Abstract
The aim of the present study was to assess whether endogenous and newly synthesized glutamate can be released from differentiating cultured cerebellar granule cells in a way compatible with a neurotransmitter role. Granule cells from 8-day-old rat cerebella were grown in basal Eagle's medium with 10% fetal calf serum for 2-12 days in vitro (DIV), then washed with Krebs-Ringer medium, and labeled for 45 min with tracer amounts of radioactive glutamine. Subsequently, the release of endogenous glutamate and of newly formed radioactive glutamate was measured in basal conditions and upon depolarization with elevated K+ concentration or veratridine. At 2 DIV, the release of endogenous and newly synthesized glutamate evoked by high K+ concentration was small and Ca2+ independent, but it progressively and steadily increased (up to 8- to 10-fold) and became Ca2+ dependent (up to 80-85%) at later stages (4, 8, and 12 DIV). Veratridine was almost ineffective with cells at 2 DIV but greatly increased glutamate release (endogenous and neosynthesized) at 8 DIV, and its action was totally antagonized by tetrodotoxin. The level and synthesis of glutamate remained fairly constant in cells from 2 to 12 DIV. γ-Aminobutyric acid synthesis from radioactive glutamine was about 3% of that of glutamate, and γ-aminobutyric acid release (endogenous and neosynthesized) was not measurable. Aspartate synthesis was about 10% of that of glutamate, and the high K+ concentration-evoked release of this amino acid was modest and scarcely affected by Ca2+. Neither high K+ concentration nor veratridine was able to induce glutamate release from confluent cerebellar astrocyte cultures at 14 DIV, although the level and synthesis of the amino acid were comparable to those in granule cells. In conclusion, the data show that a stimulus-coupled release of endogenous and neosynthesized glutamate is progressively expressed by cerebellar granule cells differentiating in culture, and this strongly supports the concept that glutamate is the neurotransmitter of these cells.
Keywords: neurotransmitter amino acids, depolarization-induced release, cerebellum, astrocyte cultures
Full text
PDF
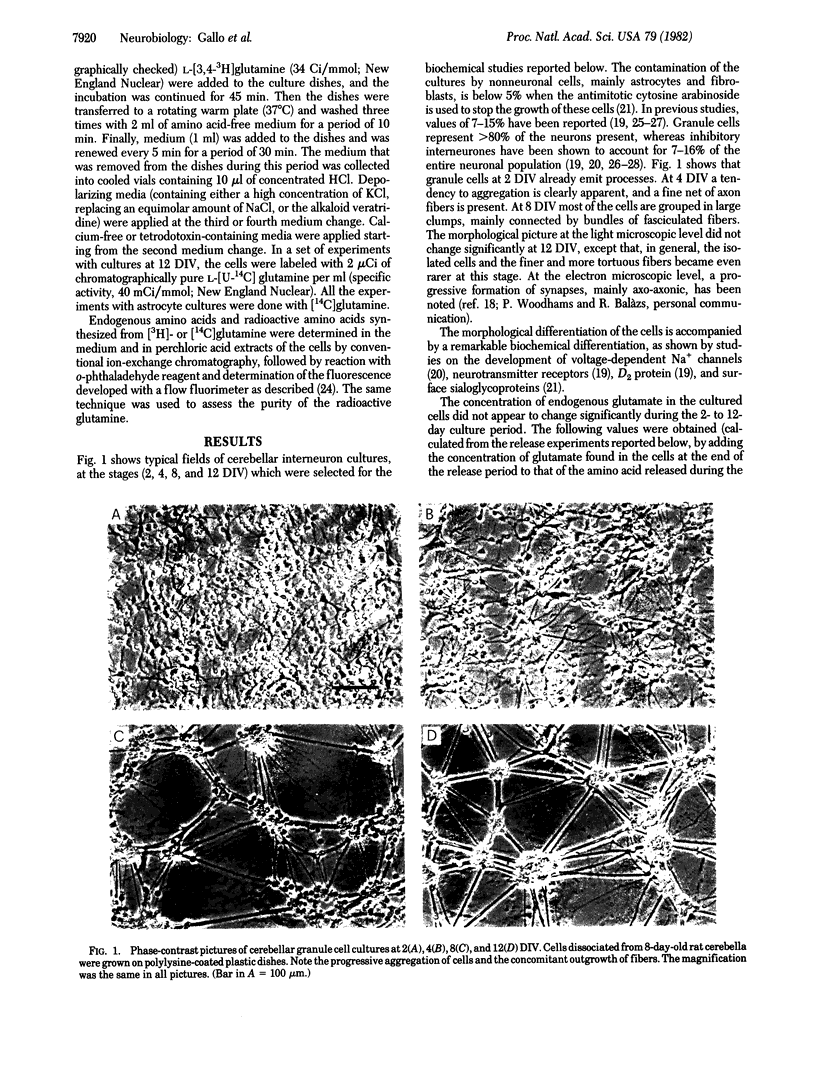
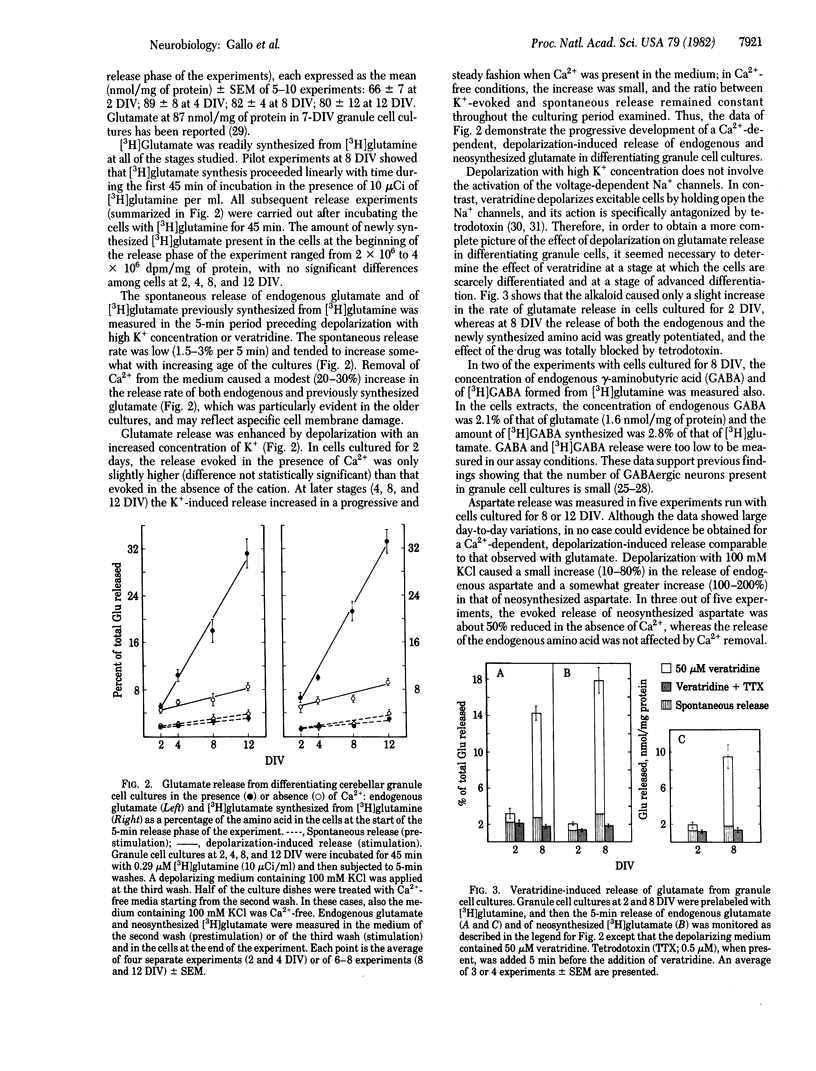


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale R., Dutton G. R., Currie D. N. An ion flux assay of action potential sodium channels in neuron- and glia-enriched cultures of cells dissociated from rat cerebellum. Brain Res. 1980 Feb 3;183(1):241–246. doi: 10.1016/0006-8993(80)90137-7. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J Physiol. 1975 Jun;247(3):617–655. doi: 10.1113/jphysiol.1975.sp010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford H. F., Ward H. K., Thomas A. J. Glutamine--a major substrate for nerve endings. J Neurochem. 1978 Jun;30(6):1453–1459. doi: 10.1111/j.1471-4159.1978.tb10477.x. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore of cultured neuroblastoma cells by veratridine and batrachotoxin. J Biol Chem. 1975 Jun 10;250(11):4053–4059. [PubMed] [Google Scholar]
- Currie D. N., Dutton G. R., Cohen J. Monolayer cultures of perikarya isolated from postnatal rat cerebellum. Experientia. 1979 Mar 15;35(3):345–347. doi: 10.1007/BF01964343. [DOI] [PubMed] [Google Scholar]
- Currie D. N., Dutton G. R. [3H]GABA uptake as a marker for cell type in primary cultures of cerebellum and olfactory bulb. Brain Res. 1980 Oct 20;199(2):473–481. doi: 10.1016/0006-8993(80)90706-4. [DOI] [PubMed] [Google Scholar]
- Dichter M. A. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978 Jun 30;149(2):279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- Drejer J., Larsson O. M., Schousboe A. Characterization of L-glutamate uptake into and release from astrocytes and neurons cultured from different brain regions. Exp Brain Res. 1982;47(2):259–269. doi: 10.1007/BF00239385. [DOI] [PubMed] [Google Scholar]
- Flint R. S., Rea M. A., McBride W. J. In vitro release of endogenous amino acids from granule cell-, stellate cell-, and climbing fiber-deficient cerebella. J Neurochem. 1981 Dec;37(6):1425–1430. doi: 10.1111/j.1471-4159.1981.tb06311.x. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Roberts P. J. Endogenous amino acid release from rat cerebellum in vitro. J Neurochem. 1980 Aug;35(2):517–519. doi: 10.1111/j.1471-4159.1980.tb06299.x. [DOI] [PubMed] [Google Scholar]
- Hamberger A. C., Chiang G. H., Nylén E. S., Scheff S. W., Cotman C. W. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979 Jun 8;168(3):513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- Hertz L., Yu A., Svenneby G., Kvamme E., Fosmark H., Schousboe A. Absence of preferential glutamine uptake into neurons--an indication of a net transfer of TCA constituents from nerve endings to astrocytes? Neurosci Lett. 1980 Jan;16(1):103–109. doi: 10.1016/0304-3940(80)90109-3. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Deutsch J. W., Carlson S. S., Wagner J. A. Biochemistry of neurotransmitter release. Annu Rev Neurosci. 1979;2:399–446. doi: 10.1146/annurev.ne.02.030179.002151. [DOI] [PubMed] [Google Scholar]
- Lasher R. S. The uptake of (3H)GABA and differentiation of stellate neurons in cultures of dissociated postnatal rat cerebellum. Brain Res. 1974 Apr 5;69(2):235–254. doi: 10.1016/0006-8993(74)90004-3. [DOI] [PubMed] [Google Scholar]
- Lasher R. S., Zagon I. S. The effect of potassium on neuronal differentiation in cultures of dissociated newborn rat cerebellum. Brain Res. 1972 Jun 22;41(2):482–488. doi: 10.1016/0006-8993(72)90521-5. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V., Ciotti T., Raiteri M. GABA fluxes in presynaptic nerve endings from immature rats. J Neurochem. 1979 Nov;33(5):1042–1053. doi: 10.1111/j.1471-4159.1979.tb05240.x. [DOI] [PubMed] [Google Scholar]
- Levi G., Gallo V. Glutamate as a putative transmitter in the cerebellum: stimulation by GABA of glutamic acid release from specific pools. J Neurochem. 1981 Jul;37(1):22–31. doi: 10.1111/j.1471-4159.1981.tb05286.x. [DOI] [PubMed] [Google Scholar]
- Levi G., Gordon R. D., Gallo V., Wilkin G. P., Balàzs R. Putative acidic amino acid transmitters in the cerebellum. I. Depolarization-induced release. Brain Res. 1982 May 13;239(2):425–445. doi: 10.1016/0006-8993(82)90520-0. [DOI] [PubMed] [Google Scholar]
- Llinás R. R. The cortex of the cerebellum. Sci Am. 1975 Jan;232(1):56–71. doi: 10.1038/scientificamerican0175-56. [DOI] [PubMed] [Google Scholar]
- Messer A. The maintenance and identification of mouse cerebellar granule cells in monolayer culture. Brain Res. 1977 Jul 8;130(1):1–12. doi: 10.1016/0006-8993(77)90838-1. [DOI] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Patel A. J., Balázs R. Effect of x-irradiation of the biochemical maturation of rat cerebellum: metabolism of [14C]glucose and [14-C]acetate. Radiat Res. 1975 Jun;62(3):456–469. [PubMed] [Google Scholar]
- Patel A. J., Hunt A., Gordon R. D., Balázs R. The activities in different neural cell types of certain enzymes associated with the metabolic compartmentation glutamate. Brain Res. 1982 May;256(1):3–11. doi: 10.1016/0165-3806(82)90091-8. [DOI] [PubMed] [Google Scholar]
- Pearce B. R., Currie D. N., Beale R., Dutton G. R. Potassium-stimulated, calcium-dependent release of [3H]GABA from neuron- and glia-enriched cultures of cells dissociated from rat cerebellum. Brain Res. 1981 Feb 16;206(2):485–489. doi: 10.1016/0006-8993(81)90552-7. [DOI] [PubMed] [Google Scholar]
- Redburn D. A., Broome D., Ferkany J., Enna S. J. Development of rat brain uptake and calcium-dependent release of GABA. Brain Res. 1978 Sep 8;152(3):511–519. doi: 10.1016/0006-8993(78)91106-x. [DOI] [PubMed] [Google Scholar]
- Roffler-Tarlov S., Sidman R. L. Concentrations of glutamic acid in cerebellar cortex and deep nuclei of normal mice and Weaver, Staggerer and nervous mutants. Brain Res. 1978 Feb 24;142(2):269–283. doi: 10.1016/0006-8993(78)90635-2. [DOI] [PubMed] [Google Scholar]
- Rohde B. H., Rea M. A., Simon J. R., McBride W. J. Effects of X-irradiation induced loss of cerebellar granule cells on the synaptosomal levels and the high affinity uptake of amino acids. J Neurochem. 1979 May;32(5):1431–1435. doi: 10.1111/j.1471-4159.1979.tb11081.x. [DOI] [PubMed] [Google Scholar]
- Sandoval M. E., Cotman C. W. Evaluation of glutamate as a neurotransmitter of cerebellar parallel fibers. Neuroscience. 1978;3(2):199–206. doi: 10.1016/0306-4522(78)90101-x. [DOI] [PubMed] [Google Scholar]
- Valcana T., Hudson D., Timiras P. S. Effects of X-irradiation on the content of amino acids in the developing rat cerebellum. J Neurochem. 1972 Sep;19(9):2229–2232. doi: 10.1111/j.1471-4159.1972.tb05133.x. [DOI] [PubMed] [Google Scholar]
- Wiklund L., Toggenburger G., Cuénod M. Aspartate: possible neurotransmitter in cerebellar climbing fibers. Science. 1982 Apr 2;216(4541):78–80. doi: 10.1126/science.6121375. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Balázs R., Wilson J. E., Cohen J., Dutton G. R. Preparation of cell bodies from the developing cerebellum: structural and metabolic integrity of the isolated cells. Brain Res. 1976 Oct 15;115(2):181–199. doi: 10.1016/0006-8993(76)90506-0. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Garthwaite J., Balázs R. Putative acidic amino acid transmitters in the cerebellum. II. Electron microscopic localization of transport sites. Brain Res. 1982 Jul 22;244(1):69–80. doi: 10.1016/0006-8993(82)90905-2. [DOI] [PubMed] [Google Scholar]
- Woodhams P. L., Wilkin G. P., Balàzs R. Rat cerebellar cells in tissue culture. II. Immunocytochemical identification of replicating cells in astrocyte-enriched cultures. Dev Neurosci. 1981;4(4):307–321. doi: 10.1159/000112770. [DOI] [PubMed] [Google Scholar]
- Young A. B., Oster-Granite M. L., Herndon R. M., Snyder S. H. Glutamic acid: selective depletion by viral induced granule cell loss in hamster cerebellum. Brain Res. 1974 Jun 14;73(1):1–13. doi: 10.1016/0006-8993(74)91002-6. [DOI] [PubMed] [Google Scholar]
- Yu A. C., Hertz L. Uptake of glutamate, GABA, and glutamine into a predominantly GABA-ergic and a predominantly glutamatergic nerve cell population in culture. J Neurosci Res. 1982;7(1):23–35. doi: 10.1002/jnr.490070104. [DOI] [PubMed] [Google Scholar]