juvenile neuronal ceroid lipofuscinosis, and 14 had genetic confirmation of disease. Of these, 12 children were homozygous for the common deletion in the ceroid-lipofuscinosis-3 gene. The common deletional mutation in the ceroid-lipofuscinosis-3 gene was analyzed using methodology previously described by this research group.17 One child was heterozygous for the deletion with a novel mutation. Two additional children had juvenile neuronal ceroid lipofuscinosis by clinical and/or electron miscroscopy criteria but either had not been studied by DNA analysis for ceroid-lipofuscinosis-3 mutations or their genetic status was provided by parental report or from another laboratory. The mean age of disease onset was 5.59 ± 0.96 years (range = 3.77-7.22 years) and the mean disease duration was 8.74 ± 2.83 years (range = 2.92-11.86 years). Based on the parents’ retrospective account on the Unified Batten Disease Rating Scale, the first noticed symptom of juvenile neuronal ceroid lipofuscinosis was vision loss in 12 children and seizure in 3 children.
As a group, children with juvenile neuronal ceroid lipofuscinosis were significantly impaired on the attention task. The average age-corrected scores for both Digits Forward and Digits Backward were significantly lower than test normative data (Digits Forward: t[14] = –27.495, P < .001; Digits Backward: t[14] = –13.539, P < .001). Using the t test for independent samples, we did not find a significant sex difference in either Digits Forward or Digits Backward performance.
Study 2: Longitudinal Study of Neuropsychological Function
To date, 18 children have enrolled in the longitudinal study of neuropsychologic function (mean age = 12.88 ± 3.59, range = 6.26-18.65; 11 males, 7 females). Of these, 5 have completed a second (annual) re-evaluation. All 18 children are Caucasian. All have clinical symptoms of juvenile neuronal ceroid lipofuscinosis and genetic confirmation of disease. Fifteen children (83.3%) are homozygous for the common deletion in ceroid-lipofuscinosis-3. Three children are heterozygous for the deletion. The mean age of disease onset is 5.79 ± 1.01 years (range = 4.25- 7.22 years), and the mean disease duration is 7.14 ± 3.51 years (range = 1.76-12.51 years). Seventeen children had a positive seizure history; in 1 child, the seizure history was unknown. Based on parent retrospective account on the Unified Batten Disease Rating Scale, the first symptom of juvenile neuronal ceroid lipofuscinosis was vision loss in 13 children and decreased cognitive function in 4 children (n = 1, unknown).
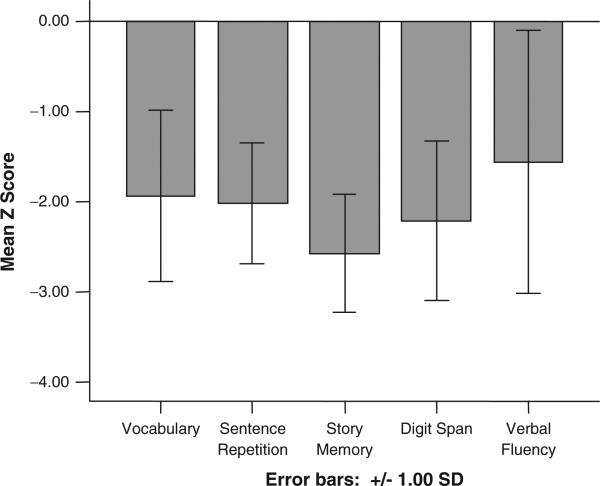
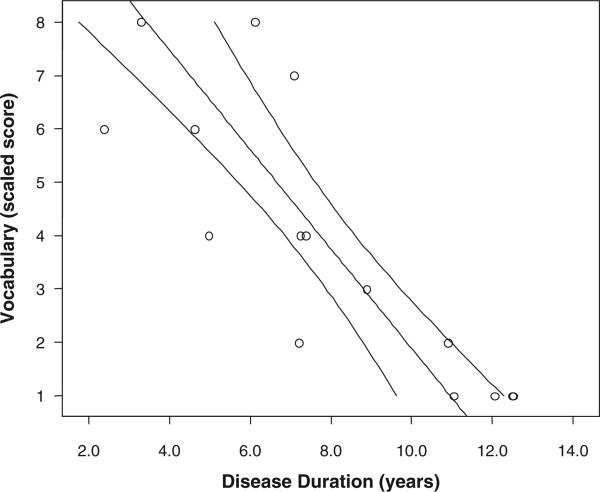
One-sample t tests showed that subjects’ test scores at baseline, in all cognitive domains, were significantly below the mean age-expected test norms (Table 2). These scores are illustrated graphically in Figure 1, which shows the mean z scores for the 5 neuropsychological tests. A series of independent-samples t tests were performed to evaluate sex differences in cognitive test performance. These results are presented in Table 3. There were no significant differences between males and females in any individual domain of cognitive function. Table 4 shows the bivariate correlations among the cognitive domains and disease duration. Disease duration was significantly inversely correlated with all neuropsychological domains except verbal repetition and most strongly correlated with estimated verbal intellectual ability (Vocabulary test). Figure 2 depicts the decline in verbal intellectual ability in association with longer disease duration.
Table 2.
Mean Cognitive Domain Scores at First Assessment
| Variable | Mean | SD | t |
|---|---|---|---|
| Vocabulary | 4.18 | 2.86 | –8.41** |
| Story Memory | 3.92 | 2.02 | –16.68** |
| Digit Span | 2.28 | 1.96 | –9.69** |
| Sentence Repetition | 3.33 | 2.66 | –10.85** |
| Verbal Fluency | 5.31 | 4.38 | –4.28* |
P < .01.
P < .001.
Figure 1.
Mean z scores across domains of neuropsychological function at first assessment.
Table 3.
Mean Cognitive Domain Scores by Sex (First Assessment)
| Variable | Sex | No. of Subjects | Mean | SD |
|---|---|---|---|---|
| Vocabulary | Male | 10 | 4.40 | 3.03 |
| Female | 7 | 3.86 | 2.79 | |
| Story Memory | Male | 11 | 2.55 | 2.42 |
| Female | 7 | 1.86 | 0.90 | |
| Digit Span | Male | 10 | 3.30 | 2.95 |
| Female | 5 | 3.40 | 2.30 | |
| Sentence Repetition | Male | 9 | 4.22 | 2.39 |
| Female | 4 | 3.25 | 0.50 | |
| Verbal Fluency | Male | 10 | 5.00 | 4.37 |
| Female | 6 | 5.83 | 4.75 |
NOTE: All comparisons were nonsignificant (t test, 2 tailed).
Table 4.
Pearson Product–Moment Correlations Among Neuropsychological Tests and Disease Duration
| Variable | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Vocabulary | |||||
| 2. Sentence Repetition | .70** | ||||
| 3. Story Memory | .81** | .92** | |||
| 4. Digit Span | .89** | .76** | .89** | ||
| 5. Verbal Fluency | .93** | .63* | .75** | .87** | |
| 6. Disease Duration | –.83** | –.51 | –.72** | –.62* | –.64* |
P < .05.
P < .01.
Figure 2.
Correlation of vocabulary score and disease duration in children with juvenile neuronal ceroid lipofuscinosis. R2 = 0.696, P < .001. Ninety-five percent confidence intervals are shown.
To examine overall neuropsychological function, a composite score was also formed by calculating the average performance across all 5 neuropsychological tests. Using this composite score, we performed a series of independent-sample t tests to examine the association between overall neuropsychological function and seizure history. There was a significant difference in the composite neuropsychological score for those with a positive seizure history (worse function) compared with those without any seizure history at the time of evaluation, t(9) = –5.34, P < .001.
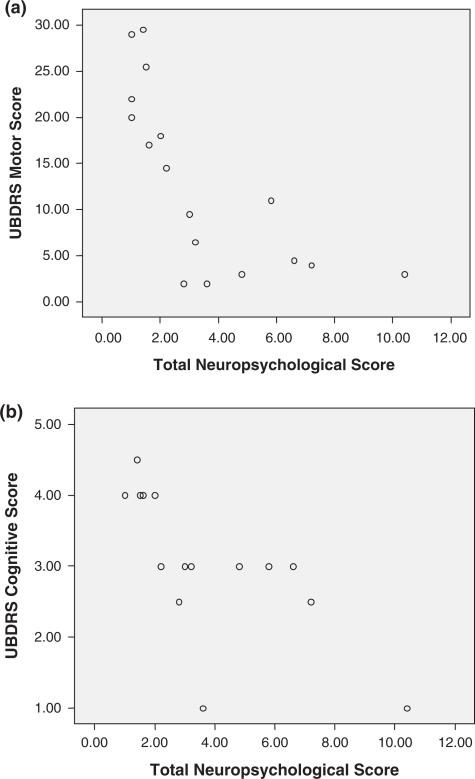
All subjects in this longitudinal study of neuropsychological function also completed the Unified Batten Disease Rating Scale examination, undergoing independent examinations by 2 or 3 neurologists. Motor neurologic function was based on the median motor score among all examiners (each item scored as 0 = normal, 4 = severely impaired). We also examined the median Clinical Global Impression score of cognitive function among all raters performing neurologic examinations (0 = normal, 4 = severely impaired). Table 5 presents the bivariate correlations among overall neuropsychological function, disease duration, and Unified Batten Disease Rating Scale–based motor score and cognitive Clinical Global Impression score (see also Figure 3a, b). The bivariate scatter plot of Unified Batten Disease Rating Scale–rated motor function and neuropsychological function suggests a considerable floor effect of the neuropsychological testing at more severe levels of motor impairment. By contrast, neuropsychological testing seems to discriminate better among subjects at milder levels of motor impairment (Figure 3a).
Table 5.
Pearson Product–Moment Correlations for Overall Neuropsychological Test Performance, Median Motor and Cognitive Function, and Disease Duration
| Variable | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Overall neuropsychological performance | ||||
| 2. Median motor function score | –.71** | |||
| 3. Median cognitive function (CGI) | –.73** | .83*** | ||
| 4. Disease duration | –.65** | .78** | .76** |
NOTE: CGI = Clinical Global Impression (from Unified Batten Disease Rating Scale).
P < .01 level (2 tailed).
P < .001 (2 tailed).
Figure 3.
(a) Bivariate scatter plot of motor function and neuropsychological function. Unified Batten Disease Rating Scale (UBDRS) motor score (median score; higher scores indicate worse function) and total neuropsychological score (age-corrected scaled scores; higher scores signify better function). At lower levels of cognitive function, there is a sharply linear association with the UBDRS motor score. At higher levels of cognitive function, motor function has greater variability. (b) Bivariate scatter plot of neurologists’ clinical impression of cognitive ability and neuropsychological function. (UBDRS cognitive score (median Clinical Global Impression score; higher scores indicate worse function) and total neuropsychological score (age-corrected scaled scores; higher scores signify better function).
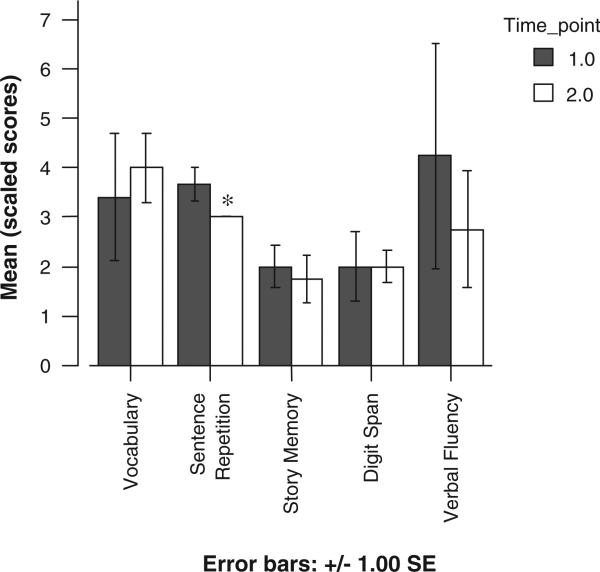
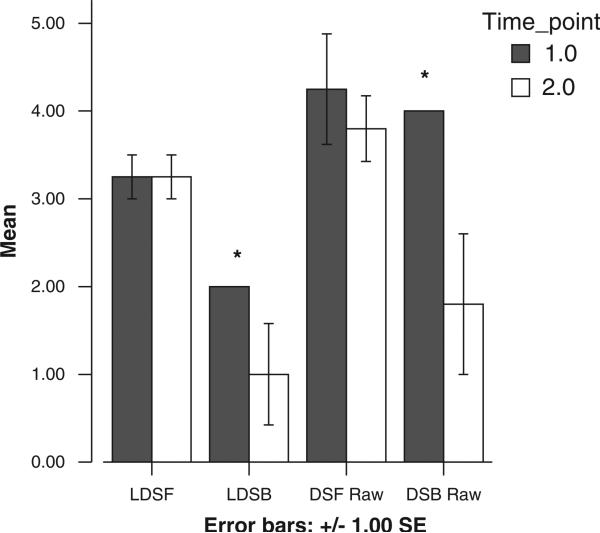
Five subjects have completed their first annual reassessment. Test scores did appear to decline in all areas except the digit span task (Figure 4); however, a series of paired t tests did not find significant differences between the 2 years in mean cognitive test scores. Although the overall digit span score did not decline, preliminary data suggest an interesting pattern of retained performance on the simple auditory attention component of digit span (digits forward). By contrast, reverse digit span, with greater demands on working memory and mental manipulation of stimuli, did decline (Figure 5). A 1-way analysis of variance test found that across the 2 years, this decline in the raw number of digits recalled (backward) was statistically significant, F(1,7) = 5.88, P <.05.
Figure 4.
Changes in cognitive test scores.
*Score is constant across subjects; no error bar.
Figure 5.
Digits Forward versus Digits Backward: patterns of change across 1 year. *Scores are constant across subjects; no error bars. LDSF = longest Digit Span Forward (scaled score); LDSB = longest Digit Span Backward (scaled score); DSF Raw = raw number of digits recalled on Digits Forward; DSB Raw = raw number of digits recalled on Digits Backward.
Discussion
Our preliminary findings highlight the substantial cognitive impairment among children with juvenile neuronal ceroid lipofuscinosis in the moderate stages of their disease. In the cross-sectional pilot study of attention skills (study 1), we found significant deficits specifically on a test of auditory attention and working memory, in contrast to age-expected levels of performance. These findings were further substantiated in the ongoing longitudinal study (study 2), which also found significant cognitive impairment in juvenile neuronal ceroid lipofuscinosis–affected children across a number of cognitive domains. Deficits in attention and working memory are particularly concerning given the reliance on auditory input by these children, all of whom had substantial vision loss or were blind. In our examination of follow-up data, the analysis of non-age-corrected raw scores is controversial as it prevents accurate comparisons between subjects of different ages. Nonetheless, our exploratory analysis of raw score on Digit Span Backward illustrates the decrement in function, contrary to an age-expected stabilization or growth in working memory span over time. A trend toward reduced cognitive performance is evident in the age-corrected scores for all other domains. Although these changes were not statistically significant, the longitudinal component of our data set is small at present. Once more subjects have completed several annual reassessments, changes in performance over time can be examined more rigorously, including better analysis of raw score data using within-subject analysis of change over time.
In our analysis of the first year's data from the longitudinal study, there was support for an association between longer disease duration and worse cognitive and motor abilities (Tables 4 and 5; Figure 2). This finding is not unexpected given that neurodegeneration in juvenile neuronal ceroid lipofuscinosis involves progressive cognitive and motor decline. Even more interesting is the possibility that the relationship between cognitive and motor symptom clusters may vary at different stages of disease; this underscores the importance of multimodal assessment of juvenile neuronal ceroid lipofuscinosis. Finally, one prior study has reported sex differences in behavioral function of Finnish children with juvenile neuronal ceroid lipofuscinosis18; these findings have not been replicated in a North American sample.19 The current study allowed us to examine this question with regard to cognitive abilities and did not find significant differences between males and females on any of the cognitive domains evaluated.
There is much still to be learned about the earliest clinical manifestations of juvenile neuronal ceroid lipofuscinosis. There is but 1 known account of 2 clinically asymptomatic children followed prospectively (identified after elder siblings were diagnosed); each had clinicopathologic confirmation of juvenile neuronal ceroid lipofuscinosis and were symptom free at their first assessment.20 In both children, scores on standardized tests of auditory attention, working memory, and verbal intellectual ability declined 1 to 2 years prior to the onset of visual symptoms. Likewise, in a pilot study by our Batten Study Group at the University of Rochester, cognitive or behavioral problems were the first apparent symptom (retrospectively described) in 19% of children evaluated and were either the second or third symptom to emerge in many other children (29% and 54%, respectively).9 These limited results are intriguing because they suggest that in some cases, neurobehavioral symptoms might emerge very early in the course of the disease and may precede physical indicators of disease such as vision loss, motor impairment, or seizures. In the current study, a positive seizure history was associated with worse cognitive function. However, there are many as yet unanswered questions regarding children's seizures, including the impact of seizure medications on cognitive abilities, effectiveness of seizure control, and frequency, severity, and recency of seizures.
Although current treatments for juvenile neuronal ceroid lipofuscinosis are symptomatic rather than neuroprotective, establishing reliable natural history data, determining assessment modalities for juvenile neuronal ceroid lipofuscinosis, and identifying appropriate neurobehavioral outcomes will be essential in preparing for future experimental therapeutics studies. Neurobehavioral assessment has become established as an integral component of therapeutics for other lysosomal and nonlysosomal storage diseases of childhood, serving a predictive role in identifying patients who are appropriate candidates for treatment, and as valid outcome measures for evaluating clinically meaningful treatment effects.21-24 Our ongoing, longitudinal research will track changes in neuropsychological abilities over time. Information on the natural history of cognitive function in juvenile neuronal ceroid lipofuscinosis can help inform daily management and educational decision making and provide a reliable assessment of cognitive change when future candidate therapies become available.
Acknowledgments
This research was funded by the Batten Disease Support and Research Association (study 1) and the Luke and Rachel Batten Foundation (study 2). The authors thank the parents and children for their participation and the Batten Disease Support and Research Association for assistance with participant recruitment. This research was conducted at the 2004 and 2005 annual meetings of the Batten Disease Support and Research Association and on an ongoing basis at the University of Rochester School of Medicine and Dentistry. A portion of this research was presented as a paper at the First International Education Conference on Batten Disease, May 3-6, 2006, Örebro, Sweden.
Footnotes
Adams HR, Kwon J, Marshall FJ, de Blieck EA, Pearce DA, Mink JW. Neuropsychological symptoms of juvenile-onset batten disease: experiences from 2 studies.
References
- 1.Santavuori P, Lauronen L, Kirveskari E, Åberg L, Sainio K, Autti T. Neuronal ceroid lipofuscinoses in childhood. Neurol Sci. 2000;21:S35–S41. doi: 10.1007/s100720070038. [DOI] [PubMed] [Google Scholar]
- 2.Rider JA, Rider DL. Thirty years of Batten disease research: present status and future goals. Mol Genet Metab. 1999;66:231–233. doi: 10.1006/mgme.1999.2827. [DOI] [PubMed] [Google Scholar]
- 3.Boustany R-M. Batten disease or neuronal ceroid lipofuscinosis. In: Moser HW, editor. Handbook of Clinical Neurology. 66. Vol. 22. Elsevier Science B.V.; Maclean, Va: 1996. pp. 671–700. [Google Scholar]
- 4.Phillips SN, Benedict JW, Weimer JM, Pearce DA. CLN3, the protein associated with batten disease: structure, function and localization. J Neurosci Res. 2005;79:573–583. doi: 10.1002/jnr.20367. [DOI] [PubMed] [Google Scholar]
- 5.Munroe PB, Mitchison HM, O'Rawe AM, et al. Spectrum of mutations in the Batten disease gene, CLN3. Am J Hum Genet. 1997;61:310–316. doi: 10.1086/514846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lou HC, Kristensen K. A clinical and psychological investigation into juvenile amaurotic idiocy in Denmark. Dev Med Child Neurol. 1973;15:313–323. doi: 10.1111/j.1469-8749.1973.tb04888.x. [DOI] [PubMed] [Google Scholar]
- 7.Lamminranta S, Åberg LE, Autti T, et al. Neuropsychological test battery in the follow-up of patients with juvenile neuronal ceroid lipofuscinosis. J Intellect Disabil Res. 2001;45:8–17. doi: 10.1046/j.1365-2788.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 8.Åberg L, Kirveskari E, Santavuori P. Lamotrigine therapy in juvenile neuronal ceroid lipofuscinosis. Epilepsia. 1999;40:796–799. doi: 10.1111/j.1528-1157.1999.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 9.Marshall FJ, de Blieck EA, Mink JW, et al. A clinical rating scale for Batten disease: reliable and relevant for clinical trials. Neurology. 2005;65:275–279. doi: 10.1212/01.wnl.0000169019.41332.8a. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds CR, Bigler ED. Test of Memory and Learning. Examiner's Manual. Pro-Ed; Austin, Tex: 1994. [Google Scholar]
- 11.Psychological Corporation . Wechsler Intelligence Scale for Children—Fourth Edition. Manual. The Psychological Corporation; San Antonio, Tex: 2003. [Google Scholar]
- 12.Psychological Corporation . WAIS-III, WMS-III. Technical Manual. The Psychological Corporation; San Antonio, Tex: 2002. [Google Scholar]
- 13.Sheslow D, Adams W. WRAML: Wide Range Assessment of Memory and Learning. Administration Manual. Wide Range; Wilmington, Del: 1990. [Google Scholar]
- 14.Wechsler D. Wechsler Memory Scale—Third Edition. The Psychological Corporation; San Antonio, Tex: 1997. [Google Scholar]
- 15.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals—Third Edition. Technical Manual. The Psychological Corporation; San Antonio, Tex: 1995. [Google Scholar]
- 16.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. Subtest Administration. The Psychological Corporation; San Antonio, Tex: 1998. [Google Scholar]
- 17.Rothberg PG, Ramirez-Montealegre D, Frazier SD, Pearce DA. Homogeneous polymerase chain reaction nucleobase quenching assay to detect the 1-kbp deletion in CLN3 that causes Batten disease. J Mol Diagn. 2004;6:260–263. doi: 10.1016/S1525-1578(10)60519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bäckman ML, Santavuori PR, Åberg LE, Aronen ET. Psychiatric symptoms of children and adolescents with juvenile neuronal ceroid lipofuscinosis. J Intellect Disabil Res. 2005;49:25–32. doi: 10.1111/j.1365-2788.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 19.Adams H, de Blieck EA, Mink JW, et al. Standardized assessment of behavior and adaptive living skills in juvenile neuronal ceroid lipofuscinosis. Dev Med Child Neurol. 2006;48:259–264. doi: 10.1017/S0012162206000570. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen K, Lou HC. Central nervous system dysfunction as an early sign of neuronal ceroid lipofuscinosis (Batten's disease). Dev Med Child Neurol. 1983;25:588–590. doi: 10.1111/j.1469-8749.1983.tb13815.x. [DOI] [PubMed] [Google Scholar]
- 21.Krivit W, Aubourg P, Shapiro E, Peters C. Bone marrow transplantation for globoid cell leukodystrophy, adrenoleukodystrophy, metachromatic leukodystrophy, and Hurler syndrome. Curr Opin Hematol. 1999;6:377–382. doi: 10.1097/00062752-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Peters C, Balthazor M, Shapiro EG, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 23.Krivit W, Peters C, Shapiro E. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Curr Opin Neurol. 1999;12:167–176. doi: 10.1097/00019052-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro E, Lockman LA, Balthazor M, Krivit W. Neuropsychological outcomes of several storage diseases with and without bone marrow transplantation. J Inherit Metab Dis. 1995;19:413–429. doi: 10.1007/BF00710053. [DOI] [PubMed] [Google Scholar]