Abstract
Pharmacological and molecular approaches have shown that an atypical β-adrenoceptor (AR), called β3-AR, that is distinct from β1-ARs and β2-ARs, exists in some tissues in heterogeneous populations such as β3a-ARs and β3b-ARs. β3-ARs belong to a superfamily of receptors linked to guanine nucleotide binding proteins (G proteins). The β3-AR gene contains two introns whereas the β1-AR and β2-AR genes are intronless, leading to splice variants. β3-ARs can couple to Gi and Gs and they are reported to be present in brown adipose tissue, vasculature, the heart, among other tissues. β3-ARs cause vasodilation of microvessels in the islets of Langerhans and may participate in the pathogenesis of cardiac failure, during which modification of β1-AR and β2-AR expression occurs. The development of β3-AR agonists has led to the elaboration of promising new drugs, including antiobesity and antidiabetic drugs. This article reviews the various pharmacological actions of β3-ARs and their clinical implications for diabetes and cardiovascular diseases.
Keywords: β3-adrenoceptors, antidiabetic, vascular smooth muscles
Introduction
The pressor effect of adrenal extracts was first shown by Oliver and Schafer in 1895. The active principle was named epinephrine by Abel in 1899. The existence of more than one adrenergic receptor was first proposed by Ahlquist in 1948. He proposed the terms α and β for receptors on smooth muscle where catecholamines produce excitatory and inhibitory responses respectively. Almost 50 years after Ahlquist first discovered evidence of the heterogeneity of adrenergic receptors [Ahlquist, 1948], the number of receptor subtypes is still unclear. β-Adrenoceptors (β-ARs) were later subdivided into β1 and β2, which are present in the myocardium and smooth muscle respectively. Pindolol, a nonselective β-AR antagonist with significant agonist activity, was found to cause relaxation of canine-isolated perfused mesenteric vessels [Clark and Bertholet, 1983] and rat aorta precontracted with potassium chloride [Doggrell, 1990]. In both instances, the vasorelaxant effect of pindolol was not significantly antagonized by propranolol, suggesting the presence of a β-AR subtype different from the conventional β1-ARs and β2-ARs. The effect of isoprenaline was ascribed not only to activation of β1-ARs and β2-ARs, but also to that of an additional adrenoceptor [Doggrell, 1990; Clark and Bertholet, 1983]. Later on, the existence of a third β-AR came into light and Gauthier et al. [1996] found that stimulation of β3-AR in human cardiac muscle, in contrast with β1- and β2-AR stimulation, resulted in a profound dose-dependent negative inotropic effect and hence suggested the participation of β3-AR in the pathogenesis of cardiac failure. Moreover, various in vivo studies have also demonstrated that positive β3-AR-related chronotropic effects were prevented by β1- or β2-AR antagonists and are likely due to baroreflex activation in response to β3-adrenoceptor agonist- induced vasodilation [Wheeldon et al. 1994; Takayama et al. 1993; Wheeldon et al. 1993; Tavernier et al 1992].
Studies using molecular and biochemical techniques are likely to provide additional new and unexpected insights into the role of AR subtypes in both normal physiologic functions and diseases. Initially the presence of β3-ARs was demonstrated in vasculature and heart, but later they were shown in adipocytes. β3-ARs mediate lipolysis in white adipose tissues and thermogenesis in brown adipose tissues [Lönnqvist et al. 1993; Langin et al. 1991; Zaagsma and Nahorski, 1990]. β3-ARs represent a heterogeneous population, such as β3a-ARs and β3b-ARs, as suggested by the studies in Chinese hamster ovary cells. Furthermore, the activation and signal transduction of such a β3a-AR and β3b-AR complex may prevent the full potency of the β3-AR agonist [Hutchinson et al. 2002]. The regulation of adrenergic receptors by receptor-specific agonists and antagonists has been actively studied for many years and is important clinically because alterations in these receptors have been suspected in many pathological states. The development of β3-AR agonists has led to the elaboration of promising new drugs and they are a target for antiobesity and antidiabetic drugs [Lowell and Flier, 1995; Pietri-Rouxel and Strosberg, 1995]. Although considerable information is available on β3-AR physiology in fat, there are many other areas in which β3-ARs are involved. The existence of an atypical β-AR, called β3-AR, distinct from β1-ARs and β2-ARs, has been demonstrated in various tissues by pharmacological [Berlan et al. 1993; Holloway et al. 1992; Tavernier et al. 1992; Langin et al. 1991; Mc Laughlin and MacDonald, 1990; Hollenga and Zaagsma, 1989; Bojanic et al. 1985] and molecular approaches [Granneman et al. 1991; Muzzin et al. 1991; Tate et al. 1991; Emorine et al. 1989]. The overview of location of β3 receptors is given in Table 1. This review aims to provide an overview of the presence of β3-ARs in various tissues, with special emphasis on the clinical implications for diabetes and cardiovascular diseases.
Table 1.
Location of β3-adrenoceptors.
| Organ | Species | Reference |
|---|---|---|
| Heart | ||
| Atria | Human | Krief et al. (1993); Berkowitz et al. (1995) |
| Ventricle | Human | Gauthier et al. (1999) |
| Vascular smooth muscles | ||
| Veins | Viard et al. (2000) | |
| Cutaneous vascular smooth muscles | Canine | Berlan et al. (1994) |
| Vasculature | Tavernier et al. (1992); Shen et al. (1994); | |
| Rohrer et al. (1999) | ||
| Thoracic aorta | Rat | Trochu et al. (1999) |
| Internal mammary artery | Human | Rozec et al. (2005) |
| Non-vascular smooth muscles | ||
| Gastrointestinal tract, brain, and prostate | Granneman et al. (1991); Emorine et al. (1989); | |
| Bensaid et al. (1993); Rodriguez et al. (1995) | ||
| Ileum | Rat | Roberts et al. (1995); Roberts et al. (1999) |
| Rectum and IAS membranes | Western blot studies | Rathi et al. (2003) |
| Urinary tract | Tomiyama et al. (1998) | |
| Near-term myometrium | Human | Bardou et al. (2000) |
| Brown adipose tissues | Emorine et al. (1994) |
Molecular structure and signal transduction mechanism of β receptors
ARs are members of a large superfamily of receptors linked to guanine nucleotide binding proteins (G proteins). All G-protein coupled receptors share structural features, such as extracellular amino terminals with sites for N-linked glycosylation, seven α-helical domains that span the plasma membrane, and intracellular carboxy terminals containing amino acid sequences that indicate probable sites of phosphorylation by one or more protein kinases. The G proteins, linked to adrenergic receptors, are heterotrimeric proteins with α, β, and γ subunits. Each subunit is part of a family consisting of multiple members [Simon et al. 1991]: approximately 20 α subunits that have been divided into four subfamilies — αs, αi, αq and α12; at least five β subunits (β1–5); and at least six γ subunits (γ1–6). Although several hundred different subunit combinations (heterotrimers) are theoretically possible, the repertoire of G proteins used by a particular receptor system is limited [Hescheler and Schultz, 1994]. Each type of G protein can be used for signaling by more than one type of receptor. β1, β2 and β3 share approximately 60% amino acid sequence identity within the presumed membrane spanning domains. The β3-AR gene contains two introns [Lelias et al. 1993; Granneman et al. 1992] in contrast to β1-AR and β2-AR genes, which are intronless. This structure leads to splice variants. The B and C isoforms contain 12 and six additional amino acids, respectively, at their C terminus in comparison with the A isoform [Lelias et al. 1993; Granneman et al. 1992]. In rat adipocytes, a unique isoform is expressed that is close to the B isoform, whereas in human brown adipocytes, the C isoform is predominant [Lelias et al. 1993; Van Spronsen et al. 1993]. It was hypothesized that the physiological response to β3-AR stimulation differs depending on the isoform expressed in a given species [Levasseur et al. 1995]. The human β3-AR exists in at least two different agonist conformations with a similar high-affinity and low-affinity pharmacology analogous to β1-AR. Both conformations are present in living cells and can be distinguished by their pharmacological characteristics [Baker, 2005].
All β-AR subtypes signal by coupling to the stimulatory G protein Gαs, leading to activation of adenyl cyclase and accumulation of the second messenger cAMP [Emorine et al. 1989; Frielle et al. 1987; Dixon et al. 1986]. Gs can directly enhance the activation of voltage-sensitive Ca2+ channels in the plasma membrane of skeletal and cardiac muscle. However, some recent studies indicate that, under certain circumstances, β 3-AR can couple to Gi as well as to Gs [Gauthier et al. 1996; Xiao et al. 1995; Chaudry et al. 1994]. Multiple mechanisms control the signaling and density of G-protein-coupled receptors. Catecholamines, which are hydrophilic, do not bind to the highly charged extracellular domains of the receptors, as might be expected, but bind in the more hydrophobic membrane-spanning domains [Caron and Lefkowitz, 1993; Jasper and Insel, 1992].
On the basis of many pharmacological and molecular studies, the existence of a fourth β-AR subtype was postulated [Brodde and Michel, 1999; Galitzky et al. 1998; Strosberg et al. 1998; Kaumann, 1997; Strosberg, 1997; Summers et al. 1997; Strosberg and Pietri-Rouxel, 1996; Barnes, 1995; Arch and Kaumann, 1993]. To date, at least nine subtypes of adrenergic receptors (three subtypes each of α1-ARs, α2-ARs, and β-ARs) have been identified. The precise function of all these receptors has not yet been defined, in part because of a dearth of highly specific agonists and antagonists. An alternative way to examine receptor function is to use molecular genetic techniques to overexpress or to knock out the expression of particular subtypes in laboratory animals [Milano et al. 1994a, 1994b; Bertin et al. 1993].
Pharmacological actions
Adipose tissue and diabetes
β3-ARs mediate lipolysis in white adipose tissues and thermogenesis in brown adipose tissues [Lönnqvist et al. 1993; Langin et al. 1991; Zaagsma and Nahorski, 1990]. The presence of the Arg64 allele in the first intracellular loop of the β3-AR gene may predispose patients to abdominal obesity, which may in turn predispose them to insulin resistance and the earlier onset of type 2 diabetes mellitus (T2DM) [Widén et al. 1995]. A naturally occurring variation, Trp64Arg β3-AR mutation, found in about 8% of Europeans and North Americans, actually restores the arginine residue in humans, which is found present in animals [Strosberg, 1997]. This variation was found to be associated with the following:
An increased capacity of obese French patients to gain weight [Clément et al. 1995].
An early onset of T2DM in obese Pima Indians by altering the balance of energy metabolism in visceral adipose tissue and tend to have a lower resting metabolic rate [Walston et al. 1995]. Similar observations were also reported in Japanese participants [Fujisawa et al. 1996].
An early onset of T2DM and clinical features of the insulin resistance syndrome in Finns [Widén et al. 1995].
The Trp64Arg β3-AR genotype is associated with mild gestational diabetes and this polymorphism is associated with increased weight gain during pregnancy [Festa et al. 1999]. The increased amount of adipose tissue after menopause is considered to elevate estradiol production, which in turn increases the risk for breast cancer. Thus, genetic traits that are related to obesity may influence the risk of postmenopausal breast cancer in an indirect manner [Huang et al. 2001]. A missense mutation in codon 64 of the β3-AR gene that results in substitution of tryptophan by arginine [Trp64 → Arg] in the first intracellular loop of the receptor protein has been reported in various ethnic groups, including the Japanese [Kadowaki et al. 1995]. A review [Arner and Hoffstedt, 1999] identified a link between obesity and the Trp64 → Arg polymorphism in 13 studies. Also a polymorphism in codon 27 of the ADRβ2 gene that features a replacement of glutamine by glutamic acid [Gln27 → Glu] is linked with obesity [Large et al. 1997]. Thus, β3-ARs may constitute a target for antiobesity and antidiabetic drugs [Lowell and Flier, 1995; Pietri-Rouxel and Strosberg, 1995].
BRL 26830 (see Table 2), a selective β3-AR agonist, caused a marked increase in blood flow to brown adipose tissue in the anesthetized rat [Takahashi et al. 1992]. The increase in blood flow may well be secondary to an augmented metabolic process [Shen and Claus, 1993], since BRL 37344 (see Table 2) causes marked increases in the plasma levels of free fatty acids and insulin. In vitro studies have demonstrated that in rat [Granneman, 1992] and dog [Galitzky et al. 1993] fat cells, catecholamines stimulate β3-ARs at higher concentrations than those required to activate β1-ARs or β2-ARs. Similar results were demonstrated in dog in vivo studies [Pelat et al. 2003]. Also, BRL 26830A causes stimulation of insulin secretion in pancreas β cells [Yoshida et al. 1991].
Table 2.
Agonists and antagonists of β3-adrenoceptors (ARs).
| β3-Agonist | Chemical name |
|---|---|
| BRL 26830A | methyl 4-[2-[[2-hydroxy-2-phenethyl]amino]propyl]benzoate-2-butanedioate |
| BRL 35135 | methyl 4-[2-[2-hydroxy-2-[3-chlorophenyl]ethylamino] propyl] phenoxyacetate |
| BRL 37344 | 4-[-[[2-hydroxy-[3-chlorophenyl] ethyl]- amino] propyl] phenoxyacetate |
| CGP 12177 | 4-[3-t-butylamino-2-hydroxypropoxy]benzimidazol-2-one |
| CGP 20712A | 2-hydroxy-5-[2-[{2-hydroxy-3-[4-[1-methyl-4-trifluoromethyl-2-imidazolyl]phenoxy]propyl}amino]ethoxy] benzamide |
| CL 316243 | disodium[R,R]-5-[2[[2-[3-chlorophenyl]-2-hydroxyethyl]-amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate |
| FR-149175 | ethyl-[[S]-8-[[R]-2-[3-chlorophenyl]-2-hydroxyethylamino]-6,7,8,9-tetrahydro-5H-benzocyclohepten-2-yloxy]acetate monohydrochloride monohydrate |
| ICI 198157 | methyl [4-[2-[[2-hydroxy-3-phenoxypropyl]amino]ethoxy]phenoxy]acetate |
| ICI D7114 | [S]-4-[2-hydroxy-3-phenoxypropylaminoethoxy]-N-[2-methoxyethyl]phenoxyacetamide |
| L 742,791 | [S]-N-[4-[2-{[3-[4-hydroxyphenoxy]-2-hydroxypropyl]amino}ethyl]phenyl]-4-iodobenzenesulfonamide |
| L 750355 | 3-pyridyloxypropanoloamine derivative |
| L755,507 | 4-[[(Hexylamino)carbonyl]amino]-N-[4-[2-[[(2S)-2-hydroxy-3-(4-hydroxyphenoxy) propyl] amino]ethyl]phenyl]-benzenesulfonamide |
| L 770,644 | [R]-4-[4-[3-cyclopentylpropyl]-4,5-dihydro-5-oxo-1 H-tetrazol-1-yl]-N-[4-[2-[[2-hydroxy-2-[3-pyridinyl]ethyl]amino] ethyl]phenyl]- benzenesulfonamide |
| L 796568 | [R]-N -[4-[2-[[2-hydroxy-2-[3-pyridinyl]ethyl]amino]ethyl]-phenyl]-4-[4-[4-[trifluoromethyl]phenyl]thiazol-2-yl]-benzenesulfonamide, dihydrochloride |
| LY 79771 | [R-[R*, S*]] alpha-[[[3-[4-hydroxyphenyl]-1-methylpropyl]amino]methyl]benzenemethanol |
| RO363 | [-]-1-[3,4-dimethoxyphenethylamino]-3-[3,4-dihdroxyphenoxy]-2-propanol] oxalate |
| SB 251023 | [4-[1-{2-[S]-hydroxy-3-[4-hydroxyphenoxy]-propylamino} cyclopentyl methyl]phenoxymethyl]phenylphosphonic acid lithium salt |
| SR 58611 | [RS]-N-[[25]-7-ethoxycarbonylmethoxy-1,2,3,4-tetrahydronapth-2-yl]-[2R]-2-[3-chlorophenyl]-2-hydroxyethanamine hydrochloride |
| SR 58611A | [N2S]-7-carbethoxymethoxy-1,2,3,4-tetrahydronaphth-2-yl-[2R]-2-hydroxy-2-chlorophenyl ethanamine hydrochloride |
| SR 59104A | N-[[6-hydroxy-1,2,3,4-tetrahydronaphthalen-[2R]-2-yl]methyl]-[2R]-2-hydroxy-2-[3-chlorophenyl]ethanamine hydrochloride |
| SR 59119A | N-[[7-methoxy-1,2,3,4-tetrahydronaphthalen-[2R]-2-yl]methyl]-[2R]-2-hydroxy-2-[3-chlorophenyl]ethanamine hydrochloride |
| ZD2079 | 4-[2-[[(2R)-2-Hydroxy-2-phenylethyl] amino]ethoxy]-benzeneacetic acid hydrochloride |
| ZD 7114 | [S]-4-[2-hydroxy-3-phenoxypropylaminoethoxy]-N-[2-methoxyethyl]phenoxyacetamide |
| ZM 215001 | [S]-4-[2-hydroxy-3-phenoxy-propylamino-ethoxy] phenoxyacetic acid [R]-N-[4-[2-[[2-hydroxy-2-[3-pyridinyl]ethyl]amino]ethyl]phenyl]-1-[4-octylthiazol-2-yl]-5-indolinesulfonamide |
| β3-Antagonist | Chemical name |
| L 748328 | [S]-N-[4-[2-{[3-[3-{aminosulfonyl}phenoxy]-2-hydroxypropyl]-amino}ethyl] phenyl]benzenesulfonamide |
| SR 59230A | 3-[2-ethylphenoxy]-1[1 S]-1,2,3,4-tetrahydronaphth-1-ylaminol-[2S]2-propanol oxalate |
In diabetic ZDF rats, CL 316243, a β3 selective agonist (see Table 2) did not reduce hyperglycemia when given under acute conditions (single intravenous injections or subcutaneous infusions for a few days). However, long-term treatment of CL 316243 progressively normalized glycemia, reduced insulinemia and decreased the levels of circulating free fatty acids in obese diabetic ZDF rats. This treatment also markedly improved their glucose and insulin responses during an intravenous glucose tolerance test [Liu et al. 1998]. Hyperinsulinemic-euglycemic clamps combined with the [2-3H]deoxyglucose method revealed that chronic CL 316243 treatment markedly increased insulin responsiveness in obese rats and that it increases glucose uptake in brown adipose tissue, white adipose tissue, the diaphragm, and skeletal muscles, but not in the heart. The maximal capacity of various tissues for glucose uptake in CL 316243-treated animals varied in the following order: brown adipose tissue > heart > diaphragm > skeletal muscles > white adipose tissue [Liu et al. 1998]. This sequence of potencies agrees with previous observations for normal rats treated with insulin [Vallerand et al. 1987] or norepinephrine [Liu et al. 1994] as well as with cold-exposed animals [Vallerand et al. 1990]. Acute treatment of obese rodents with CL 316243 causes a number of diverse metabolic effects, including an increase in oxygen consumption and insulin levels, and a decrease in food intake. The mechanism by which CL 316243 increases insulin secretion in rodents is not known. Nevertheless, the stimulus for insulin secretion is extremely potent as it causes a 50 to 100-fold increase in insulin levels, which remain elevated (24-fold increase) despite the presence of hypoglycemia. This effect cannot be mediated by the direct effects of CL 316243 on pancreatic β cells because pancreatic islets appear not to express detectable levels of β3-AR mRNA and because insulin secretion is not stimulated following addition of CL 316243 to cultured pancreatic islets [Grujic et al. 1997].
In another study by Fu and colleagues [Fu et al. 2007], CL 316243 and BRL 37344 downregulated adiponectin, but upregulated adiponectin receptor 2 (not receptor 1) in epididymal and/or subcutaneous white adipose tissue and in brown adipose tissue. Tumor necrosis factor-α (TNF-α) expression was upregulated only in epididymal adipose tissue, which suggests that upregulation of TNF-α and downregulation of adiponectin by β -AR activation may contribute to the pathogenesis of catecholamine-induced insulin resistance, and that upregulation of adiponectin receptor 2 may be a feedback result of reduced adiponectin [Fu et al. 2007]. In addition, the effects of CL 316243 were investigated in obese diabetic KKAy mice by Fu and colleagues [Fu et al. 2008]. Two weeks’ subcutaneous administration of CL 316243 reduced serum levels of glucose, insulin, triglyceride, free fatty acid and TNF-α, and increased adiponectin. CL 316243 recovered the mRNA expressions of adiponectin, adiponectin receptors and β3-ARs, which were reduced in epididymal white adipose tissue in KKAy mice. Meanwhile, CL 316243 suppressed the overexpressed mRNA level of TNF-a in both epididymal white adipose tissue and brown adipose tissue. These data suggest that the normalization of adiponectin, adiponectin receptors and TNF-α may result in the amelioration of obesity-induced insulin resistance [Fu et al. 2008].
β3-Agonists appear to be of significance not only in obesity but also in terms of the risks of cardiovascular disorders because visceral obesity is the most dangerous form of regional fat accumulation, the form of obesity that is more directly linked to β3-AR activity [Arner, 1995].
Vascular smooth muscles
β3-ARs produce sustained peripheral vasodilation that is predominant in skin and fat [Shen et al. 1994; Berlan et al. 1993]. A study showed that the relaxation of rat thoracic aorta was caused by selective β3-AR agonists like CGP 12177 [Mohell and Dicker, 1989], cyanopindolol [Engel et al. 1981], ZD 2079 [Grant et al. 1994], ZM 215001 [Tesfamariam and Allen, 1994], and SR 58611 [Trochu et al. 1999] (see Table 2), further supporting the presence of β3-ARs [Brawley et al. 2000a, 2000b]. A β3-AR-mediated vasorelaxation was also observed in the canine pulmonary artery, an effect that was exerted through a cAMP-dependent pathway [Tagaya et al. 1999]. In the rat carotid artery, the selective β3-AR agonist BRL 37344 and the selective β2-AR agonist, salbutamol, were not antagonized by propranolol (100 nM), and pretreatment of the artery segments with BRL 37344 did not desensitize the tissue to the relaxant effect of isoprenaline and salbutamol [Oriowo, 1994]. In the same tissue, MacDonald and colleagues [MacDonald et al. 1999] confirmed the presence of β3-AR by the relaxant effects of two selective β3-AR agonists, BRL 37344 and ZD 2079. In normal dogs, the infusion of BRL 37344 or CL 316243 or CGP 12177 induced an increase in heart rate and cutaneous blood flow (evaluated in the internal part of the ear) [Berlan et al. 1994].
The Trp64Arg mutation of β3-AR has been suggested to confer susceptibility to essential hypertension [Morris et al. 1994] and this was confirmed by Tonolo and colleagues [Tonolo et al. 1999]. These authors concluded that the Trp64Arg polymorphism of the β3-AR gene is associated more often with high blood pressure than with normal blood pressure. Isoproterenol, BRL 37344 and CGP 12177 are reported to produce a reduction in arterial blood pressure. In sinoaortic denervated animals, isoproterenol infusion provoked tachycardia and hypotension [Tavernier et al. 1992]. It was demonstrated that a higher dose of isoproterenol is required to obtain in vivo β3-mediated vasodilation than that necessary for β1-or β2-mediated vasodilation. The reason for this may be that β3-AR is a ‘back-up’ receptor activated during extreme or stressful conditions [Pelat et al. 2003].
Significant increases in systolic blood pressure and Doppler stroke distance occurred with BRL 37344 and salbutamol, which were unaffected by pretreatment with bisoprolol and completely blocked by nadolol, in keeping with the β 2-mediated effects. BRL 37344 and salbutamol produced significant chronotropic effects, which were unaffected by β1-AR blockade [Wheeldon et al. 1994]. In a clinical study, isoprenaline produced an increase in systolic blood pressure and left ventricular stroke distance that was not attenuated by a dose of nadolol, which produced complete blunting of β1- and β2-mediated responses but not of β3-mediated effects [Wheeldon et al. 1993].
Experimental in vivo studies have demonstrated that positive β3-AR-related chronotropic effects were prevented by β1-AR or β2-AR antagonists and are likely due to baroreflex activation in response to β3-AR agonist-induced vasodilation [Tavernier et al. 1992; Takayama et al. 1993]. Apart from this, positive chronotropic effects were not observed in denervated animals and thus it was concluded that tachycardia resulted from a baroreceptor-mediated reflex in response to a drop in blood pressure caused by the vasodilating action of β3-AR agonists [Berlan et al. 1994; Shen et al. 1994]. In normal dogs, infusion of isoproterenol, BRL 37344 or CGP 12177 increased heart rate with the following order of potency: BRL 37344 > isoproterenol >> CGP 12177. Isoproterenol stimulated adenylate cyclase activity in heart membranes from normal dogs, whereas CGP 12177 and BRL 37344 were without any stimulating action [Tavernier et al. 1992]. In vitro studies are more suitable in analyzing the cardiac effects of β3-ARs. A typical example of the masking effects of baroreflex activation lies with 1,4-dihydropyridines, which induce a negative inotropic effect in vitro, but a positive chronotropic and inotropic effect in vivo as a consequence of vasodilation [Piepho, 1991].
The presence of β3-ARs has also been reported in veins. In the rat portal vein, activation of β3-ARs stimulates L-type Ca2+ channels through a Gαs-induced stimulation of the cyclic AMP/ protein kinase, a pathway and the subsequent phosphorylation of the channels [Viard et al. 2000]. In rats, the selective β-AR agonist CL 316243 induced marked increases in islet blood flow and plasma insulin level, and these increases were stopped by bupranolol, a β1-AR, β2-AR and β3-AR antagonist, but not by nadolol, a β1-AR and β2-AR antagonist, indicating that β3-ARs caused a vasodilation of microvessels in the islets of Langerhans [Atef et al. 1996].
Cardiac effects
β3-AR stimulation of the human cardiac muscle, in contrast with β1-AR and β2-AR stimulation, resulted in a profound dose-dependent negative inotropic effect. This unexpected finding suggests that β3-ARs may participate in the pathogenesis of cardiac failure, during which modification of β1-AR and β2-AR expression occurs [Brodde, 1993]. Functional β3-ARs stimulation, which occurs in the normal left ventricle, causes direct inhibition on (Ca2+)iT and ICa,L(L-type Ca channels) and produces a negative inotropic action [Cheng et al. 2001]. In another study, it was found that β3-AR activation inhibits the L-type Ca2+ channel in both normal and heart failure rat myocytes. In heart failure, β3-AR stimulation-induced inhibition of Ca2+ channels is enhanced, which is responsible for reduced inotropic response [Zhang et al. 2005]. β3-AR agonists induce negative inotropic effects with the following order of potency: BRL 37344 > SR 58611 = CL 316243 > CGP 12177, similar to that observed in Chinese hamster ovary cells transfected with human β3-ARs [Pietri-Rouxel and Strosberg, 1995; Dolan et al. 1994]. In another study, the mechanical effects of BRL 37344 were not modified by pretreatment with metoprolol (β1-AR antagonist) or nadolol, indicating that this effect was not mediated by β1-ARs or β2-ARs. By contrast, bupranolol, which possesses β3-AR antagonist properties [Pietri-Rouxel and Strosberg, 1995; Galitzky et al. 1993; Sugasawa et al. 1992], antagonized the negative inotropic effects of BRL 37344 with a pA2 value similar to that determined in adipocytes [Galitzky et al. 1998; Pietri-Rouxel and Strosberg, 1995].
In heart failure, increased activity of the sympathetic nervous system leads to downregulation of cardiac β1-ARs and β2-ARs [Brodde, 1993] resulting from their phosphorylation by cAMP-dependent protein kinase or β-AR kinase. Reduced β1-ARs and β2-ARs lead to a decrease in the contractile response to β-AR agonists [Strosberg, 1993]. Contrary to β1-ARs and β 2-ARs, the abundance of the negatively inotropic β3-ARs increases in the failing myocardium [Moniotte et al. 2001]. β3-ARs lack phosphorylation sites for cAMP-dependent protein kinase or β-AR kinase [Strosberg, 1993], and thus may not be downregulated in heart failure. According to this hypothesis, the high adrenoceptor tone during heart failure may alter the cardiac contractile activity as a result of unmasked β3-AR stimulation in the presence of reduced β1-ARs and β2-ARs [Gauthier et al. 1996]. Overstimulation of the relatively desensitization-resistant β3-AR [Liggett et al. 1993] after increased sympathetic tone and norepinephrine release in the setting of heart failure in humans may further decrease cardiac inotropy [Moniotte et al. 2001]. The levels of β3-AR mRNA and proteins show an increase in the failing heart compared with the nonfailing heart. The β3-AR agonist BRL 37344 was found to markedly aggravate the cardiac function and stimulate cardiac myocytes apoptosis in the failing heart. If the levels of β3-AR are too high, they might contribute to the loss of cardiac function and be the foundation of the functional degradation of heart failure [Kong et al. 2004]. Moreover, another study in isoproterenol-induced chronic heart failure rats suggests that the myocardial upregulation of β3-AR in heart failure is associated with increased oxidative stress [Kong et al. 2010]. These studies open the perspective for correcting the disordered adrenergic regulation of the failing heart with specific antagonists of the human cardiac β 3-AR. By contrast, Rasmussen et al. (2009) reported that as increased intracellular myocyte Na+ levels represent a key adverse pathophysiological feature of heart failure, and the β3-AR mediates the stimulation of the only export route for Na+- the Na+-K+ pump — the upregulation of this receptor may also represent a useful compensatory mechanism. Data from animal studies and circumstantial observations from clinical trials suggest that β3-AR activation is beneficial in severe heart failure, and that β3-AR agonists are a promising therapeutic option for the treatment of this disease (Rasmussen et al. 2009). In transgenic mice with cardiac-specific overexpression of protein of the human β3-AR (TGβ3 mice), the human β3-AR is quiescent until stimulated with a selective agonist L 755,507, at which point there is a marked augmentation in left ventricular contractility. In addition, because β3-AR is relatively insensitive to catecholamines, it would be minimally activated by endogenous catecholamines. This approach could have important therapeutic potential in patients with heart failure, in which delivery of the human β3-AR by gene therapy could provide a functionally inactive signaling protein that becomes activated only when a highly selective agonist is exogenously administered [Kohout et al. 2001].
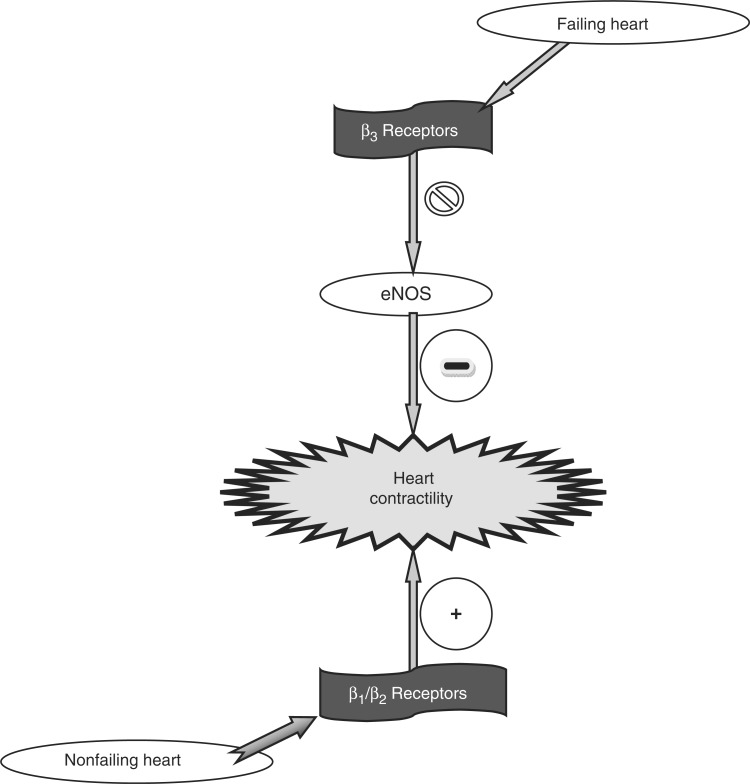
In congestive heart failure (CHF), β3-AR expression is increased. This augmentation is proposed to exacerbate the dysfunctional [Ca2+]i regulation, enhance inhibition of cardiac contraction and relaxation, and lead to worsening of cardiac failure [Cheng et al. 2001]. In CHF, when marked increases in sympathetic tone and cardiac norepinephrine release have rendered the positive inotropic β1-AR system relatively unresponsive, the upregulated β3-AR pathways would continue to exhibit a negative inotropic effect. This altered balance between opposing inotropic influences of β1-ARs and β3-ARs in CHF may contribute to progressive cardiac dysfunction in CHF. The enhanced response to β3-AR stimulation in CHF may also be related to increased numbers of β3-ARs or an altered signal transduction [Cheng et al. 2001]. As shown in Figure 1, in CHF, NO-cGMP signaling may be altered [Mohan et al. 1996], thereby altering CHF myocyte response to β3-AR stimulation [Cheng et al. 2001]. The enhanced contractile response to β3-AR stimulation in CHF myocytes of dogs may be coupled to Gi through both NO-dependent and NO-independent mechanisms [Cheng et al. 2001]. The activation of Gi also has the potential to couple β3-ARs to other important signaling pathways such as mitogen-activated protein kinase [Soeder et al. 1999]. The increase in other neurohormonal activation, such as TNF-α, endothelin 1, and angiotensin II, may also differentially modulate β3-AR expression and function. These studies indicate that using β3-AR agonists for the treatment of obesity and diabetes [Arch et al. 1984] may have cardiac side effects, especially in patients with CHF [Cheng et al. 2001]. Also, these studies suggest several novel therapeutic strategies for the treatment of CHF, such as the use of β3-AR antagonists or Gi inhibitors. Gan and colleagues [Gan et al. 2007a] reported that a β3-AR antagonist SR 59230A can block the β3-AR—nitric oxide synthase (NOS)—cyclic GMP pathway and improve cardiac function in heart failure in rats if administered long term. SR 59230A can also attenuate cardiac remodeling by inhibition of interstitial fibrosis to a certain degree, which may help to improve cardiac function in heart failure [Gan et al. 2007b]
Figure 1.
Postulated changes in β-adrenoceptor signaling in cardiomyocytes from nonfailing to failing myocardium. eNOS, endothelial nitric oxide synthase.
β3-ARs are involved in the vasomotor control of the internal mammary artery and thus open new fields of investigation in coronary bypass graft management, heart failure, and hypertension [Rozec et al. 2005]. In the hearts of long-term diabetic rats, the expression of β1-ARs decreases, whereas that of β3-ARs increases. This may suggest that a decrease in β1-AR together with an increase in β3-AR expression might be involved in the development of diabetes-induced cardiac dysfunction [Dinçer et al. 2001].
In cardiac myocytes, repolarization of the action potential is produced by several potassium currents [Barry and Nerbonne, 1996] like very slow activating and deactivating delayed rectifier potassium current (IKs). This current represents the predominant repolarizing current during increased heart rate [Zeng et al. 1995; Jurkiewicz and Sanguinetti, 1993; Varnum et al. 1993]. The channel underlying IKs is formed by the assembly of two transmembrane proteins, the KvLQT1 and MinK protein [Barhanin et al. 1996; Sanguinetti et al. 1996]. The IKs current amplitude in the heart is increased by catecholamines, which are mediated by β-ARs [Lo and Numann, 1998; Sanguinetti et al. 1991; Duchatelle-Gourdon et al. 1989]. Catecholamines develop negative inotropic effects and shorten the human cardiac action potential through β3-ARs [Gauthier et al. 1996]. The shortening of the human cardiac action potential under β3-AR stimulation may be because they can couple functionally to the KvLQT1/MinK potassium channel in the Xenopws oocyte expression system, which involves G proteins [Kathofer et al. 2000]. The shortening of cardiac action potentials is expected to affect the repolarization process, thereby potentially triggering arrhythmias. The coupling of the KvLQT1/MinK channel to the β3-AR may have important implications for arrhythmogenesis in the heart and thus may open new perspectives for the prevention and treatment of cardiac arrhythmias [Kathofer et al. 2000]. The findings of Zhou and colleagues [Zhou et al. 2008] suggested that β-AR blocking agents with β3-AR agonist properties might be useful for cardiac arrhythmia control after myocardial infarction, especially in treating ventricular tachycardia storms.
Endothelium
After L-NAME treatment or removal of endothelium, relaxant responses to isoprenaline were found to be unaffected by propranolol, suggesting that they were mediated by β3-ARs and/or the low-affinity state of β1-ARs, formerly proposed as putative β4-ARs [Brawley et al. 1998].
In the rat thoracic aorta, β3-ARs act in conjunction with β1-ARs and β2-ARs to mediate relaxation through activation of an NOS pathway and subsequent increase in cyclic GMP levels [Trochu et al. 1999]. In human vessels, β3-AR relaxation was also found to be mediated partly through NO production. This was evidenced by its complete abrogation by NOS inhibition under circumstances when both prostanoids and endothelium-derived hyperpolarizing factors (EDHFs) are inoperative (that is, after cyclooxygenase inhibition and preconstriction with high potassium chloride respectively) [Dessy et al. 2004]. This may be caused by functional coupling of β3-AR agonists to NO production in whole human ventricular muscle through Gai proteins [Moniotte et al. 2001; Gauthier et al. 1998]. Endothelial cells produce a hyperpolarization leading to vascular muscle relaxation through activation of calcium-dependent K+ channels [Busse et al. 2002]. Dessy and colleagues [Dessy et al. 2004] demonstrated vessel hyperpolarization in response to β3-AR agonists and the abrogation of β3-AR-mediated relaxation after vessel pretreatment with the K+ channel inhibitors charybdotoxin and apamin, two signatures of an EDHF response. These results are also in agreement with the recent proposition of β3-AR-mediated relaxation through K+ channel activation in rat aorta [Rautureau et al. 2002]. Functional β3-AR vasorelaxation mediated in part by EDHFs in human coronary resistance arteries may have a major bearing on our understanding of regulating coronary perfusion in circumstances such as dyslipidemia, diabetes and atherosclerosis, all associated with decreased NO production and/or bioavailability.
Conclusions
Almost 50 years after Ahlquist first uncovered evidence of the heterogeneity of adrenergic receptors, the number of receptor subtypes is still unclear, although nine subtypes are well documented (three subtypes each of α1-ARs, α2-ARs and β-ARs). Adrenergic receptors are members of a large superfamily of receptors linked to G proteins. The identification of new subtypes of receptors offers the promise of new therapeutic agents. β3-ARs, which are found at unique sites such as in brown adipose tissue and the gallbladder, are potential targets for antiobesity drugs. Although considerable information is available on β3-AR physiology in fat, there are many other areas in which β3-ARs are involved. The presence of β3-ARs in vasculature and heart provides new avenues for the development of innovative type-specific drugs. Since alterations in adrenergic receptors have a role in many clinical settings, the development of such agonists and antagonists may give therapeutic potential for the treatment of various disorders, including diabetes mellitus, hypertension, dyslipidemia, cardiac arrythmias, heart failure and diabetes-induced cardiac dysfunction. It is known that using β3-AR agonists to treat obesity and diabetes may have cardiac side effects, especially in patients with CHF. However, with the knowledge that there are two types of β3-ARs (β3a and β3b), it may be possible to develop subtype-specific drugs that are more effective and have fewer side effects than those currently available.
Footnotes
This article received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Ahlquist R.P. (1948) A study of the adrenotropic responses. Am J Physiol 153: 586–600 [DOI] [PubMed] [Google Scholar]
- Arch J.R., Ainsworth A.T., Cawthorne M.A., Piercy V., Sennitt M.V., Thody V.E., et al. (1984) Atypical β-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature 309: 163–165 [DOI] [PubMed] [Google Scholar]
- Arch S.J., Kaumann A.J. (1993) Beta 3 and atypical beta-adrenoceptors. Med Res Rev 13: 663–729 [DOI] [PubMed] [Google Scholar]
- Arner P. (1995) The β3-adrenergic receptor-a cause and a cure of obesity?. N Engl J Med 333: 382–383 [DOI] [PubMed] [Google Scholar]
- Arner P., Hoffstedt J. (1999) Adrenoceptor genes in human obesity. J Intern Med 245: 667–672 [DOI] [PubMed] [Google Scholar]
- Atef N., Lafontan M., Doublé A., Hélary C., Ktorza A., Pénicaud L. (1996) A specific β3-adrenoceptor agonist induces increased pancreatic islet blood flow and insulin secretion in rats. Eur J Pharmacol 298: 287–292 [DOI] [PubMed] [Google Scholar]
- Baker J. (2005) Evidence for a secondary state of the human β3-adrenoceptor. Mol Pharmacol 68: 1645–1655 [DOI] [PubMed] [Google Scholar]
- Bardou M., Loustalot C., Cortijo J., Simon B., Naline E., Dumas M., et al. (2000) Functional, biochemical and molecular biological evidence for a possible β3-adrenoceptor in human near-term myometrium. Br J Pharmacol 130: 1960–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romex G. (1996) KvLQT1 and IsK (minK) proteins associate to form the IKS cardiac potassium current. Nature 384: 78–80 [DOI] [PubMed] [Google Scholar]
- Barnes P.J. (1995) Beta-adrenergic receptors and their regulation. Am J Respir Crit Care Med 152: 838–860 [DOI] [PubMed] [Google Scholar]
- Barry D.M., Nerbonne J.M. (1996) Myocardial potassium channels: Electrophysiological and molecular diversity. Annu Rev Physiol 58: 363–394 [DOI] [PubMed] [Google Scholar]
- Bensaid M., Kaghad M., Rodriguez M., Le Fur G., Caput D. (1993) The rat beta 3-adrenergic receptor gene contains an intron. FEBS Lett 318: 223–226 [DOI] [PubMed] [Google Scholar]
- Berkowitz D.E., Nardone N.A., Smiley R.M., Price D.T., Kreutter D.K., Fremeau R.T., et al. (1995) Distribution of β3-adrenoceptor mRNA in human tissues. Eur J Pharmacol 289: 223–228 [DOI] [PubMed] [Google Scholar]
- Berlan M., Galitzky J., Bousquet-Melou A., Lafontan M., Montastruc J.L. (1993) β3-Adrenoceptor-mediated increase in cutaneous blood flow in the dog. J Pharmacol Exp Ther 268: 1444–1451 [PubMed] [Google Scholar]
- Berlan M., Galitzky J., Bousquet-Melou A., Lafontan M., Montastruc J.L. (1994) Beta-3 adrenoceptor-mediated increase in cutaneous blood flow in the dog. J Pharmacol Exp Ther 268: 1444–1451 [PubMed] [Google Scholar]
- Bertin B., Mansier P., Makeh I., Briand P., Rostene W., Swynghedauw B., et al. (1993) Specific atrial overexpression of G protein coupled human beta 1 adrenoceptors in transgenic mice. Cardiovasc Res 27: 1606–1612 [DOI] [PubMed] [Google Scholar]
- Bojanic D., Jansen J., Nahorski S., Zaagsma J. (1985) Atypical characteristics of the β-adrenoceptor mediating cyclic AMP generation and lipolysis in the rat adipocyte. Br J Pharmacol 84: 131–137 [PMC free article] [PubMed] [Google Scholar]
- Brawley L., MacDonald S., Shaw A.M. (1998) Role of endothelium in classical and atypical β-adrenoceptor-mediated vasorelaxation in rat isolated aorta. Br J Pharmacol 122: 395 [Google Scholar]
- Brawley L., Shaw A.M., MacDonald A. (2000a) β1-β2- and atypical β-adrenoceptor-mediated relaxation in rat isolated aorta. Br J Pharmacol 129: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley L., Shaw A.M., MacDonald A. (2000b) Role of endothelium/nitric oxide in atypical β-adrenoceptor-mediated relaxation in rat isolated aorta. Eur J Pharmacol 398: 285–296 [DOI] [PubMed] [Google Scholar]
- Brodde O.E. (1993) β-Adrenoceptors in cardiac disease. Pharmacol Ther 60: 405–430 [DOI] [PubMed] [Google Scholar]
- Brodde O.E., Michel M. (1999) Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev 51: 651–689 [PubMed] [Google Scholar]
- Busse R., Edwards G., Félétou M., Fleming I., Vanhoutte P.M., Weston A.H. (2002) EDHF: Bringing the concepts together. Trends Pharmacol Sci 23: 374–380 [DOI] [PubMed] [Google Scholar]
- Caron M.G., Lefkowitz R.J. (1993) Catecholamine receptors: Structure, function, and regulation. Recent Prog Horm Res 48: 277–290 [DOI] [PubMed] [Google Scholar]
- Chaudry A., MacKenzie R.G., Georgic L.M., Granneman J.G. (1994) Differential interaction of beta (1)- and beta (3)-adrenergic receptors with G (i) in rat adipocytes. Cell Signal 6: 457–465 [DOI] [PubMed] [Google Scholar]
- Cheng H., Zhang Z., Onishi K., Ukai T., Sane D., Cheng C. (2001) Upregulation of functional β3-adrenergic receptor in the failing canine myocardium. Circ Res 89: 599. [DOI] [PubMed] [Google Scholar]
- Clark B.J., Bertholet A. (1983) Effects of pindolol on vascular smooth muscle. Gen Pharmacol 14: 117–119 [DOI] [PubMed] [Google Scholar]
- Clément K., Vaisse C., Manning B.S.J., Basdevant A., Guy-Grand B., Ruiz J., et al. (1995) Genetic variation in the β3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity. N Engl J Med 333: 352–354 [DOI] [PubMed] [Google Scholar]
- Dessy C., Moniotte S., Ghisdal P., Havaux X., Noirhomme P., Balligand J.L. (2004) Endothelial β3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. Circulation 110: 948–954 [DOI] [PubMed] [Google Scholar]
- Dinçer U.D., Bidasee K., Güner S., Tay A., Özçelikay A.T., Altan V.M. (2001) The effect of diabetes on expression of β1-, β2-, and β3-adrenoreceptors in rat hearts. Diabetes 50: 455–461 [DOI] [PubMed] [Google Scholar]
- Dixon R.A., Kobilka B.K., Strader D.J., Benovic J.L., Dohlman H.G., Frielle T., et al. (1986) Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature (Lond) 321: 75–79 [DOI] [PubMed] [Google Scholar]
- Doggrell S.A. (1990) Relaxant and beta 2-adrenoceptor blocking activities of (±)-,(+)- and (—)-pindolol on the rat isolated aorta. J Pharm Pharmacol 42: 444–446 [DOI] [PubMed] [Google Scholar]
- Dolan J.A., Muenkel H.A., Burns M.G., Pellegrino S.M., Fraser C.M., Pietri F., et al. (1994) Beta-3 adrenoceptor selectivity of the dioxolane dicarboxylate phenethanolamines. J Pharmacol Exp Ther 269: 1000–1006 [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Hartzell H.C., Lagrutta A.A. (1989) Modulation of the delayed rectifier potassium current in frog cardiomyocytes by beta-adrenergic agonists and magnesium. J Physiol (Lond) 415: 251–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emorine L., Blin N., Strosberg A.D. (1994) The human β3-adrenoceptor: The search for a physiological function. Trends Pharmacol Sci 15: 3–7 [DOI] [PubMed] [Google Scholar]
- Emorine L.J., Marullo S., Briend-Sutren M.M., Patey G., Tate K., Delavier-Klutchko C., et al. (1989) Molecular characterization of the human β3-adrenergic receptor. Science (Wash DC) 245: 1118–1121 [DOI] [PubMed] [Google Scholar]
- Engel G., Hoyer D., Bertold R., Wagner H. (1981) (±)(125Iodo)cyanopindolol, a new ligand for β-adrenoceptors: Identification and quantification of subclasses of β-adrenoceptors in guinea pig. Naunyn-Schmiedeberg's Arch Pharmacol 317: 277–285 [DOI] [PubMed] [Google Scholar]
- Festa A., Krugluger W., Shnawa N., Hopmeier P., Haffner S., Schernthaner G. (1999) Trp64Arg Polymorphism of the β3-adrenergic receptor gene in pregnancy: Association with mild gestational diabetes mellitus. J Clin Endocrinol Metab 84(5): 1695–1699 [DOI] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K.W., Caron M.G., Lefkowitz R.J., Kobilka B.K. (1987) Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A 84: 7920–7924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Isobe K., Zeng Q., Suzukawa K., Takekoshi K., Kawakami Y. (2007) β-adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-α expression in adipocytes. Eur J Pharmacol 569: 155–162 [DOI] [PubMed] [Google Scholar]
- Fu L., Isobe K., Zeng Q., Suzukawa K., Takekoshi K., Kawakami Y. (2008) The effects of beta(3)-adrenoceptor agonist CL-316,243 on adiponectin, adiponectin receptors and tumor necrosis factor-alpha expressions in adipose tissues of obese diabetic KKAy mice. Eur J Pharmacol 584(1): 202–206 [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Ikegami H., Yamato E., Takekawa K., Nakagawa Y., Hamada Y., et al. (1996) Association of Trp64Arg mutation of the beta3-adrenergic-receptor with NIDDM and body weight gain. Diabetologia 39: 349–352 [DOI] [PubMed] [Google Scholar]
- Galitzky J., Langin D., Montrastruc J.L., Lafontan M., Berlan M. (1998) On the presence of a putative fourth β-adrenoceptor in human adipose tissue. Trends Pharmacol Sci 19: 164–165 [DOI] [PubMed] [Google Scholar]
- Galitzky J., Reverte M., Carpene C., Lafontan M., Berlan M. (1993) β3-Adrenoceptors in dog adipose tissue: Studies on their involvement in the lipomobilizing effect of catecholamines. J Pharmacol Exp Ther 266: 358–366 [PubMed] [Google Scholar]
- Gan R.T., Li W.M., Wang X., Wu S., Kong Y.H. (2007a) Effect of beta3-adrenoceptor antagonist on the cardiac function and expression of endothelial nitric oxide synthase in a rat model of heart failure. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 19(11): 675–678 [PubMed] [Google Scholar]
- Gan R.T., Li W.M., Xiu C.H., Shen J.X., Wang X., Wu S., et al. (2007b) Chronic blocking of beta 3-adrenoceptor ameliorates cardiac function in rat model of heart failure. Chin Med J (Engl) 120(24): 2250–2255 [PubMed] [Google Scholar]
- Gauthier C., Leblais V., Kobzik L., Trochu J.N., Khandoudi N., Bril A., et al. (1998) The negative inotropic effect of β3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102: 1377–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C., Tavernier G., Charpentier F., Langin D., Le Marec H. (1996) Functional β3-adrenoceptor in the human heart. J Clin Invest 98: 556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C., Tavernier G., Trochu J., Leblais V., Laurent K., Langin D., et al. (1999) Interspecies differences in the cardiac negative inotropic effects of β3-adrenoceptor agonists. Pharmacol Expt Ther 290(2): 687–693 [PubMed] [Google Scholar]
- Granneman J. (1992) Effects of agonist exposure on the coupling of β1-β3-adrenergic receptors to adenylyl cyclase in isolated adipocytes. J Pharmacol Exp Ther 261: 638–642 [PubMed] [Google Scholar]
- Granneman J., Lahners K., Chaudhry A. (1991) Molecular cloning and expression of the rat β-adrenergic receptor. Mol Pharmacol 40: 895–899 [PubMed] [Google Scholar]
- Granneman J., Lahners K., Rao D. (1992) Rodent and human beta 3-adrenergic receptor genes contain an intron within the protein-coding block. Mol Pharmacol 42: 964–970 [PubMed] [Google Scholar]
- Grant T.L., Mayers R.M., Quayle S.P., Briscoe M.G., Howe R., Rao B.S., et al. (1994) Zeneca ZD2079 is a novel β3-adrenoceptor agonist. Br J Pharmacol 112: 213P [Google Scholar]
- Grujic D., Susulic V., Harper M.E., Himms-Hagen J., Cunningham B., Corkey B., et al. (1997) β3-Adrenergic receptors on white and brown adipocytes mediate β3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. JBC online 272: 17686–17693 [DOI] [PubMed] [Google Scholar]
- Hescheler J., Schultz G. (1994) Heterotrimeric G proteins involved in the modulation of voltage-dependent calcium channels of neuroendocrine cells. Ann N Y Acad Sci 733: 306–312 [DOI] [PubMed] [Google Scholar]
- Hollenga C., Zaagsma J. (1989) Direct evidence for the atypical nature of functional beta-adrenoceptors in rat adipocytes. Br J Pharmacol 98: 1420–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B., Howe R., Rao B., Stribling D. (1992) A novel selective adrenoceptor agonist of brown fat and thermogenesis. Am J Clin Nutr 55: 262S–264S [DOI] [PubMed] [Google Scholar]
- Huang X., Hamajima N., Saito T., Matsuo K., Mizutani M., Iwata H., et al. (2001) Possible association of β2- and β3-adrenergic receptor gene polymorphisms with susceptibility to breast cancer. Breast Cancer Res 3: 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson D.S., Bengtsson T., Evans B.A., Summers R.J. (2002) Mouse β3a- and β 3b-adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signalling pathways. Br J Pharmacol 135: 1903–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper J.R., Insel P.A. (1992) Evolving concepts of partial agonism: The beta-adrenergic receptor as a paradigm. Biochem Pharmacol 43: 119–130 [DOI] [PubMed] [Google Scholar]
- Jurkiewicz N.K., Sanguinetti M.C. (1993) Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res 72: 75–83 [DOI] [PubMed] [Google Scholar]
- Kadowaki H., Yasuda K., Iwamoto K., Otabe S., Shimokawa K., Silver K., et al. (1995) A mutation in the β3-adrenergic receptor gene is associated with obesity and hyperinsulinemia in Japanese subjects. Biochem Biophys Res Commun 215: 555–560 [DOI] [PubMed] [Google Scholar]
- Kathofer S., Zhang W., Karle C., Thomas D., Schoels W., Kiehn J. (2000) Functional coupling of human β3-adrenoreceptors to the KvLQT1/MinK potassium channel. J Biol Chem 275: 26743–26747 [DOI] [PubMed] [Google Scholar]
- Kaumann A.J. (1997) Four β-adrenoceptor subtypes in mammalian heart. Trends Pharmacol Sci 18: 70–76 [DOI] [PubMed] [Google Scholar]
- Kohout T., Takaoka H., McDonald P., Perry S., Mao L., Lefkowitz R., et al. (2001) Augmentation of cardiac contractility mediated by the human β3-adrenergic receptor overexpressed in the hearts of transgenic mice. Circulation 104: 2485. [DOI] [PubMed] [Google Scholar]
- Kong Y.H., Li W.M., Tian Y. (2004) Effect of beta3-adrenoreceptors agonist on beta3-adrenoreceptors expression and myocyte apoptosis in a rat model of heart failure. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 16(3): 142–147 [PubMed] [Google Scholar]
- Kong Y.H., Zhang Y., Li N., Zhang L., Gao Y.H., Xue H.J., et al. (2010) Association between beta3-adrenergic receptor and oxidative stress in chronic heart failure rats. Zhonghua Xin Xue Guan Bing Za Zhi 38(5): 435–439 [PubMed] [Google Scholar]
- Krief S., Lönnqvist F., Raimbault S., Baude B., Van Spronsen A., Arner P., et al. (1993) Tissue distribution of β3-adrenoceptor receptor mRNA in man. J Clin Invest 91: 344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langin D., Portillo M.P., Saulnier-Blache J.S., Lafontan M. (1991) Coexistence of three β -adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol 199: 291–301 [DOI] [PubMed] [Google Scholar]
- Large V., Hellstrom L., Reynisdottir S., Lonnqvist F., Eriksson P., Lannfeld L., et al. (1997) Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Invest 100: 3005–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelias J.M., Kaghad M., Rodriguez M., Chalon P., Bonnin J., Dupre I., et al. (1993) Molecular cloning of a human beta 3-adrenergic receptor cDNA. FEBS Lett 324: 127–130 [DOI] [PubMed] [Google Scholar]
- Levasseur S., Pigeon C., Reyl-Desmars F., Caput D., Lewin M.J.M. (1995) Adenylyl cyclase stimulation by the human and rat β3-adrenergic receptor isoforms expressed in the CHO cell. Gastroenterol Clin Biol 19: 668–672 [PubMed] [Google Scholar]
- Liggett S.B., Freedman N.J., Schwinn D.A., Lefkowitz R.J. (1993) Structural basis for receptor subtype-specific regulation revealed by a chimeric β3/β2-adrenergic receptor. Proc Natl Acad Sci U S A 90: 3665–3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Pérusse F., Bukowiecki L.J. (1994) Chronic norepinephrine infusion stimulates glucose uptake in white and brown adipose tissues. Am J Physiol Regul Integr Comp Physiol 266: R914–R920 [DOI] [PubMed] [Google Scholar]
- Liu X., Pérusse F., Bukowiecki L.J. (1998) Mechanisms of the antidiabetic effects of the β3-adrenergic agonist CL-316243 in obese Zucker-ZDF rats. Am J Physiol Regul Integr Comp Physiol 274: R1212–R1219 [DOI] [PubMed] [Google Scholar]
- Lo C.F., Numann R. (1998) Independent and exclusive modulation of cardiac delayed rectifying K+ current by protein kinase C and protein kinase A. Circ Res 83: 995–1002 [DOI] [PubMed] [Google Scholar]
- Lönnqvist F., Krief S., Strosberg A.D., Nyberg B., Emorine L.J., Arner P. (1993) Evidence for a functional β3-adrenoceptor in man. Br J Pharmacol 110: 929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell B.B., Flier J.S. (1995) The potential significance of β3-adrenoceptor receptors. J Clin Invest 95: 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A., McLean M., MacAuly L., Shaw A.M. (1999) Effects of propranolol and L-NAME on beta-adrenoceptor-mediated relaxation in rat carotid artery. J Auton Pharmacol 19: 145–149 [DOI] [PubMed] [Google Scholar]
- Mc Laughlin D., MacDonald A. (1990) Evidence for the existence of ‘atypical’ β-adrenoceptors β-adrenoceptors) mediating relaxation in the rat distal colon. Br J Pharmacol 101: 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano C.A., Allen L.F., Rockman H.A., Dolber P.C., McMinn T.R., Chien K.R., et al. (1994a) Enhanced myocardial function in transgenic mice overexpressing the beta2-adrenergic receptor. Science 264: 582–586 [DOI] [PubMed] [Google Scholar]
- Milano C.A., Dolber P.C., Rockman H.A., Bond R.A., Venable M.E., Allen L.F., et al. (1994b) Myocardial expression of a constitutively active alpha1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc Natl Acad Sci USA 91: 10109–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan P., Brutsaert D.L., Paulus W.J., Sys S.U. (1996) Myocardial contractile response to nitric oxide and cGMP. Circulation 93: 1223–1229 [DOI] [PubMed] [Google Scholar]
- Mohell N., Dicker A. (1989) The β-adrenergic radioligand (3H)-CGP-12177, generally classified as an antagonist, is a thermogenic agonist in brown tissue. Biochem J 261: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniotte S., Kobzik L., Feron O., Trochu J., Gauthier C., Balligand J.L. (2001) Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 103: 1649–1655 [DOI] [PubMed] [Google Scholar]
- Morris A.D., Petrie J.R., Connel J.M.C. (1994) Insulin and hypertension. J Hypertens 12: 633–642 [PubMed] [Google Scholar]
- Muzzin P., Revelli J., Khune F., Gocayne J., Combie W.M., Venter J., et al. (1991) An adipose tissue specific β-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem 266: 24053–24058 [PubMed] [Google Scholar]
- Oriowo M.A. (1994) Atypical beta-adrenoceptors in the rat isolated common carotid artery. Br J Pharmacol 113: 699–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelat M., Verwaerde P., Galitzky J., Lafontan M., Berlan M., Senard J., et al. (2003) High isoproterenol doses are required to activate β3-adrenoceptor-mediated functions in dogs. Pharmacol Expt Ther 304: 246–253 [DOI] [PubMed] [Google Scholar]
- Piepho R.W. (1991) Heterogeneity of calcium channel blockers. Hosp Pharm 26: 856–864 [Google Scholar]
- Pietri-Rouxel F., Strosberg A.D. (1995) Pharmacological characteristics and species-related variations of β3-adroneceptor receptors. Fundam Clin Pharmacol 9: 211–218 [DOI] [PubMed] [Google Scholar]
- Rasmussen H.H., Figtree G.A., Krum H., Bundgaard H. (2009) The use of beta3-adrenergic receptor agonists in the treatment of heart failure. Curr Opin Investig Drugs 10(9): 955–962 [PubMed] [Google Scholar]
- Rautureau Y., Toumaniantz G., Serpillon S., Jourdon P., Trochu J.N., Gauthier C. (2002) Beta3-adrenoceptor in rat aorta: Molecular and biochemical characterization and signalling pathway. Br J Pharmacol 137:153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi S., Kazerounian S., Banwait K., Schulz S., Waldman S., Rattan S. (2003) Functional and molecular characterization of β-adrenoceptors in the internal anal sphincter. Pharmacol Expt Ther 305(2): 615–624 [DOI] [PubMed] [Google Scholar]
- Roberts S.J., Papaionanou M., Evans B.A., Summers R.J. (1999) Characterization of β-adrencoceptor mediated smooth muscle relaxation and the detection of mRNA for β1-, β2- and β3-adrenoceptors in rat ileum. Br J Pharmacol 127: 949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.J., Russel F.D., Molenaar P., Summers R.J. (1995) Characterization and localization of atypical β-adrenoceptors in rat ileum. Br J Pharmacol 116: 2549–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Carillon C., Coquerel A., Le Fur G., Ferrara P., Caput D., et al. (1995) Evidence for the presence of β3-adrenergic receptor m-RNA in the human brain. Mol Brain Res 29: 369–375 [DOI] [PubMed] [Google Scholar]
- Rohrer D.K., Chruscinski A., Schauble E.H., Bernstein D., Kobilka B.K. (1999) Cardiovascular and metabolic alterations in mice lacking both β1 and β2-adrenergic receptors. J Biol Chem 274: 16701–16708 [DOI] [PubMed] [Google Scholar]
- Rozec B., Serpillon S., Toumaniantz G., Sèze C., Rautureau Y., Baron O., et al. (2005) Characterization of beta3-adrenoceptors in human internal mammary artery and putative involvement in coronary artery bypass management. J Am Coll Cardiol 46: 351–359 [DOI] [PubMed] [Google Scholar]
- Sanguinetti M.C., Curran M.E., Zou A., Shen J., Spector P.S., Atkinson D.L., et al. (1996) Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKS potassium channel. Nature 384: 80–83 [DOI] [PubMed] [Google Scholar]
- Sanguinetti M.C., Jurkiewicz N.K., Scott A., Siegl P.K.S. (1991) Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action. Circ Res 68: 77–84 [DOI] [PubMed] [Google Scholar]
- Shen Y.T., Claus T.H. (1993) Potential mechanisms of β3-adrenoceptor-induced peripheral vasodilation in conscious dogs. Clin Res 41: 348A [Google Scholar]
- Shen Y.T., Zhang H., Vatner S.F. (1994) Peripheral vascular effects of beta-3 adrenergic receptor stimulation in conscious dogs. J Pharmacol Exp Ther 268: 466–473 [PubMed] [Google Scholar]
- Simon M.I., Strathmann M.P., Gautam N. (1991) Diversity of G proteins in signal transduction. Science 252: 802–808 [DOI] [PubMed] [Google Scholar]
- Soeder K.J., Snedden S.K., Cao W., Della Rocca G.J., Daniel K.W., Luttrell L.M., et al. (1999) The β3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism. J Biol Chem 274: 12017–12022 [DOI] [PubMed] [Google Scholar]
- Strosberg A.D. (1993) Structure, function, and regulation of adrenoceptor receptors. Protein Sci 2: 1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg A.D. (1997) Structure and function of the β3-adrenoceptor. Ann Rev Pharmacol Toxicol 37: 421–450 [DOI] [PubMed] [Google Scholar]
- Strosberg A.D., Pietri-Rouxel F. (1996) Function and regulation of the three β-adrenoceptors. Trends Pharmacol Sci 17: 373–381 [DOI] [PubMed] [Google Scholar]
- Strosberg A.D., Gerhardt C.C., Gros J., Jockers R., Pietri-Rouxel F. (1998) Reply on the putative existence of a fourth β-adrenoceptor: Proof is still missing. Trends Pharmacol Sci 19: 165–166 [Google Scholar]
- Sugasawa T., Matsuzaki M., Morooka S., Foignant N., Blin N., Strosberg A.D. (1992) In vitro study of a novel atypical beta-adrenoceptor agonist, SM-1044. Eur J Pharmacol 216: 207–215 [DOI] [PubMed] [Google Scholar]
- Summers R.J., Kompa A., Roberts S.J. (1997) β -Adrenoceptor subtypes and their desensitization mechanisms. J Auton Pharmacol 17: 331–343 [DOI] [PubMed] [Google Scholar]
- Tagaya E., Tamaoki J., Takemura H., Isono K., Nagai A. (1999) Atypical adrenoceptor-mediated relaxation of canine pulmonary artery through a cyclic adenosine monophosphate-dependent pathway. Lung 177: 321–332 [DOI] [PubMed] [Google Scholar]
- Takahashi H., Yoshida T., Nishimura N., Nakanishi T., Kondo M., Yoshimura M. (1992) Beta3-adrenergic agonist, BRL-26830A, and alpha/beta blocker, arotinolol, markedly increase regional blood flow in the brown tissue in anesthetized rats. Jap Circ J 56: 936–942 [DOI] [PubMed] [Google Scholar]
- Takayama S., Furukawa Y., Ren L.M., Inoue Y., Sawaki S., Chiba S. (1993) Positive chronotropic and inotropic responses to BRL 37344, a β3-adrenoceptor agonist in isolated, blood-perfused dog atria. Eur J Pharmacol 231: 315–321 [DOI] [PubMed] [Google Scholar]
- Tate K., Briend-Sutren M., Emorine L.J., Klutchko C., Marullo S., Strosberg A.D. (1991) Expression of three human β-adrenergic-receptor subtypes in transfected Chinese ovary cells. Eur J Biochem 196: 357–361 [DOI] [PubMed] [Google Scholar]
- Tavernier G., Galitzky J., Bousquet-Melou A., Montastruc J.L., Berlan M. (1992) The positive chronotropic effect induced by BRL 37344 and CGP 12177, two beta-3 adrenoceptor agonists, does not involve cardiac beta adrenoceptors but bar-oreflex mechanisms. J Pharmacol Exp Ther 263: 1083–1090 [PubMed] [Google Scholar]
- Tavernier G., Galitzky J., Bousquet-Melou A., Montastruc J.L., Berlan M. (1992) The positive chronotropic effect induced by BRL 37344 and CGP 12177, two β3-adrenergic agonists, does not involve cardiac β-adrenoceptors but reflex mechanisms. J Pharmacol Exp Ther 91: 344–349 [PubMed] [Google Scholar]
- Tesfamariam B., Allen G.T. (1994) β1- and β2-adrenoceptor antagonist activities of ICI-215001, a putative β3-adrenoceptor agonist. Br J Pharmacol 112: 55–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama Y., Hayakawa K., Shinagawa K., Akahane M., Ajisawa Y., Park Y.C., Kurita T. (1998) β -Adrenoceptor subtypes in the ureteral smooth muscle of rats, rabbits and dogs. Eur J Pharmacol 352: 269–278 [DOI] [PubMed] [Google Scholar]
- Tonolo G., Melis M.G., Secchi G., Atzeni M.M., Angius M.F., Carboni A., et al. (1999) Association of Trp64Arg β3-adrenergic-receptor gene polymorphism with essential hypertension in the Sardinian population. J Hypertens 17: 33–38 [DOI] [PubMed] [Google Scholar]
- Trochu J.N., Leblais V., Rautureau Y., Bévérelli F., Le Marec H., Berdeaux A., Gauthier C. (1999) Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracia aorta. Br J Pharmacol 128: 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallerand A.L., Pérusse F., Bukowiecki L.J. (1987) Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol Endocrinol Metab 253: E179–E186 [DOI] [PubMed] [Google Scholar]
- Vallerand A.L., Pérusse F., Bukowiecki L.J. (1990) Stimulatory effects of cold exposure and cold acclimation on glucose uptake in rat peripheral tissues. Am J Physiol Regul Integr Comp Physiol 259: R1043–R1049 [DOI] [PubMed] [Google Scholar]
- Van Spronsen A., Nahmias C., Krief S., Briend-Sutren M.M., Strosberg A.D., Emorine L.J. (1993) The promoter and intron/exon structure of the human and mouse beta 3-adrenergic-receptor genes. Eur J Biochem 213: 1117–1124 [DOI] [PubMed] [Google Scholar]
- Varnum M.D., Busch A.E., Bond C.T., Maylie H., Adelman J.P. (1993) The Min K channel underlies the cardiac potassium current IKs and mediates species-specific responses to protein kinase C. Proc Natl Acad Sci U S A 90: 11528–11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard P., Macrez N., Coussin F., Morel J.L., Mironneau J. (2000) Beta-3 adrenergic stimulation of L-type Ca2+ channels in rat portal vein myocites. Br J Pharmacol 129: 1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J., Silver K., Bogardus C., Knowler W., Celi P., Austin S., et al. (1995) Time of onset of non-insulin-dependent diabetes mellitus and genetic variation in the β3-adrenergic-receptor gene. New Engl J Med 333(6): 343–347 [DOI] [PubMed] [Google Scholar]
- Wheeldon N.M., McDevitt D.G., Lipworth B.J. (1993) Investigation of putative cardiac β3-adrenoceptors in man. Q J Med 86: 255–261 [PubMed] [Google Scholar]
- Wheeldon N.M., McDevitt D.G., Lipworth B.J. (1994) Cardiac effects of the β3-adrenoceptor agonist BRL 35135 in man. Br J Clin Pharmacol 37: 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widén E., Lehto M., Kanninen T., Walston J., Shuldiner A.R., Groop L.C. (1995) Association of a polymorphism in the β3-adrenergic-receptor gene with features of the insulin resistance syndrome in Finns. N Engl J Med 333: 348–351 [DOI] [PubMed] [Google Scholar]
- Xiao R.P., Ji X., Lakatta E.G. (1995) Functional coupling of beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol 47: 322–329 [PubMed] [Google Scholar]
- Yoshida T., Hiraoka N., Kondo M. (1991) Effect of a β3-adrenoceptor agonist, BRL 26830A, on insulin and glucagon release in mice. Endocrinol J pn 38: 641–646 [DOI] [PubMed] [Google Scholar]
- Zaagsma J., Nahorski S.R. (1990) Is the adipocyte β-adrenoceptor a prototype for the recently cloned atypical beta 3-adrenoceptor?. Trends Pharmacol Sci 11: 3–7 [DOI] [PubMed] [Google Scholar]
- Zeng J., Laurita K.R., Rosenbaum D.S., Rudy Y. (1995) Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type: Theoretical formulation and their role in repolarization. Circ Res 77: 140–152 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Cheng H., Onishi K., Ohte N., Wannenburg Y., Cheng C. (2005) Enhanced inhibition of L-type Ca2+ current by β3-adrenergic stimulation in failing rat heart. J Pharmacol Exp Ther 315: 1203–1211 [DOI] [PubMed] [Google Scholar]
- Zhou S., Tan A.Y., Paz O., Ogawa M., Chou C., Hayashi H. (2008) Antiarrhythmic effects of beta3-adrenergic receptor stimulation in a canine model of ventricular tachycardia. Heart Rhythm 5: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]