Abstract
Fatty acids (FAs) are important as metabolic substrates and as structural components of biological membranes. However, they also function as signalling molecules. Recently, a series of G protein-coupled receptors (GPRs) for FAs has been described and characterized. These receptors have differing specificities for FAs of differing chain length and degree of saturation, for FA derivatives such as oleoylethanolamide, and for oxidized FAs. They are a critical component of the body's nutrient sensing apparatus, and small molecule agonists and antagonists of these receptors show considerable promise in the management of diabetes and its complications. Agonists of the long-chain free fatty acid receptors FFAR1 and GPR119 act as insulin secretagogues, both directly and by increasing incretins. Although, drugs acting at short-chain FFA receptors (FFAR2 and FFAR3) have not yet been developed, they are attractive targets as they regulate nutrient balance through effects in the intestine and adipose tissue. These include regulation of the secretion of cholecystokinin, peptide YY and leptin. Finally, GPR132 is a receptor for oxidized FAs, which may be a sensor of lipid overload and oxidative stress, and which is involved in atherosclerosis. Regulation of its signalling pathways with drugs may decrease the macrovascular risk experienced by diabetic patients. In summary, FA receptors are emerging drug targets that are involved in the regulation of nutrient status and carbohydrate tolerance, and modulators of these receptors may well figure prominently in the next generation of antidiabetic drugs.
Keywords: atherosclerosis, diabetes, drug therapies, free fatty acid, G protein-coupled receptor
Introduction
The first three decades of the third millennium will see a doubling in global prevalence of diabetes to over 350 million. The resulting morbidity and premature mortality from microvascular, macrovascular and other complications is driving research into the underlying mechanisms and the quest for nutritional and pharmacological interventions. The role of free fatty acids (FFAs) in glycaemic regulation and type 2 diabetes pathogenesis is well established [Bergman and Ader, 2000], but the recent identification of a family of G protein-coupled receptors (GPRs) whose natural ligands are fatty [Stoddart et al. 2008; Covington et al. 2006] has awakened interest in how the effects of fatty acids (FAs) are mediated and has alerted us to the possibility of new therapeutic approaches. The recent demonstration that palmitoleate (C16: 1n7) secreted by adipose tissue enhances insulin sensitivity in other tissues lends further stimulus to the study of messenger functions of FAs [Cao et al. 2008]. In this review, we discuss what is known about FA receptors, and speculate on how modulation of these signalling pathways may help lessen the burden of diabetes and its complications.
A family of GPRs for FFAs
The majority of signalling across cell membranes occurs following the binding of messengers to their cognate receptors. In most cases, receptor activation is coupled to proteins with GTPase activity (G proteins) which, in turn activate or inhibit intracellular messengers [Millar and Newton, 2010]. The α subunits of G proteins differ in ability to modulate pathways: Gαs, activation leads to increased cyclic adenosine monophosphate (cAMP); Gαi/o, inhibition of cAMP production; Gαq/11, by activating phospholipase C, increases inositol-1,4,5-trisphosphate, which increases cellular calcium and also diacylglycerol, which activates protein kinase C; this Gα subtype is pertussis toxin sensitive; Gαq12/13, activates the Rho family of GTPases. The human genome contains over 800 GPRs divided into five families (i.e. rhodopsin, secretin, adhesion, glutamate and taste). GPRs mediate the effect of about one third of the drugs in current use. The family of receptors for FFAs, which belong to the rhodopsin family, are summarized in Table 1. Like other GPRs, these are proteins of 300–400 amino acids comprising an extracellular domain, seven transmembrane loops and an intracellular domain. The receptors have specificities for FAs of differing chain length. GPR119 is included here although its ligand is oleoylethanolamide (OEA), an FA derivative rather than an FFA. The FFA receptors form part of the body's nutrient sensing apparatus, which includes the gastrointestinal tract, pancreas, adipose tissue, leucocytes and parts of the central nervous system. Unlike the classic ‘lock and key’ relationship between receptors and their ligands, nutrient receptors are considered to be promiscuous in that they can be activated by a range of ligands [Wellendorph et al. 2009].
Table 1.
A family of fatty acid receptors.
| Ligand | Gα subunit | Chromosome | Tissue expression | |
|---|---|---|---|---|
| FFAR1 | C16–C22 Thiazolidinedione | Gαq/11 | 19q13.1 | β cell |
| FFAR2 | C2–C4 | Gαq/11, Gαi/o | 19q13.1 | Adipocyte, entero-endocrine cells, immune cells |
| FFAR3 | C3>C4>C2 | Gαi/o | 19q13.1 | Adipocyte |
| GPR84 | C9–C14 | Gαi/o | 12q13.13 | Spleen, monocytes, macrophages |
| GPR119 | Oleoylethanolamide, lysophosphatidylcholine | Gαs | Xq26.1 | β cell, entero-endocrine cells |
| GPR120 | C14–C22 | Gαq/11 | 10q23.3 | Entero-endocrine cells |
| GPR132 | 9-hydroxyoctadecadienoic acid | Gαq/11, Gαi/o, Gα12/13 | 14q32.3 | Macrophages |
FFAR1 (GPR40)
FFAs stimulate insulin secretion acutely, but chronic elevation leads to lipotoxicity with decreased β-cell function and peripheral insulin resistance. FFAR1 may be involved in both effects, and it remains controversial whether FFAR1 agonists or antagonists are most likely to be useful pharmacologically [Morgan and Dhayal, 2009; Brownlie et al. 2008]. FFAR1 was described independently by three groups [Briscoe et al. 2003; Itoh et al. 2003; Kotarsky et al. 2003]. The receptor is activated by medium- and long-chain saturated and unsaturated FAs [Ichimura et al. 2009]. Pentadecanoic (C15) and palmitic (C16) acids are the most potent saturated ligands, while chain length and degree of saturation are not major determinants of receptor affinity for unsaturated FAs. The most important sites for FFAR1 expression are the insulin-secreting β cells, the K and L cells of the small and large intestine respectively, splenocytes and peripheral blood mononuclear cells [Morgan et al. 2009; Parker et al. 2009]. FFAR1 is also expressed in the α cells in the pancreatic islets, where it is involved in regulating glucagon secretion in response to unsaturated FAs [Flodgren et al. 2007]. Although expressed in areas of the central nervous system, including the hippocampus, its role is not known at present [Ma et al. 2008]. The EC50 for receptor activation is in the micromolar range, while the FFA is probably in the nanomolar range physiologically (most FAs being bound to albumin). It has thus been difficult to assess the physiological significance of FFAR1 activation in vivo. Local generation of FFAs by lipoprotein lipase may increase local FFA delivery.
Steneberg and colleagues reported that mice deficient in FFAR1 are protected from obesity-induced hyperinsulinaemia, dyslipidaemia and glucose intolerance, while overexpression of the receptor leads to hyperinsulinaemia and glucose intolerance [Steneberg et al. 2005]. However, subsequent studies [Morgan and Dhayal, 2009] have not suggested that FFAR1 knockdown has a major protective effect. Recently developed small molecule antagonists such as DC260126 may not, therefore, be useful in the treatment of diabetes. It now seems that agonists and not antagonists may prove to be of greater clinical use [Bharate et al. 2009; Brownlie et al. 2008; Kebede et al. 2008], which is consistent with the observation that FFAR1 overexpression increased insulin secretion and improved glucose tolerance in both normal and diabetic mice [Nagasumi et al. 2009]. The receptor can be activated by thiazolidinedione drugs, as well as FFAs [Kotarsky et al. 2003]. A small molecule agonist (GW9508) of FFAR1 and GPR120 has been described [Briscoe et al. 2006]. Both GW9508 and linoleic acid increased intracellular calcium in HEK-293 cells transfected with FFAR1, and GW9508 also enhanced insulin secretion from MIN6 cells. Christiansen and colleagues described a series of 4-phenethynyldihydrocinnamic acid derivatives that were FFAR1 agonists and also could act as insulin secretagogues [Christiansen et al. 2008]. A series of diacylphloroglucinol compounds with FFAR1 agonist activity has also been studied [Bharate et al. 2008]. There are also prospects for altering receptor activation by limited genetic manipulations [Sum et al. 2009]. None of the above insulin secretagogues has yet reached the stage of being subjected to human clinical trials.
FFAR2 (GPR43)
One of a cluster of FA receptor genes located at chromosome 19q13.1, FFAR2 is preferentially activated by propionate and is coupled to both Gαi/o and Gαq/11. FFAR2 is highly expressed in leucocytes, and may have a role in the differentiation and activation of monocytes and polymorphonuclear cells. It is not known whether these actions are relevant to diabetes although patients with diabetes have increased monocyte activation and this is a precursor to tissue infiltration (including adipose and the arterial wall) and their subsequent differentiation into macrophages. These processes are important in regulating the low-grade inflammation that accompanies obesity and in atherogenesis. FFAR2 is also expressed in the L cells of the ileum and colon [Karaki et al. 2006]. These entero-endocrine cells synthesize and secrete peptide YY. The latter is known to be increased in response to short-chain FAs [Cherbut et al. 1998], and its release is accompanied by decreased appetite and decreased food intake through actions in the central nervous system [Batterham et al. 2003]. Short-chain FAs are produced in the gut by microbial fermentation of indigestible carbohydrate. Using probiotics, the gut flora can be altered and this has recently been proposed as a means of regulating appetite, decreasing insulin resistance and decreasing risk of nonalcoholic fatty liver disease [Musso et al. 2010]. Use of probiotics increases intestinal release of glucagon-like peptide (GLP)-1 and GLP-2 (incretins) and also peptide YY [Musso et al. 2010; Cani et al. 2009]. Finally, FFAR2 has been reported to be expressed in adipocytes, and its expression upregulated in mice fed a high fat diet [Hong et al. 2005]. Silencing of the FFAR2 gene using siRNA interfered with adipocyte differentiation in the 3T3-L1 cell line. These authors did not detect FFAR3 (GPR41) in adipose tissue or 3T3-L1 cells. In our hands, FFAR3 is highly expressed in 3T3-L1 preadipocytes and adipocytes, while FFAR2 is only expressed in fully differentiated cells. Ge and colleagues reported that FFAR2 activation decreased lipolysis in vivo, an effect that was not present in FFAR2 knockout mice [Ge et al. 2008]. Furthermore, in vivo FFAR2 activation decreased plasma FFA levels. Clearly, FFAR2 activation could improve some features of metabolic syndrome. However, as with thiazolidinediones, this may be achieved by decreasing circulating FFAs through increased adipocyte differentiation and thus increased levels of adiposity. Lee and colleagues described a number of small molecule phenylacetamides that acted as selective allosteric agonists at FFAR2 [Lee et al. 2008]. The synthetic ligands were more potent than endogenous ligands (acetate and propionate), and treatment of adipocytes with these agonists inhibited lipolysis.
FFAR3 (GPR41)
Propionate, butyrate and pentanoate are all equally potent in activating FFAR3, which couples with Gαi/o. GPR42 shares 98% homology with FFAR3 but is biologically inactive. Xiong and colleagues reported that FFAR3 was highly expressed in both an adipocyte cell line and primary adipocyte cultures [Xiong et al. 2004]. Propionate, acting through FFAR3, increased leptin secretion by adipocytes and propionate administration to mice increased circulating leptin levels. Short-chain FAs may thus regulate appetite and energy homeostasis through FFAR3 expressed on adipocytes. Previous studies [Ge et al. 2008; Hong et al. 2005] suggested that FFAR2 but not FFAR3 was expressed on mature adipocytes. Clearly this inconsistency, which may arise from use of different cell and animal models in different studies, needs to be resolved. To date, small molecule synthetic ligands for FFAR3 have not been described. FFAR2 and FFAR3 have about 50% structural homology [Brown et al. 2003]. Their relative importance and their differing physiological roles are poorly understood. There is, however, considerable interest currently in the regulatory effects of short-chain FA in nutrient intake, energy balance, glucose homeostasis and cardiovascular risk: modest alcohol intake decreases risk of cardiovascular events through improved lipid profile and glucose tolerance as well as having beneficial effects on inflammatory and haemostatic parameters [Djousse et al. 2009]. The effect of ethanol ingestion, which increases circulating acetate, to improve glucose homeostasis is due to effects on both insulin production and insulin sensitivity [Player et al. 2010; Hafko et al. 2009]. In a recent study [Gao et al. 2009], butyrate administration to mice led to improved systemic insulin sensitivity. Furthermore, short-chain FAs directly stimulate differentiation of stem cells into insulin-secreting β cells [Ren et al. 2010; Li et al. 2008]. Butyrate has long been known to influence adipocyte maturation, and this was presumed to be due to its action as a histone deacetylase (HDAC) inhibitor. Recent data, however, suggest that butyrate enhances adipogenesis while other HDAC inhibitors attenuate the process [Kim et al. 2009]. Finally, fermentation of complex carbohydrates in the colon yields very high local levels of short-chain FAs but also increases circulating short-chain FA levels. Recent studies demonstrate that administration of complex carbohydrates improves the lipid profile, decreases postprandial FFA excursions, improves insulin sensitivity and increases circulating GLP-1 [Freeland et al. 2010; Priebe et al. 2010; Tarini and Wolever, 2010; Queenan et al. 2007]. It is hard to imagine that FFA receptors are not relevant to the above physiological and clinical observations. FFAR1 and FFAR2 may be expressed in β cells [Covington et al. 2006], although this has only been alluded to in patent applications and the suggestion is not supported by peer-reviewed publications.
GPR84
This receptor for medium-chain FFAs is highly expressed on immune cells and is activated particularly by capric (C10), undecanoic (C11) and lauric (C12) acids [Ichimura et al. 2009]. There are at present no data to support a role for this receptor in nutrient balance, and pharmacological modulators have not been described. Medium-chain triglycerides and FAs decrease energy intake (probably through actions on hepatic intermediary metabolism) [Ooyama et al. 2009; de Sousa et al. 2006]. However, we cannot assume that this is GPR84 mediated since the effects of medium-chain FAs on immune cell activation are not mediated by GPR84 [Versleijen et al. 2009].
GPR119
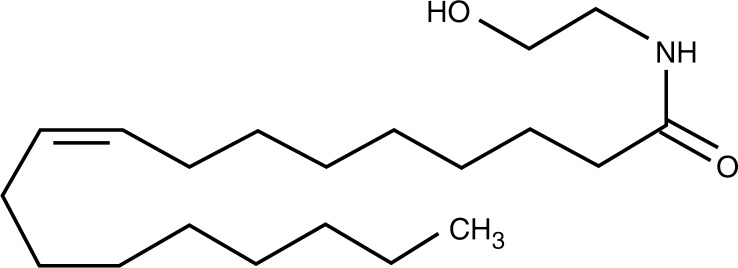
Although not strictly an FFA receptor, GPR119 is considered here because its natural ligands are FA derivatives and there is a real prospect for receptor agonists to be used for diabetes treatment [Overton et al. 2008]. GPR119 is highly expressed on the pancreatic β cell where agonists act as insulin secretagogues. The natural ligand is OEA (Figure 1) [Overton et al. 2006], an analogue of the cannabinoid anandamide. OEA is an activator of peroxisome proliferator-activated receptor (PPAR)-α, and systemic administration of OEA decreases feeding and body weight. Lysophosphatidylcholine (LPC) is also an endogenous ligand. Both OEA and LPC are produced by cell surface phospholipases in a variety of tissues including the small intestine.
Figure 1.
Oleoylethanolamide.
Chu and colleagues showed that the GPR119-specific agonist AR231453 increased cellular cAMP and insulin release in HIT-T15 cells and in rodent islets [Chu et al. 2007]. The agonist was inactive when the GPR119 gene was silenced. AR231453 increased insulin release and improved glucose tolerance in wild-type mice and diabetic KK/A(y) mice. In a further study [Chu et al. 2010], they screened a series of lipid amides for the ability to activate GPR119 in cells transfected to express the receptor. N-oleoyldopamine (OLDA), an endovanilloid, proved to be as potent an activator as either OEA or AR231453. Furthermore, OLDA enhanced secretion in insulin-producing cells and also improved glucose tolerance when administered orally to mice. There was no response when OLDA was administered to GPR119-deficient mice. Synthetic GPR119 agonists share a common mechanism of action to OEA but some agents may additionally activate other pathways in insulin-secreting cells [Ning et al. 2008].
GPR119 is also expressed in entero-endocrine cells, which secrete glucose-dependent insulinotropic peptide (GIP) or GLP-1, and OEA signalling through GPR119 leads to increased GLP-1 secretion from these cells [Lauffer et al. 2009]. Administration of AR231453 increases secretion of both incretins, contributing to improved glucose tolerance in mice [Chu et al. 2010, 2008]. Release of GLP-1 and improved glucose tolerance was further enhanced when AR231453 was administered along with the dipeptidyl peptidase-IV inhibitor sitagliptin [Chu et al. 2010]. While GPR119 is the mediator for the insulin secretory and incretin effects of OEA, recent experiments with GPR119 knockout mice suggest that the receptor does not mediate the appetite-suppressing effects [Lan et al. 2009]. GPR119 agonists represent an exciting new class of agents with considerable potential to improve glucose tolerance. Several agents of this class are now in phase 1 clinical trials [Jones et al. 2009; Shah, 2009; Semple et al. 2008]. Although GPR119 may not mediate the central effects of lipid mediators on energy homeostasis, it may well be that the orally active agonists help preserve β cells, either directly, or indirectly by increasing levels of GIP and GLP-1 [Overton et al. 2008].
GPR120
This receptor shares only 10% sequence homology with FFAR1, and is optimally activated by saturated FAs of chain length 14–18 and unsaturated FAs of chain length 16–22. GPR120 is expressed in monocytes, adipocytes and various cells of the gastrointestinal tract. The role of the receptor in immune cells is not known. In our hands, GPR120 expression decreases on monocyte activation while expression of the oxidized FA receptor GPR132 (described in the following) increases. GPR120 is expressed during adipocyte maturation and may have a role in regulating adipogenesis [Gotoh et al. 2007]. It certainly seems logical for a long-chain FA receptor regulating differentiation of cells whose function is to store energy as fat. FFAR1 is not expressed in adipocytes [Gotoh et al. 2007]. The major site of GPR120 expression is in the gastrointestinal tract where it is involved in FA sensing at various locations: work in rats has demonstrated a high level of expression in the taste buds of the tongue [Matsumura et al. 2009, 2007]. GPR120 expression has also been reported in pancreatic β cells, although this has not been a consistent finding [Morgan and Dhayal, 2009]. There is expression in the entero-endocrine cells (K cells) of the duodenum and jejunum where GPR120 is thought to regulate secretion of cholecystokinin (CCK) [Tanaka et al. 2008a] and GIP [Parker et al. 2009; Tanaka et al. 2008b]. In these cells, it is colocalized with FFAR1 and GPR119. GPR120 is the dominant long-chain FA receptor in the large intestine and regulates GLP-1 secretion from the L cells [Hirasawa et al. 2005; Katsuma et al. 2005]. We do not know what the most important natural ligands for GPR120 are but omega-3 FA appear to be active in this regard: α-linolenic acid is a potent stimulator of GLP-1 secretion [Tanaka et al. 2008b], and colon-targeted eicosapentaenoic acid and docosahexaenoic acid have also been shown to be potent GLP-1 secretagogues [Morishita et al. 2008]. The small molecule agonist G9508 activates GPR120 [Moore et al. 2009; Briscoe et al. 2006]. Other agonists have been identified from the screening of PPAR-γ activators [Suzuki et al. 2008] and natural compounds including grifolic acid and its derivatives [Hara et al. 2009]. There are no compounds that have been shown to be of value as human pharmaceuticals at this stage.
GPR132 (G2A)
GPR132 is a stress-inducible receptor, expression of which is increased in lymphocytes on exposure of cells to DNA-damaging agents. Expression of the receptor is associated with a block in cell-cycle progression in the G2/M phase of the cycle (G2A1/4G2 Accumulation) [Obinata et al. 2009]. Activation of the receptor leads to increased intracellular calcium and activation of kinases, including mitogen-activated protein kinases (particularly JNK). There has been considerable controversy regarding its pathophysiological role and its natural ligands. The highest expression is in leucocytes followed by the spleen, lung and heart. GPR132 is highly expressed in the macrophages of atherosclerotic plaque. Strong evidence has emerged that GPR132 functions as a receptor for oxidized FAs with 9-hydroxyoctadecadienoic acid (9-HODE) being the most potent ligand [Obinata et al. 2009, 2005; Yin et al. 2009]. 9-HODE is an oxidized derivative of linoleic acid produced nonenzymatically in advanced atherosclerotic lesions. Regulation of chemotaxis and immune function through GPR132 is important in a variety of disease states caused by inflammation, infection or autoimmunity [Yang et al. 2005]. A recent study provides evidence that 9-HODE is a pro-inflammatory mediator in the skin and that this action is mediated through GPR132 [Hattori et al. 2008]. The latter was expressed in normal human epidermal keratinocytes and in a keratinocyte line. Culture of the cells with 9-HODE led to increased intracellular calcium with inhibition of cell proliferation and increased secretion of inflammatory mediators.
GPR132 is expressed in the macrophages of atherosclerotic plaques, both in humans and experimental rabbits [Rikitake et al. 2002]. Genetic manipulation studies in mice have shed light on how GPR132 may contribute to the process of atherosclerosis. Bolick and colleagues used GPR132 knockout mice (G2A−/−) to study the interaction between monocytes and endothelial cells [Bolick et al. 2007]. The endothelial cells from GPR132-deficient mice showed increased intercellular adhesion molecule-1 and E-selectin expression, as well as secreting more interleukin (IL)-6 and monocyte chemotactic protein-1 compared with cells from control animals. Restoring GPR132 expression in knockout animals reversed the above changes and decreased monocyte interaction with endothelial cells. The conclusion from this study, which used animals with early atheroma, was that GPR132 was protective against atherosclerosis. A more recent study considered the effect of GPR132 deficiency in macrophages from apoE−/− mice [Bolick et al. 2009]. Macrophages from G2−/− A animals had lower levels of apoptosis, a more pro-inflammatory M1 phenotype and decreased phagocytic activity. GPR132 deficiency was associated with an increase in size of the atherosclerotic lesions in the aortic root and increased numbers of infiltrating macrophages (presumably due to attenuated apoptosis).
Parks and colleagues used LDLR−/− mice fed a high-fat diet and demonstrated that loss of GPR132 was associated with increased macrophage accumulation, probably due to inhibition of apoptosis [Parks et al. 2005]. However, GPR132 deficiency attenuated lesion progression and was associated with lower numbers of intimal macrophages, and higher levels of high-density lipoprotein (HDL) cholesterol. Thus GPR132, in this animal model, appeared to have a detrimental effect on atherosclerosis. In a very recent study [Parks et al. 2009], the pro-atherogenic effect of GPR132 was confirmed with G2A−/− animals secreting more Apo1 and ApoE in their HDL fractions compared with control animals having normal GPR132. The study suggested that alterations in lipid status rather than monocyte chemotactic function were responsible for the apparent protective effect of GPR132 deficiency. The effect of GPR132 on hepatic lipid metabolism is consistent with another recent study, which showed that GPR132 activation may help to protect against gallstones [Johnson et al. 2008]. There are no published studies detailing pharmacological manipulation of GPR132 activity. Thus, studies to date confirm a role for GPR132 in atherosclerosis but beneficial pharmacological modulation of receptor activity will only be possible when the precise role of the receptor is better understood.
Conclusion
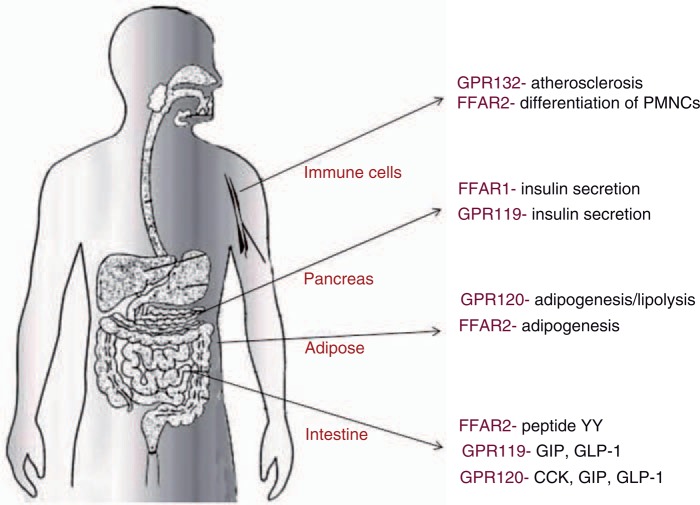
Nutrient-sensing GPRs are critical in the body's regulation of food intake and energy balance. Receptors for FFAs have emerged as an important component of this apparatus and evidence is emerging that pharmacological manipulation of these pathways could contribute to the management of type 2 diabetes (Figure 2). The controversy of whether FFAR1 agonists or antagonists might be beneficial is being resolved. Small molecule agonists may be useful as insulin secretagogues with direct actions on the β cell as well as indirect actions increasing secretion of incretins. Activation of FFAR2 decreases food intake by increasing peptide YY secretion. Short-chain FFAs have also been shown to increase adipogenesis, decrease lipolysis and increase leptin secretion through actions on short-chain FA receptors on adipocytes. A clearer understanding of the molecular species and mechanisms involved in the protective effects of short-chain FAs will lead to better probiotic, nutritional and pharmacological interventions. GPR119 agonists act as insulin secretagogues and several agents in this class have entered clinical trials. Their action is both direct on the β cell and indirectly by increasing GIP and GLP-1 secretion. In the latter, they synergize with dipeptidyl peptidase-IV inhibitors. GPR120 has roles in taste, incretin secretion and adipogenesis. Small molecule agonists have been identified. GPR132 is a receptor for oxidized lipids, which is expressed in the macrophages of advanced atherosclerotic lesions. It can be viewed as a sensor of lipid overload and oxidative stress, both of which are features of the diabetic state. While there are many uncertainties about the role of this receptor, it may well emerge as a therapeutic target in high-risk atherosclerosis-prone patients. In summary, FAs, through a series of recently described receptors, have important roles regulating insulin secretion, nutrient intake (through effects on incretins, CCK, peptide YY and leptin), as well as lipid storage and flux in adipose tissue. Targeting these signalling functions with small molecule agonists and antagonists is beginning to show considerable promise in helping us to prevent the rising burden of diabetes and its complications.
Figure 2.
Effects mediated through free fatty acid receptors. CCK, cholecystokinin; FFAR1, free fatty acid receptor-1; FFAR2, free fatty acid receptor-2; GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide-1; PMNCs, peripheral blood mononuclear cells.
Funding
This article received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- Batterham R.L., Cohen M.A., Ellis S.M., Le Roux C.W., Withers D.J., Frost G.S., et al. (2003) Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 349: 941–948 [DOI] [PubMed] [Google Scholar]
- Bergman R.N., Ader M. (2000) Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 11: 351–356 [DOI] [PubMed] [Google Scholar]
- Bharate S.B., Nemmani K.V., Vishwakarma R.A. (2009) Progress in the discovery and development of small-molecule modulators of G-protein-coupled receptor 40 (GPR40/FFA1/FFAR1): An emerging target for type 2 diabetes. Expert Opin Ther Pat 19: 237–264 [DOI] [PubMed] [Google Scholar]
- Bharate S.B., Rodge A., Joshi R.K., Kaur J., Srinivasan S., Kumar S.S., et al. (2008) Discovery of diacylphloroglucinols as a new class of GPR40 (FFAR1) agonists. Bioorg Med Chem Lett 18: 6357–6361 [DOI] [PubMed] [Google Scholar]
- Bolick D.T., Skaflen M.D., Johnson L.E., Kwon S.C., Howatt D., Daugherty A., et al. (2009) G2 A deficiency in mice promotes macrophage activation and atherosclerosis. Circ Res 104: 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolick D.T., Whetzel A.M., Skaflen M., Deem T.L., Lee J., Hedrick C.C. (2007) Absence of the G protein-coupled receptor G2 A in mice promotes monocyte/endothelial interactions in aorta. Circ Res 100: 572–580 [DOI] [PubMed] [Google Scholar]
- Briscoe C.P., Peat A.J., McKeown S.C., Corbett D.F., Goetz A.S., Littleton T.R., et al. (2006) Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: Identification of agonist and antagonist small molecules. Br J Pharmacol 148: 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe C.P., Tadayyon M., Andrews J.L., Benson W.G., Chambers J.K., Eilert M.M., et al. (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311 [DOI] [PubMed] [Google Scholar]
- Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., et al. (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319 [DOI] [PubMed] [Google Scholar]
- Brownlie R., Mayers R.M., Pierce J.A., Marley A.E., Smith D.M. (2008) The long-chain fatty acid receptor, GPR40, and glucolipotoxicity: Investigations using GPR40-knockout mice. Biochem Soc Trans 36: 950–954 [DOI] [PubMed] [Google Scholar]
- Cani P.D., Lecourt E., Dewulf E.M., Sohet F.M., Pachikian B.D., Naslain D., et al. (2009) Gut micro-biota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 90: 1236–1243 [DOI] [PubMed] [Google Scholar]
- Cao H., Gerhold K., Mayers J.R., Wiest M.M., Watkins S.M., Hotamisligil G.S. (2008) Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134: 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbut C., Ferrier L., Roze C., Anini Y., Blottiere H., Lecannu G., et al. (1998) Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol 275: G1415–G1422 [DOI] [PubMed] [Google Scholar]
- Christiansen E., Urban C., Merten N., Liebscher K., Karlsen K.K., Hamacher A., et al. (2008) Discovery of potent and selective agonists for the free fatty acid receptor 1 (FFA(1)/GPR40), a potential target for the treatment of type II diabetes. J Med Chem 51: 7061–7064 [DOI] [PubMed] [Google Scholar]
- Chu Z.L., Carroll C., Alfonso J., Gutierrez V., He H., Lucman A., et al. (2008) A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 149: 2038–2047 [DOI] [PubMed] [Google Scholar]
- Chu Z.L., Carroll C., Chen R., Alfonso J., Gutierrez V., He H., et al. (2010) N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol Endocrinol 24: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z.L., Jones R.M., He H., Carroll C., Gutierrez V., Lucman A., et al. (2007) A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 148: 2601–2609 [DOI] [PubMed] [Google Scholar]
- Covington D.K., Briscoe C.A., Brown A.J., Jayawickreme C.K. (2006) The G-protein-coupled receptor 40 family (GPR40–GPR43) and its role in nutrient sensing. Biochem Soc Trans 34: 770–773 [DOI] [PubMed] [Google Scholar]
- de Sousa J.U.L., Arnold M., Langhans W., Geary N., Leonhardt M. (2006) Caprylic acid infusion acts in the liver to decrease food intake in rats. Physiol Behav 87: 388–395 [DOI] [PubMed] [Google Scholar]
- Djousse L., Lee I.M., Buring J.E., Gaziano J.M., Djousse L., Lee I.M., et al. (2009) Alcohol consumption and risk of cardiovascular disease and death in women: Potential mediating mechanisms. Circulation 120: 237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgren E., Olde B., Meidute-Abaraviciene S., Winzell M.S., Ahren B., Salehi A., et al. (2007) GPR40 is expressed in glucagon producing cells and affects glucagon secretion. Biochem Biophys Res Commun 354: 240–245 [DOI] [PubMed] [Google Scholar]
- Freeland K.R., Wilson C., Wolever T.M. (2010) Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr 103: 82–90 [DOI] [PubMed] [Google Scholar]
- Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., et al. (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58: 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Li X., Weiszmann J., Wang P., Baribault H., Chen J.L., et al. (2008) Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149: 4519–4526 [DOI] [PubMed] [Google Scholar]
- Gotoh C., Hong Y.H., Iga T., Hishikawa D., Suzuki Y., Song S.H., et al. (2007) The regulation of adipogenesis through GPR120. Biochem Biophys Res Commun 354: 591–597 [DOI] [PubMed] [Google Scholar]
- Hafko R., Orecna M., Bacova Z., Kollarikova G., Lacik I., Strbak V., et al. (2009) Mechanism of etha-nol-induced insulin secretion from INS-1 and INS-1E tumor cell lines. Cell Physiol Biochem 24: 441–450 [DOI] [PubMed] [Google Scholar]
- Hara T., Hirasawa A., Sun Q., Sadakane K., Itsubo C., Iga T., et al. (2009) Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch Pharmacol 380: 247–255 [DOI] [PubMed] [Google Scholar]
- Hattori T., Obinata H., Ogawa A., Kishi M., Tatei K., Ishikawa O., et al. (2008) G2 A plays proinflammatory roles in human keratinocytes under oxidative stress as a receptor for 9-hydroxyoctadecadienoic acid. J Invest Dermatol 128: 1123–1133 [DOI] [PubMed] [Google Scholar]
- Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., et al. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94 [DOI] [PubMed] [Google Scholar]
- Hong Y.H., Nishimura Y., Hishikawa D., Tsuzuki H., Miyahara H., Gotoh C., et al. (2005) Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146: 5092–5099 [DOI] [PubMed] [Google Scholar]
- Ichimura A., Hirasawa A., Hara T., Tsujimoto G. (2009) Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat 89: 82–88 [DOI] [PubMed] [Google Scholar]
- Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., et al. (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176 [DOI] [PubMed] [Google Scholar]
- Johnson L.E., Elias M.S., Bolick D.T., Skaflen M.D., Green R.M., Hedrick C.C. (2008) The G protein-coupled receptor G2A: Involvement in hepatic lipid metabolism and gallstone formation in mice. Hepatology 48: 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Leonard J.N., Buzard D.J., Lehmann J. (2009) GPR119 agonists for the treatment of type 2 diabetes. Expert Opin Ther Pat 19: 1339–1359 [DOI] [PubMed] [Google Scholar]
- Karaki S., Mitsui R., Hayashi H., Kato I., Sugiya H., Iwanaga T., et al. (2006) Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res 324: 353–360 [DOI] [PubMed] [Google Scholar]
- Katsuma S., Hatae N., Yano T., Ruike Y., Kimura M., Hirasawa A., et al. (2005) Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem 280: 19507–19515 [DOI] [PubMed] [Google Scholar]
- Kebede M., Alquier T., Latour M.G., Semache M., Tremblay C., Poitout V., et al. (2008) The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 57: 2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.N., Choi H.Y., Kim Y.K. (2009) Regulation of adipocyte differentiation by histone deacetylase inhibitors. Arch Pharm Res 32: 535–541 [DOI] [PubMed] [Google Scholar]
- Kotarsky K., Nilsson N.E., Flodgren E., Owman C., Olde B. (2003) A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun 301: 406–410 [DOI] [PubMed] [Google Scholar]
- Lan H., Vassileva G., Corona A., Liu L., Baker H., Golovko A., et al. (2009) GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol 201: 219–230 [DOI] [PubMed] [Google Scholar]
- Lauffer L.M., Iakoubov R., Brubaker P.L. (2009) GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 58: 1058–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Schwandner R., Swaminath G., Weiszmann J., Cardozo M., Greenberg J., et al. (2008) Identification and functional characterization of allo-steric agonists for the G protein-coupled receptor FFA2. Mol Pharmacol 74: 1599–1609 [DOI] [PubMed] [Google Scholar]
- Li L., Lili R., Hui Q., Min W., Xue W., Xin S., et al. (2008) Combination of GLP-1 and sodium butyrate promote differentiation of pancreatic progenitor cells into insulin-producing cells. Tissue Cell 40: 437–445 [DOI] [PubMed] [Google Scholar]
- Ma D., Lu L., Boneva N.B., Warashina S., Kaplamadzhiev D.B., Mori Y., et al. (2008) Expression of free fatty acid receptor GPR40 in the neurogenic niche of adult monkey hippocampus. Hippocampus 18: 326–333 [DOI] [PubMed] [Google Scholar]
- Matsumura S., Eguchi A., Mizushige T., Kitabayashi N., Tsuzuki S., Inoue K., et al. (2009) Colocalization of GPR120 with phospholipase-Cbeta2 and alpha-gustducin in the taste bud cells in mice. Neurosci Lett 450: 186–190 [DOI] [PubMed] [Google Scholar]
- Matsumura S., Mizushige T., Yoneda T., Iwanaga T., Tsuzuki S., Inoue K., et al. (2007) GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res 28: 49–55 [DOI] [PubMed] [Google Scholar]
- Millar R.P., Newton C.L. (2010) The year in G protein-coupled receptor research. Mol Endocrinol 24: 261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K., Zhang Q., Murgolo N., Hosted T, Duffy R. (2009) Cloning, expression, and pharmacological characterization of the GPR120 free fatty acid receptor from cynomolgus monkey: Comparison with human GPR120 splice variants. Comp Biochem Physiol B Biochem Mol Biol 154: 419–426 [DOI] [PubMed] [Google Scholar]
- Morgan N.G., Dhayal S. (2009) G-protein coupled receptors mediating long chain fatty acid signalling in the pancreatic beta-cell. Biochem Pharmacol 78: 1419–1427 [DOI] [PubMed] [Google Scholar]
- Morishita M., Tanaka T., Shida T, Takayama K. (2008) Usefulness of colon targeted DHA and EPA as novel diabetes medications that promote intrinsic GLP-1 secretion. J Control Release 132: 99–104 [DOI] [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M. (2010) Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: Mechanisms and implications for metabolic disorders. Curr Opin Lipidol 21: 76–83 [DOI] [PubMed] [Google Scholar]
- Nagasumi K., Esaki R., Iwachidow K., Yasuhara Y., Ogi K., Tanaka H., et al. (2009) Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 58: 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y., O'Neill K., Lan H., Pang L., Shan L.X., Hawes B.E., et al. (2008) Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol 155: 1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinata H., Hattori T., Nakane S., Tatei K., Izumi T. (2005) Identification of 9—hydroxyoctadeca-dienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem 280: 40676–40683 [DOI] [PubMed] [Google Scholar]
- Obinata H., Izumi T., Obinata H., Izumi T. (2009) G2 A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat 89: 66–72 [DOI] [PubMed] [Google Scholar]
- Ooyama K., Kojima K., Aoyama T, Takeuchi H. (2009) Decrease of food intake in rats after ingestion of medium-chain triacylglycerol. J Nutr Sci Vitaminol 55: 423–427 [DOI] [PubMed] [Google Scholar]
- Overton H.A., Babbs A.J., Doel S.M., Fyfe M.C., Gardner L.S., Griffin G., et al. (2006) Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 3: 167–175 [DOI] [PubMed] [Google Scholar]
- Overton H.A., Fyfe M.C., Reynet C. (2008) GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol 153(Suppl 1): S76–S81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H.E., Habib A.M., Rogers G.J., Gribble F.M., Reimann F. (2009) Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks B.W., Gambill G.P., Lusis A.J., Kabarowski J.H.S. (2005) Loss of G2 A promotes macrophage accumulation in atherosclerotic lesions of low density lipoprotein receptor-deficient mice. J Lipid Res 46: 1405–1415 [DOI] [PubMed] [Google Scholar]
- Parks B.W., Srivastava R., Yu S., Kabarowski J.H. (2009) ApoE-dependent modulation of HDL and atherosclerosis by G2A in LDL receptor-deficient mice independent of bone marrow-derived cells. Arterioscler Thromb Vasc Biol 29: 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Player M.S., Mainous 3rd A.G., King D.E., Diaz V.A., Everett C.J. (2010) Moderate alcohol intake is associated with decreased risk of insulin resistance among individuals with vitamin D insufficiency. Nutrition 26: 100–105 [DOI] [PubMed] [Google Scholar]
- Priebe M.G., Wang H., Weening D., Schepers M., Preston T., Vonk R.J., et al. (2010) Factors related to colonic fermentation of nondigestible carbohydrates of a previous evening meal increase tissue glucose uptake and moderate glucose-associated inflammation. Am J Clin Nutr 91: 90–97 [DOI] [PubMed] [Google Scholar]
- Queenan K.M., Stewart M.L., Smith K.N., Thomas W., Fulcher R.G., Slavin J.L., et al. (2007) Concentrated oat beta-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterol-emic adults in a randomized controlled trial. Nutr J 6: 6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Yan L., Shang C.Z., Cao J., Lu L.H., Min J., et al. (2010) Effects of sodium butyrate on the differentiation of pancreatic and hepatic progenitor cells from mouse embryonic stem cells. J Cell Biochem 109: 236–244 [DOI] [PubMed] [Google Scholar]
- Rikitake Y., Hirata K.-i., Yamashita T., Iwai K., Kobayashi S., Itoh H., et al. (2002) Expression of G2A, a receptor for lysophosphatidylcholine, by macrophages in murine, rabbit, and human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22: 2049–2053 [DOI] [PubMed] [Google Scholar]
- Semple G., Fioravanti B., Pereira G., Calderon I., Uy J., Choi K., et al. (2008) Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J Med Chem 51: 5172–5175 [DOI] [PubMed] [Google Scholar]
- Shah U. (2009) GPR119 agonists: A promising new approach for the treatment of type 2 diabetes and related metabolic disorders. Curr Opin Drug Discov Devel 12: 519–532 [PubMed] [Google Scholar]
- Steneberg P., Rubins N., Bartoov-Shifman R., Walker M.D., Edlund H. (2005) The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 1: 245–258 [DOI] [PubMed] [Google Scholar]
- Stoddart L.A., Smith N.J., Milligan G. (2008) International Union of Pharmacology LXXI. Free fatty acid receptors FFA1, −2, and −3: Pharmacology and pathophysiological functions. Pharmacol Rev 60: 405–417 [DOI] [PubMed] [Google Scholar]
- Sum C.S., Tikhonova I.G., Costanzi S., Gershengorn M.C. (2009) Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation. J Biol Chem 284: 3529–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Igari S., Hirasawa A., Hata M., Ishiguro M., Fujieda H., et al. (2008) Identification of G protein-coupled receptor 120—selective agonists derived from PPARgamma agonists. J Med Chem 51: 7640–7644 [DOI] [PubMed] [Google Scholar]
- Tanaka T., Katsuma S., Adachi T., Koshimizu T.A., Hirasawa A., Tsujimoto G., et al. (2008a) Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol 377: 523–527 [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yano T., Adachi T., Koshimizu T.A., Hirasawa A., Tsujimoto G., et al. (2008b) Cloning and characterization of the rat free fatty acid receptor GPR120: In vivo effect of the natural ligand on GLP-1 secretion and proliferation of pancreatic beta cells. Naunyn Schmiedebergs Arch Pharmacol 377: 515–522 [DOI] [PubMed] [Google Scholar]
- Tarini J., Wolever T.M. (2010) The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab 35: 9–16 [DOI] [PubMed] [Google Scholar]
- Versleijen M.W., van Esterik J.C., Roelofs H.M., van Emst-de Vries S.E., Willems P.H., Wanten G.J., et al. (2009) Parenteral medium-chain triglyceride-induced neutrophil activation is not mediated by a pertussis toxin sensitive receptor. Clin Nutr 28: 59–64 [DOI] [PubMed] [Google Scholar]
- Wellendorph P., Johansen L.D., Brauner-Osborne H. (2009) Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol 76: 453–465 [DOI] [PubMed] [Google Scholar]
- Xiong Y., Miyamoto N., Shibata K., Valasek M.A., Motoike T., Kedzierski R.M., et al. (2004) Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A 101: 1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.V., Radu C.G., Wang L., Riedinger M., Witte O.N. (2005) Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2 A. Blood 105: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Yin H., Chu A., Li W., Wang B., Shelton F., Otero F., et al. (2009) Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 284: 12328–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]