Abstract
Hypercalcaemia is commonly seen in the context of parathyroid dysfunction and malignancy and, when severe, can precipitate Life-threatening sequelae. The differential of hypercalcaemia is broad and can be categorized based on parathyroid hormone (PTH) Levels. The acute management of severe hypercalcaemia is discussed along with a brief review of therapeutic advances in the field.
Keywords: hypercalcaemia, hypercalcaemic crisis, hyperparathyroidism, malignancy-associated hypercalcaemia
Introduction
Hypercalcaemia (serum calcium >2.6mmol/l [>10.5 mg/dl]) affects about 0.5% of hospitalized patients and is usually well tolerated if calcium levels are <3.0 mmol/l (<12 mg/dl). Calcium levels above this threshold are associated with increasingly severe volume contraction, neurological, cardiac and gastrointestinal dysfunction, and require urgent treatment to prevent life-threatening consequences.
Pathophysiology
Calcium, phosphate and magnesium are the major divalent cations in the body [Carroll and Matfin, 2010; Matfin, 2009; Selby, 2002]. Calcium is ingested in the diet, absorbed from the intestine, filtered in the glomerulus of the kidney, reabsorbed in the renal tubules and eliminated in the urine. Approximately 99% of total body calcium is found in bone. Most of the remainder is located in the intracellular compartment with only a small amount present in extracellular fluid (ECF). Calcium homeostasis is directly or indirectly regulated by vitamin D and parathyroid hormone (PTH) [Carroll and Matfin, 2010; Matfin, 2009; Selby, 2002].
PTH is secreted by the parathyroid glands. A unique calcium receptor on the parathyroid cell membrane (extracellular calcium-sensing receptor [CsR]) responds rapidly to changes in plasma calcium levels [Ayuk et al. 2010]. When the plasma calcium level is high, appropriate inhibition of PTH release should occur, whilst a decrease in plasma calcium prompts a rapid adaptive increase in PTH release. The main function of PTH is to maintain the calcium concentration of the ECF by promoting the release of calcium from bone, increasing the activation of vitamin D and stimulating calcium conservation by the kidney while increasing phosphate excretion. The major action of the activated form of vitamin D, also called calcitriol (1,25-dihydroxy-vitamin D), is to increase the absorption of calcium from the intestine. Calcitriol also sensitizes bone to the resorptive actions of PTH.
At least one of the following mechanisms is involved in the pathophysiology of hypercalcaemia: (1) increased intestinal calcium absorption; (2) increased bone resorption; and (3) increased renal calcium reabsorption or decreased calcium excretion. Hypercalcaemia predominantly results from increased mobilization of calcium from the bone via the final common pathway of activation of RANK (receptor activator of nuclear factor-kappa B) receptors on the surface of osteoclasts by RANK-ligand (RANKL) derived from osteo-blasts [Tanaka et al. 2005]. Increased delivery of calcium to the nephron results in nephrogenic diabetes insipidus with impaired urine concentrating ability, due to both effects on vasopressin binding with aquaporin downregulation and renal interstitial sodium concentration [Shoback et al. 2007]. In addition, volume depletion can result from associated vomiting. The resultant intravascular volume contraction and subsequent reduction in glomerular filtration rate (GFR), severely limits the ability of the kidneys to clear calcium. If continued calcium mobilization occurs, hypercalcaemia can rapidly escalate. This chain of events understates the extreme importance of volume resuscitation in the management of hypercalcaemia.
Aetiology
The causes of hypercalcaemia can be conveniently divided into those associated with an elevated or inappropriately normal PTH level, and those where PTH output is appropriately suppressed (Table 1). Notable exceptions to this paradigm include hypercalcaemia due to familial hypocalciuric hypercalcaemia (FHH), where PTH output is appropriate to the level of ambient calcium sensed by the abnormal CsR, thiazide diuretics and lithium. In an ambulatory population, primary hyperparathyroidism (PHPT) accounts for the vast majority of detected hypercalcaemia (>90%) [Joshie et al. 2009]. Inappropriate autonomous PTH secretion is found in the context of parathyroid adenomas which may be solitary or multiple. Parathyroid adenomas are most commonly sporadic but may be part of an endocrine neoplastic syndrome such as multiple endocrine neoplasia (MEN)-1 or MEN-2a, especially if numerous or found in the young. Parathyroid hyperplasia without an obvious physiological stimulus can also occur, and usually involves all of the glands. Rarely, inappropriate PTH secretion may result from a parathyroid carcinoma. Secondary hyperparathyroidism (SHPT) is an appropriate physiological adaptation to many situations in which hypocalcaemia is seen, including vitamin D deficiency, advanced chronic kidney disease (CKD) and gastrointestinal malabsorption of calcium [Carroll and Matfin, 2010; Selby, 2002]. However, parathyroid autonomy often develops if the stimulus persists, and when hypercalcaemia results the condition is redefined as tertiary hyperparathyroidism (THPT). Vitamin D deficiency is typically severe and longstanding before THPT develops. Lithium therapy produces biochemistry that mimics FHH as the intracellular calcium concentration threshold at which PTH continues to be produced and secreted is raised, whilst hypocalciuria is also seen.
Table 1.
Aetiology of hypercalcaemia.
| Elevated or inappropriately normal PTH | Suppressed PTH |
|---|---|
| Primary hyperparathyroidism (PHPT) | Malignancy |
| Solitary adenoma | Humoral mediators (e.g. PTH-related protein) |
| Multiple adenoma | Vitamin D mediated |
| Parathyroid hyperplasia | Multiple myeloma |
| Parathyroid carcinoma | Lytic bone metastases |
| Tertiary hyperparathyroidism (THPT) | |
| Advanced chronic kidney disease (CKD) | Drug induced |
| Severe vitamin D deficiency | Calcium supplementation |
| Malabsorption of calcium (e.g. celiac disease) | Milk-alkali syndrome |
| Vitamin D intoxication | |
| Vitamin A intoxication | |
| Thiazide diuretics | |
| Lithium therapy | |
| Miscellaneous | |
| Familial hypocalciuric hypercalcaemia (FHH) | Endocrinopathies |
| Thyrotoxicosis | |
| Addison's disease | |
| Phaeochromocytoma | |
| VIPoma | |
| Immobilization | |
| Granulomatous disorders including sarcoidosis |
PTH, parathyroid hormone.
Malignancy related hypercalcaemia is the most common cause of inpatient hypercalcaemic crises (>50%) and complicates 10–30% of malignancies. Hypercalcaemia secondary to malignancy usually presents in the context of advanced clinically obvious disease. It results either from humoral mediated bone resorption, increased calcitriol production due to increased 1α-hydroxylase activity in some lymphomas or direct destruction of bone either in myeloma or lytic metastatic disease. The majority of humoral hypercalcaemia of malignancy (>80%) is induced by PTH-related protein (PTHrP), a peptide with significant homology to PTH [Broadus et al. 1988]. PTHrP induces bone resorption by binding to PTH receptor type 1 and also induces hypercalciuria and phosphaturia. Increased expression of 1α-hydroxylase by lymphoproliferative tissues including lymphoma occasionally results in clinically significant hyper-calcaemia as a result of significantly increased activation of vitamin D [Hewison et al. 2007]. Many solid tumours are associated with hypercalcaemia and include squamous cell carcinomas of the lung, head, neck and oesophagus, renal cell carcinoma and breast carcinoma. Humoral-mediated bone resorption accounts for the majority of hypercalcaemia in these malignancies even when lytic metastatic bone disease is present. Finally, haematological malignancies such as multiple myeloma can be associated with hypercalcaemia via locally produced osteolytic peptides (i.e. paracrine effects).
A number of administered drugs can cause hypercalcaemia. Thiazide diuretics reduce renal calcium excretion and mild hypercalcaemia is frequently seen. The effect of lithium is discussed above. Calcium supplementation rarely causes hypercalcaemia if normal physiological mechanisms of calcium regulation are intact. In milkalkali syndrome, a high intake of milk or calcium carbonate (used to treat dyspepsia or more commonly now osteoporosis) may lead to hypercalcaemia mediated by the high calcium intake plus metabolic alkalosis, which augments calcium reabsorption in the distal tubule. Hypercalcaemia in the context of vitamin D intoxication is well recognised but rare [Holick, 2007]. Prolonged immobilization may be associated with hypercalcaemia due to a marked increase in bone resorption. Patients with underlying high bone turnover states are at particular risk [Shoback et al. 2007]. Granuloma-associated macrophages occasionally express 1α-hydroxylase with consequent increased active vitamin D levels (i.e. calcitriol), and hypercalcaemia will complicate over 10% of cases of sarcoidosis. Finally, a number of endocrinopathies are associated with hypercalcaemia. Hypercalcaemia in thyrotoxicosis is postulated to be secondary to increased bone resorption [Igbal et al. 2003]. Volume depletion in Addison's disease promotes hypercalcaemia, whilst PTHrP secretion by phaeochromocytomas occasionally results in clinically significant hypercalcaemia. The very rare vasoactive intestinal peptide (VIP)-secreting tumour, VIPoma, is associated with hypercalcaemia possibly through VIP-mediated stimulation of the PTH receptor.
Diagnostic considerations
Severe hypercalcaemia is defined as a serum calcium level of >3.5mmol/l (>14mg/dl) [Stewart, 2005]. Forty percent of total serum calcium is protein bound with the majority bound to albumin, whilst 50% is ionized and active. Albumin levels should therefore be considered when assessing hypercalcaemia, although most laboratories now provide a corrected calcium level. However, in states of acute albumin fluctuations such as sepsis, measurement of ionized calcium (hypercalcaemia >1.4mmol/l [>5.6mg/dl]) may be a more reliable assessment of calcium status.
Severe hypercalcaemia, suspected clinically or detected biochemically, should prompt immediate treatment. The first step in the evaluation of hypercalcaemia is to establish whether the process is PTH dependent. Other tests include measurement of serum creatinine, 25-hydroxyvitamin D, thyroid function, serum electrophoresis and urinary Bence—Jones protein, and bone markers such as alkaline phosphatase as soon as possible to direct specific treatment. PTHrP can be assayed in many laboratories but adds little to the management if malignancy-associated hypercalcaemia is suspected, especially if PTH levels are suppressed. Assessment of 1,25-dihydroxyvitamin D is only required if the patient is on synthetic versions, or has a confirmed or suspected diagnosis of granulomatous disease or lymphoproliferative disorders.
PTH is an unstable peptide and therefore blood samples should be delivered to the laboratory promptly. The ‘intact’ molecule is now assayed routinely and was thought to have eliminated the previous problem of measured bioinactive fragments (especially in patients with impaired renal function, which is common in patients with acute hypercalcaemia). However, it has recently become clear that even the ‘intact’ assay measures large PTH fragments which are biologically inactive and which can also accumulate with impaired renal function. Despite the introduction of new ‘whole’ assays (which includes measurement of the extreme N-terminal amino acids), these have not been shown to offer any unique clinical usefulness compared with the older ‘intact’ assays and therefore these assays continue to be routinely used. It should be remembered that PHPT is a common condition and is therefore occasionally the cause of hypercalcaemia in patients with concurrent cancer [Stewart, 2005].
Other investigations should be directed by the clinical situation and include electrocardiogram (ECG) and imaging tests as required.
Clinical signs and features
The symptoms and signs of hypercalcaemia predominantly relate both to the volume contraction that accompanies this finding, and the neuromuscular dysfunction that occurs. Aside from underlying specific features (e.g. bone pain in metastatic neoplastic disease), the symptoms are the same irrespective of the aetiology, and relate more to the level of hypercalcaemia. Overt symptoms are unlikely to occur if the corrected calcium is <3.0 mmol/l (<12 mg/dl), although a thorough search for symptoms in patients with milder degrees of hypercalcaemia will often elicit abdominal or neuropsychiatric features not necessarily volunteered by the patient. Whilst the differential diagnosis of hypercalcaemia is broad, a finding of calcium levels elevated to the extent that overt symptoms are present nearly always indicates either PHPT/THPT or malignancy. Acute hypercalcaemia strongly favours a diagnosis of neoplastic disease, although sudden volume contraction secondary to diarrhoea, vomiting, surgery or immobilization can dangerously exacerbate pre-existing hypercalcaemia.
As a consequence of hypercalcaemia induced nephrogenic diabetes insipidus, the initial symptoms relate to polyuria and the resultant adaptive increased thirst. Neurological dysfunction, secondary to the central neuronal depressant effect of increased calcium, is prominent and may manifest as confusion, drowsiness, agitation, stupor or coma. Myopathy is occasionally seen. Hypertension as a consequence of calcium-mediated vasoconstriction can occur in chronic disease [Ayuk et al. 2010] but is less likely in the acute volume-contracted state. Bradyarrhythmias or heart block are frequently seen in severe hypercalcaemia, however, and relate to detrimental effects on the cardiac action potential as a consequence of increased extracellular calcium. Gastrointestinal symptoms resulting in part from smooth muscle hypotonicity include constipation, nausea, anorexia, vomiting and abdominal pain, and are often severe. Renal stones and pancreatitis can occur. The term hypercalcaemic crisis is frequently used to describe the severely compromised patient with profound volume depletion, altered sensorium which may be manifest as coma, cardiac decompensation and abdominal pain that may mimic an acute abdomen.
The diagnosis of hypercalcaemic crisis can sometimes be difficult to make clinically when associated with malignancy. This is because the patient may already be debilitated, anorexic, nauseated, constipated, weak or confused, from the underlying malignancy, concurrent medications, complications of chemotherapy or radiotherapy, as well as comorbid disorders. Clinical vigilance is crucial in this setting to prevent unnecessary morbidity and mortality.
Acute intervention
Once severe hypercalcaemia is recognized, the patient should be managed in an appropriate location such as an Acute Medical Unit (AMU), high-dependency area or intensive care unit. As with all acute medical patients, prompt assessment and management of the ABCDEs should occur (airway; breathing; circulation; disability, i.e. conscious level; and examination and evaluation).
The acute management of hypercalcaemia will depend on a number of factors including severity of symptoms, comorbidities that may affect treatment options and the patient's prognosis. In malignancy-related severe hypercalcaemia it may be appropriate to adopt a palliative approach that will emphasize comfort cares and symptom control.
General supportive care
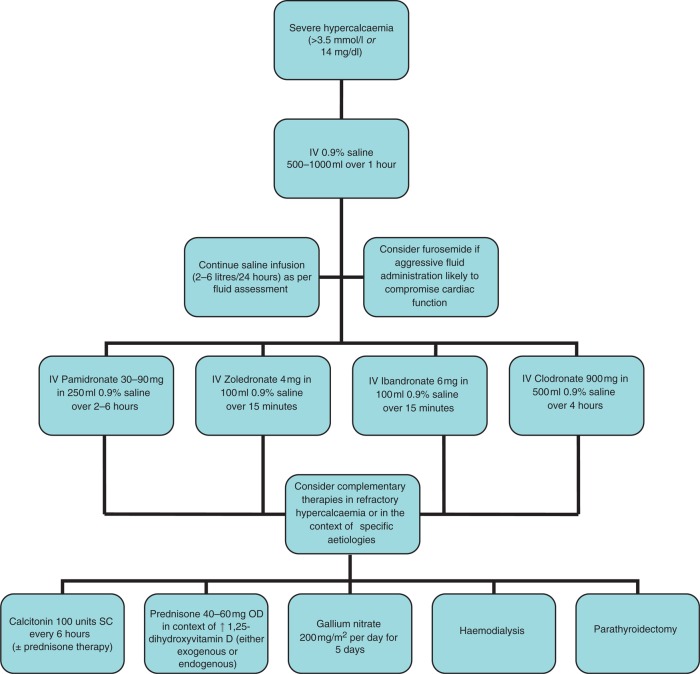
The cornerstone of acute management of hypercalcaemia is fluid resuscitation with correction of the volume state. As described above, hypercalcaemia potently induces a diuresis, and the subsequent volume contraction and reduction in GFR compounds renal calcium clearance. Appropriate fluid administration should depend on an assessment of volume depletion, but in most situations of hypercalcaemic crises 500–1000 ml of 0.9% saline should be given over the first hour, and 2–6 litres over the first 24 hours. This regimen should be continued for several days. Whilst historical approaches advocated the use of loop diuretics (e.g. furosemide) to induce further renal calcium losses (i.e. calciuresis) in the acute management, more recent treatment strategies have warned of the risk of aggravating volume contraction if these medications are used [LeGrand et al. 2008]. However, loop diuretics do have a role in those patients where vigorous fluid resuscitation may provoke cardiogenic fluid overload. In these situations, once euvolaemia has been attained, aggressive fluid administration (i.e. 3 litres 0.9% saline over 24 hours) should be balanced with intravenous (IV) furosemide treatment (20–40 mg every 2–4 hours) to maintain a neutral fluid balance. In most circumstances this can be achieved by inducing a forced diuresis of 2.5 litres over 24 hours and allowing for 500 ml of insensible fluid loss. Potassium and magnesium levels should be cautiously monitored whenever furosemide is used, and replaced appropriately if required. Central line insertion with central venous pressure (CVP) measurements should be considered in patients where external features of fluid state are difficult to assess, or in those who poorly tolerate initial attempts at aggressive fluid administration.
Figure 1.
An algorithm for the acute management of hypercalcaemia.
Any offending agents causing hypercalcaemia should be discontinued as soon as possible. Immobilization promotes osteoclastic bone resorption, hence early ambulation should be encouraged if possible. Dietary calcium restriction is only warranted in patients with vitamin D dependent hypercalcaemia.
In general, for more severe hypercalcaemia, multiple therapies should be started as soon as possible. For example, rapid-acting (hours) approaches including rehydration, forced diuresis and calcitonin, together with the most effective antiresorptive agents (i.e. bisphosphonates) may all be used in the first few days.
Calcium-specific therapy
Irrespective of the aetiology, severe hypercalcaemia predominantly results from increased mobilization of calcium from bone. Bisphosphonate therapy directly addresses this feature by inhibiting osteoclast activity [Reszka, 2010]. Several IV bisphosphonate formulations are licensed for use in Europe: the nitrogen-containing zoledronate, pamidronate and ibandronate; and the nonnitrogen-based clodronate. Zoledronate and pamidronate are licensed in the US. Pooled analyses of two randomized controlled trials (RCTs) suggest a superior effect of zoledronate over pamidronate in the management of malignancy-related severe hypercalcaemia [Major et al. 2001]. However, the significance of these findings has been debated and treatment choice should be based on local availability [Stewart, 2005]. Intravenous bisphosphonates (Table 2) should be administered as soon as severe hypercalcaemia is detected because there is latency until peak effect of 2–5 days. The dose of pamidronate depends on the level of hypercalcaemia (i.e. 30 mg over 2 hours with calcium <3 mmol/l [< 12 mg/dl] or significant renal impairment [as described in the following]; 60 mg over 4 hours with calcium 3.0–3.4mmol/l [12–14mg/dl]; and 90 mg over 6 hours with calcium >3.4mmol/l [>14mg/dl]). Caution should be exercised in renal impairment, and both pamidronate and zoledronate are relatively contraindicated if the GFR is less than 30ml/min/1.73m2 [British National Formulary, 2010]. However, the clinician should assess the perceived benefit of treatment in severe hypercalcaemia, and must also consider that renal impairment may be a consequence of the hypercalcaemia itself. In such cases, renal impairment may be seen to improve as the hypercalcaemia abates. Another potential risk of IV bisphosphonate use is osteonecrosis of the jaw (ONJ) [Bilezikian, 2006]. ONJ is more likely in the setting of malignancy-related hypercalcaemia as these patients may have had previous high-dose IV bisphosphonate treatment of skeletal metastases or multiple myeloma.
Table 2.
Medical therapy in severe hypercalcaemia.
| Drug | Dose | Infusion rate | Notes |
|---|---|---|---|
| Bisphosphonates | |||
| Pamidronate [British National Formulary, 2010] | 90mg | 90 mg in 250 ml 0.9% saline over 2–6 hours | Flu-like symptoms. Effect may last 3–4 weeks [Purohit et al. 1995] |
| Zoledronate [British National Formulary, 2010] | 4mg | 15 minutes in 100 ml 0.9% saline | Flu-like symptoms. Effect may last >12 months |
| Ibandronate [von Moos et al. 2008] | 6mg | 15 minutes in 100 ml 0.9% saline | Flu-like symptoms, nausea, vomiting. Effect may last 2–4 weeks [Ralston et al. 1997] |
| Clodronate [Atula el al. 2006] | 900mg | 4 hours in 500 ml 0.9% saline | Nausea, diarrhoea, skin reactions, bronchospasm. Effect may last 2 weeks [Purohit et al. 1995] |
| Additional options | |||
| Calcitonin [British National Formulary, 2010] | 100 units QDS | IM/SC | Hypersensitivity reaction (Test dose required). Flushing, nausea, vomiting |
| 10 units/kg | 6 hours in 500 ml 0.9% saline | ||
| Prednisone [British National Formulary, 2010] | 40–60 mg OD | Oral | Hyperglycaemia, neutrophilia, Adrenal suppression, Psychosis |
| Hydrocortisone [British National Formulary, 2010] | 100–300 mg/day | IV | |
| Gallium nitrate [Cvitkovic et al. 2006] | 200mg/m2 | 24 hours in 1000 ml 0.9% saline. Repeated 5 consecutive days | Nephrotoxicity seen if more rapid infusion used |
IM, intramuscularly; SC, subcutaneously; IV, intravenously.
Following an infusion, 60–90% of patients will have a marked improvement in calcium levels, and ongoing bisphosphonate treatment should be considered if the hypercalcaemia is likely to recur at a later date (i.e. malignancy-related hypercalcaemia). Owing to the long duration of effect of these agents, second doses are usually not required for some time (at least 7 days) and should be based on the prescribing information for the specific agent used.
Bisphosphonates are not generally necessary in patients with PHPT as they often respond to rehydration. Bisphosphonates should be particularly avoided if parathyroid surgery is imminent as their use can result in profound postoperative hypocalcaemia.
Calcitonin acts by inhibiting osteoclast action and therefore calcium mobilization from bone [Chesnut et al. 2008]. It can be given as a subcutaneous (SC) or intramuscular (IM) injection (100 units every 6 hours [QDS]) or as an IV infusion in emergencies (10 units/kg over 6 hours). A test dose should precede treatment as hypersensitivity reactions are reported. Flushing, nausea and vomiting can occur as milder side effects. Tachyphylaxis is often seen with calcitonin administration and can be minimised by the co-administration of glucocorticoid therapy. Calcitonin administration can result in more rapid decreases in serum calcium, but its role at present is in patients with refractory hypercalcaemia as the absolute effect on serum calcium is small [Stewart, 2005]. In addition to the role described above, glucocorticosteroid therapy may also be useful in cases of hypercalcaemia resulting from increased exogenous (i.e. vitamin D toxicity) or endogenous 1,25-dihydroxyvitamin D (i.e. granulomatous or lymphoproliferative disorders), due to increased metabolism of vitamin D in the context of glucocorticoid therapy [Holick, 2007]. Prednisone used at a dose of 40–60 mg once-daily (OD) or if IV therapy is required, hydrocortisone at a dose of 100–300 mg/day, are commonly used. Glucocorticoids may also be useful in hypercalcaemia associated with malignancies involving cytokine release (e.g. some myelomas).
Less frequently used medications that interfere with osteoclast action and therefore bone resorption include gallium nitrate and mithramycin. Gallium nitrate appears to be at least as effective as pamidronate in achieving reductions in calcium levels, but is limited by the long duration of infusion [Cvitkovic et al. 2006]. Over 24 hours, 200 mg/m2 of body surface area is administered for 5 consecutive days. Significant nephrotoxicity seen with rapid infusions is ameliorated by the longer duration of infusion [Chitambar, 2010]. Mithramycin (Plicamycin) is a tumouricidal antibiotic that has significant renal and hepatic toxicity. It is currently only available for research purposes but has significant hypocalcaemic properties. Phosphate infusion efficiently reduces the serum calcium level within minutes of administration, but the resultant tissue deposition of calcium phosphate makes it generally inappropriate for use in hypercalcaemia. The only indications are for life-threatening cardiac arrhythmias or severe encephalopathy when dialysis is not immediately available.
Haemodialysis against a low or zero calcium dialysate is effective and should be considered in any patient already on dialysis therapy. In refractory hypercalcaemia it should be considered as an additional therapy even in those without underlying renal dysfunction. Urgent parathyroidectomy can be considered in all patients with hypercalcaemic crises as a result of hyperparathyroidism but is more safely performed in the stabilized elective patient. However, initial curative success rates differ only marginally between elective and urgent cases, and long-term outcomes appear similar [Cannon et al. 2010].
Treatment of precipitating illness
After the acute treatment of severe hypercalcaemia (serum calcium can be decreased by 0.7–2.2 mmol/l [3–9mg/dl] within 24–48 hours in most patients), the underlying cause should be established. Further therapy will be determined by the diagnosis.
All patients with underlying parathyroid disease (e.g. parathyroid adenoma, hyperplasia or carcinoma), who present with hypercalcaemic crisis, should be considered for elective parathyroidectomy at the earliest safest opportunity unless there is good reason not to (i.e. comorbidities, poor prognosis, nonlocalizable disease or strong patient preference). Definitive treatment of a primary solid tumour with expression of PTHrP may prevent further hypercalcaemic events. Occasionally patients with nonhumoral hypercalcaemia as a result of lytic bone metastases may find improvement with radiation therapy directed at the lesion. Treatment of granulomatous disorders with standard therapy including glucocorticoids and immunosuppressants may reduce circulating 1,25-dihydroxyvitamin D levels.
In the rare occasions where severe hypercalcaemia is felt to be secondary to drug therapy, the offending drug must be stopped and serum calcium levels monitored for 3–6 months. This may be particularly difficult in those on lithium therapy. However, newer psychotropic agents effective in bipolar disease can effect this change more safely than in the past. Drug cessation must also occur in the more common scenario where a drug is felt to have contributed to the hypercalcaemic state (i.e. thiazide therapy in a patient with malignancy-associated hypercalcaemia), unless significant benefit-to-risk ratios can be demonstrated.
Emerging treatments
Cinacalcet, a calcimimetic which activates the CsR thereby reducing PTH secretion, is in current use [Ayuk et al. 2010]. It is licensed in the US for THPT in the context of advanced CKD, parathyroid carcinoma and additionally PHPT deemed unsuitable for surgery in the UK. It reduces serum calcium levels significantly in most patients with PHPT, and in approximately two thirds of those with parathyroid carcinoma [Ayuk et al. 2010; Marcocci et al. 2009; Silverberg et al. 2007]. The use of cinacalcet in the management of hypercalcaemic crisis has not yet, to the best of the authors' knowledge, been the subject of a RCT, but many case studies and reports demonstrate safety and effectiveness of its application in the context of refractory hyperparathyroid disease. It is commenced at a dose of 30 mg OD orally and titrated to a maximum dose of 90 mg QDS (180 mg per day in renal dialysis patients).
Osteoclast recruitment with resultant bone resorption is in part mediated by the RANKL system [Tanaka et al. 2005]. Activation of the RANK receptor located on immature osteoclasts by osteoblast-derived RANKL promotes maturation and differentiation of the osteoclast. Denosumab, a monoclonal antibody which binds to RANKL preventing binding to the RANK receptor, has been shown to reduce bone resorption in metastatic bone disease and appears to have a good safety profile [Body et al. 2010]. The cytokine osteoprotegrin (OPG) binds to RANKL preventing the activation of osteoclast precursors, and synthetic versions have shown some promise in the treatment of bone malignancy and malignancy-associated hypercalcaemia [Fili et al. 2009]. Further trials specifically examining the role of these two agents in the management of hypercalcaemia are awaited. Monoclonal antibodies directed against PTHrP have been produced and have been shown to be useful for controlling bone metastases in patients with small cell lung cancer [Yamada et al. 2009]. Whether this will translate into a useful additional treatment for the management of severe hypercalcaemia secondary to humoral mechanisms remains to be seen.
Conclusions
Severe hypercalcaemia is an endocrine emergency that requires prompt action to prevent severe neurological, cardiac and renal consequences. The diagnosis should be considered in any patient with known parathyroid disease or malignancy who presents with acute deterioration, especially in the context of neurological dysfunction. The cornerstone of treatment is appropriate volume resuscitation, followed by the administration of calcium-specific therapies including bisphosphonates. Emerging therapies, including calcimimetics and drugs that affect the RANKL system, are likely to become more widely used in the future although further studies are required to define their role.
Funding
This article received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
None declared.
References
- Atula S.T., Tahtela R.K., Nevalainen J.I., Pylkkanen L.H. (2006) Clodronate as a single-dose intravenous infusion effectively provides short-term correction of malignant hypercalcemia. Acta Oncologica 42: 735–740 [DOI] [PubMed] [Google Scholar]
- Ayuk J., Cooper M.S., Gittoes N.J.L. (2010) New perspectives in the management of primary hyperparathyroidism. Ther Adv Endocrin Metab, doi:10.1177/2042018810382326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian J.P. (2006) Osteonecrosis of the jaw — do bisphosphonates pose a risk? N Eng J Med 355: 2278–2281 [DOI] [PubMed] [Google Scholar]
- Body J.J., Lipton A., Gralow J., Steger G., Gao G., Yeh H., et al. (2010) Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res 25: 440–446 [DOI] [PubMed] [Google Scholar]
- British National Formulary (2010) British National Formulary 60, Online edition, http://www.bnf.org
- Broadus A.E., Mangin M., Ikeda K., Insogna K.L., Weir E.C., Burtis W.J., et al. (1988) N Engl J Med 319: 556–563 [DOI] [PubMed] [Google Scholar]
- Cannon J., Lew J., Solorzano J. (2010) Parathyroidectomy for hypercalcemic crisis: 40 years' experience and long-term outcomes. Surgery 148: 807–813 [DOI] [PubMed] [Google Scholar]
- Carroll R., Matfin G. (2010) Endocrine and metabolic emergencies: hypocalcaemia. Ther Adv Endocrinol Metab 1: 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut C.H., Azria M., Silverman S., Engelhardt M., Olson M., Mindeholm M. (2008) Salmon calcitonin: a review of current and future therapeutic indications. Osteoporos Int 19: 479–491 [DOI] [PubMed] [Google Scholar]
- Chitambar C.R. (2010) Medical applications and toxicities of gallium compounds. Int J Environ Res Public Health 7: 2337–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvitkovic F., Armand J.-P., Tubiana-Hulin M., Rossi J.-F., Warrell R. (2006) Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J 12: 47–53 [DOI] [PubMed] [Google Scholar]
- Fili S., Karalaki M., Schaller B. (2009) Therapeutic implications of osteoprotegrin. Cancer Cell Int 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M., Burke F., Evans K.N., Lammas D.A., Sansom D.M., Liu P., et al. (2007) Extra-renal 25-hydroxyvitamin D3-1-hydroxylase in human health and disease. J Steroid Biochem 103: 316–321 [DOI] [PubMed] [Google Scholar]
- Holick M.F. (2007) Vitamin D deficiency. N Engl J Med 357: 266–281 [DOI] [PubMed] [Google Scholar]
- Igbal A.A., Burgess E.H., Gallina D.L., Nanes M.S., Cook C.B. (2003) Hypercalcemia in hyperthyroidism: patterns of serum calcium, parathyroid hormone, and 1,25-dihydroxyvitamin D3 levels during management of thyrotoxicosis. Endocr Pract 9: 517–521 [DOI] [PubMed] [Google Scholar]
- Joshie D., Center J.R., Eisman J.A. (2009) Investigation of incidental hypercalcaemia. BMJ 339: b4613. [DOI] [PubMed] [Google Scholar]
- LeGrand S.B., Leskuski D., Zama I. (2008) Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med 149: 259–263 [DOI] [PubMed] [Google Scholar]
- Major P., Lortholary A., Hon J., Abdi E., Mills G., Menssen H.D., et al. (2001) Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 19: 558–567 [DOI] [PubMed] [Google Scholar]
- Marcocci C., Chanson P., Shoback D., Bilezikian J., Fernandez-Cruz L., Orgiazzi J., et al. (2009) Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism. J Clin Endocr Metab 94: 2766–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matfin G. (2009) Disorders of fluid balance and electrolytes, In: Porth C.M., Matfin G. (eds). Pathophysiology: Concepts of Altered Health States, 8th edn, Wolters Kluwer Health: Philadelphia, PA [Google Scholar]
- Purohit O.P., Radstone C.R., Anthony C., Kanis J.A., Coleman R.E. (1995) A randomised double-blind comparison of intravenous pamidronate and clodronate in the hypercalcaemia of malignancy. Brit J Cancer 72: 1289–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston S.H., Thiebaud D., Herrmann Z., Steinhauer E.U., Thurlimann B., Walls J., et al. (1997) Dose—response study of ibandronate in the treatment of cancer-associated hypercalcaemia. Brit J Cancer 75: 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reszka A.A. (2010) Bisphosphonate mechanisms of action. Contemporary Endocrinology: Osteoporosis 2nd edn Humana Press: New York, NY [Google Scholar]
- Selby P. (2002) Normal calcium homeostasis. Clin Rev Bone Miner Metab 1: 3–9 [Google Scholar]
- Shoback D., Sellmeyer D., Bikle D.D. (2007) Metabolic bone disease, In: Shoback D., Gardner D.G. (eds). Greenspan's Basic and Clinical Endocrinology, 8th edn McGraw Hill: New York [Google Scholar]
- Silverberg S.J., Rubin M.R., Faiman C., Peacock M., Shoback D.M., Smallridge R.C., et al. (2007) Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocr Metab 92: 3803–3808 [DOI] [PubMed] [Google Scholar]
- Stewart A. (2005) Hypercalcaemia associated with cancer. N Engl J Med 352: 373–379 [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nakamura K., Takahasi N., Suda T. (2005) Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL—RANK signaling system. Immunol Rev 208: 30–49 [DOI] [PubMed] [Google Scholar]
- von Moos R., Caspar C.B., Thurlimann B., Angst R., Inauen R., Greil R., et al. (2008) Renal safety profiles of ibandronate 6 mg infused over 15 and 60 min: a randomized, open-label study. Ann Oncol 19: 1266–1270 [DOI] [PubMed] [Google Scholar]
- Yamada T., Muguruma H., Yano S., Ikuta K., Ogino H., Kakiuchi S., et al. (2009) Intensification therapy with anti-parathyroid hormone-related protein antibody plus zoledronic acid for bone metastases of small cell lung cancer cells in severe combined immunodeficient mice. Mol Cancer Ther 8: 119–126 [DOI] [PubMed] [Google Scholar]