Abstract
A continuous search for a permanent cure for diabetes mellitus is underway with several remarkable discoveries over the past few decades. One of these is the potential of pancreatic stem/progenitor cells to rejuvenate functional β cells. However, the existence of these cell populations is still obscure and a lack of phenotype characterization hampers their use in clinical settings. Cellular reprogramming through induced pluripotent stem (iPS) cell technology can become an alternative strategy to generate insulin-producing cells in a relatively safe (autologous-derived cells, thus devoid of rejection risk) and efficient way (high cellular proliferation) but retain a precise morphological and genetic composition, similar to that of the native β cells. iPS cell technology is a technique of transducing any cell types with key transcription factors to yield embryonic-like stem cells with high clonogenicity and is able to give rise into all cell lineages from three germ layers (endoderm, ectoderm, and mesoderm). This approach can generate β-like pancreatic cells that are fully functional as proven by either in vitro or in vivo studies. This novel proof-of-concept stem cell technology brings new expectations on applying stem cell therapy for diabetes mellitus in clinical settings.
Keywords: cellular reprogramming, diabetes mellitus, induced pluripotent stem (iPS) cells, pancreatic β cells
Introduction
Diabetes mellitus (DM) is a global emerging disease with progressive incidence worldwide. There were 171 million people in the world suffering with DM in 2000 and is projected to increase to 366 million people by 2030 [Wild et al. 2004]. Two distinct types of DM are well characterized, i.e. type 1 (T1DM) and type 2 (T2DM), in which T1DM results from progressive β cell destruction mostly due to autoimmunity [Gillespie, 2006] and T2DM that is mainly caused by a combination of insulin resistance and inadequate insulin secretion [Ali and Dayan, 2009]. As a consequence, β cell mass is reduced to about 50% in the later stages [Gallwitz, 2008], causing 20–30% of T2DM patients to initiate insulin therapy. T1DM and T2DM are associated with long-term major microvascular and macrovascular complications despite intensive insulin treatment [Ali and Dayan, 2009]. Matching subcutaneous insulin dose to control blood glucose level is challenging for both diabetic types [Ali and Dayan, 2009; Efrat, 2008; Limbert et al. 2008; Eisenbarth, 2007], therefore it is difficult to maintain a long-term control [Gallwitz, 2008]. Considering these problems have lead to the initiative of β cell replacement by islets allograft transplantation. However, this therapeutic approach is hindered by limited cadaveric donors, constant destruction by autoimmune response, and toxicity due to chronic use of immunosuppressants [Eisenbarth, 2007], as well as the fact that only 10% of the transplanted patients successfully maintain insulin independence within 5 years due to graft cell loss [Ryan et al. 2005].
Hence, regeneration of functional β cell mass from human stem cells represent the most promising approach for cure in T1DM nowadays. Patients with T2DM who require exogenous insulin may also benefit from β cell replacement therapy, considering the occurrence of progressively worsening β cell failure [Efrat, 2008]. The efforts to regenerate functional β cells from adult pancreatic stem cells have been widely explored. However, the progress is slow due to the lack of a phenotype definition for pancreatic stem/progenitor cells. The use of human embryonic stem cells (ESCs) is limited by ethical issues and a great risk of tumorigenicity [Yao et al. 2006; Assady et al. 2001; Soria et al. 2000]. At present, cellular reprogramming through induced pluripotent stem (iPS) cell technology represents a remarkable breakthrough in the generation of insulin-producing pancreatic β cells. The process involves administration of four transcription factors associated with pluripotency into various cell types which will trigger the cell to dedifferentiate into a pluripotent state in which it was redifferentiated to become β cells. Cellular reprogramming can be initiated across cell lineage boundaries (e.g. fibroblast to β cells) [Aguayo-Mazzucato and Bonner-Weir, 2010]. The corresponding cellular technology offers solutions to many limited aspects of stem cell therapy which have hampered its use to date, including the generation of safe, efficient, and effective insulin-secreting cells, no risk of graft rejection, and lack of ethical concerns. This review aims to elaborate on the steps of iPS-based technology, molecular mechanisms of cellular reprogramming, similarities between iPS and ESCs, evidence of insulin-secreting β cells from fibroblast-transformed iPS cells, as well as elucidating their major obstacles and future strategies to solve these problems.
Overview of the iPS cells: A major breakthrough in cellular reprogramming
History of iPS cells
Through a remarkable technology of so-called cellular reprogramming, it is now possible to generate pluripotent stem cells from terminally differentiated cells (e.g. skin fibroblasts) simply by modifying their epigenetic profiles. This can be achieved by deliberately inducing the expression of pluripotency-associated genes (i.e. turning on) while repressing the expression of differentiation-associated genes (i.e. turning off) with the end result is the reacquisition of embryonic traits [Aasen et al. 2008; Reik, 2007]. Subsequently, the resulting pluripotent stem cells can be directed to re-differentiate into cells of all three germ layers, thus crossing the cell lineage boundaries (fibroblasts to insulin-producing β cells). Of course, this technological breakthrough has significant implications for overcoming the ethical issues associated with human ESC derivation from human embryos that hamper its clinical use nowadays. These brand new pluripotent stem cells have been officially termed iPS cells. The generation of iPS cells from differentiated somatic cells was first demonstrated in 2006 by Takahashi and Yamanaka [Takahashi and Yamanaka, 2006]. In that pioneer study in an animal model, they reprogrammed adult and embryonic mouse fibroblasts by transfecting the cells with plasmids containing four selected transcription factors (Oct4, Sox2, cMyc, and Klf4) which are known to be involved in the regulation of expression of self-renewal and pluripotency genes in ESCs. They reported that the transfection was sufficient to induce the dedifferentiation of fibroblasts into ESC-like iPS cells. The result was soon validated by other studies that used the same combination of ectopic stemness factor to reprogram fibroblasts into ESC-like iPS cells [Maherali and Hochedlinger, 2008; Okita et al. 2007; Wernig et al. 2007]. Furthermore, with the more optimized culture medium and transduction methods, iPS cells have been successfully generated from human somatic cells, such as dermal fibroblasts [Lowry et al. 2008; Takahashi et al. 2007; Yu et al. 2007], and keratinocytes [Aasen et al. 2008]. Evidence from numerous studies on iPS cells have proved that iPS cells can be generated from somatic cells of all three germ layers. Further studies also reported that iPS cells can be generated by using a safer combination of ectopic stemness factor (Oct4, Sox2, Lin28, and Nanog) [Yu et al. 2007].
The characteristics of iPS cells
Plenty of evidence from both in vitro and in vivo studies has shown that the characteristics of iPS cells are highly similar to those of ES cells. The morphologic characteristics of iPS cells are similar to those of ESCs [Takahashi et al. 2007; Yu et al. 2007]. iPS cells also express surface antigens that are specifically expressed by human ESCs such as SSEA-3, SSEA-4, TRA-1–60, TRA-1-81, TRA-2-49/6E (alkaline phosphatase), and Nanog [Lowry et al. 2008; Park et al. 2008; Takahashi et al. 2007; Yu et al. 2007]. In regard to its self-renewal capacity, iPS cells have been shown able to maintain the normal telomere length despite unlimited replication [Stadtfeld et al. 2008; Takahashi et al. 2007].
With regard to its pluripotent capacity, in vitro studies have proved that iPS cells can differentiate into cells of all three germ layers. When cultured in the suspension medium used to culture and differentiate ESCs, iPS cells spontaneously aggregate into cluster of cells known as an embryoid body (EB) in a manner similar to those of human ESCs and differentiate into cells expressing the gene markers of endoderm (Foxa2, Sox17, GATA 4/6, α-fetoprotein, and albumin), mesoderm (Brachyury-T, Msp1/2, Isl-1, α-actin, £-globin, and Runx2), and ectoderm (Sox1, Nestin, Pax6, GFAP, Olig2, neuro-filament, and β-III Tubulin) cells [Maehr et al. 2009; Lowry et al. 2008; Takahashi and Yamanaka, 2006]. Furthermore, under specific guided differentiation protocols, iPS cells can also differentiate into certain lineages of cells, such as erythrocytes [Ye et al. 2009], neurons [Hu et al. 2010; Wernig et al. 2008], cardiomyocytes [Moretti et al. 2010; Nelson et al. 2009; Zhang et al. 2009b; Zwi et al. 2009], and insulin-producing β-like cells [Alipio et al. 2010; Maehr et al. 2009; Zhang et al. 2009a; Tateishi et al. 2008].
Further evidence of the pluripotent capacity of iPS also comes from numerous in vivo studies. When injected subcutaneously into an immuno-deficient mice, human iPS cells differentiate and develop teratoma consisting of cells from three germ layers, such as epithelial cells, myocytes, chondrocytes, adipocytes, neurons, and epidermis [Okita et al. 2007; Takahashi et al. 2007]. The pluripotent capacity of iPS cells are also investigated in utero in animal models. Theoretically, in order to prove the in utero pluripotent capacity of iPS cells, the injected iPS cells should be able to integrate with the conventional diploid embryos (usually at a morula stage, so-called tetraploid complementation) and should be well sustained throughout the embryonic development until eventually generated a chimeric organism [Martinez-Fernandez et al. 2009]. Indeed, when murine iPS cells were injected into the mice blastocyst, it contributed to the formation of germline competent chimeric mice [Okita et al. 2007]. Further analysis revealed that iPS cell-derived progeny cells were distributed evenly in various tissues of mice, such as brain, lungs, liver, kidneys, muscles, skins, and gonadal tissues. One experiment even had successfully generated a germline competent mouse which was able to reproduce a viable offspring [Zhao et al. 2009]. Furthermore, the genomic profile between iPS cells and ESCs were similar. iPS cells retained a normal karyotype [Maehr et al. 2009; Takahashi et al. 2007; Wernig et al. 2007; Yu et al. 2007] and also expressed hTERT and telomerase, which are the characteristic of pluripotent cells. iPS cells actively expressed genes that involved in the regulation of self-renewal and pluripotency state (e.g. Oct4, Sox2, Nanog, Rex1, Gdf3, Fgf4, Esg1, DPPA2, DPPA4, hTERT) which are also known as specific gene markers for human ESCs [Maehr et al. 2009; Takahashi et al. 2007]. Moreover, the expression of lineage-specific or differentiation-associated genes into the three germ layers (e.g. Brachyury, Mesp1, Sox17, Foxa2, NeuroD1, Pax6) had been shown to be depressed or silenced in iPS cells just like human ES cells.
The profiles were also similar, such as the DNA methylation profiles [Mikkelsen et al. 2008; Yu et al. 2007; Bernstein et al. 2006] and the histone modification profiles [Bernstein et al. 2007, 2006; Mikkelsen et al. 2007]. Genomic sequencing analysis had revealed the presence of CpG islands demethylation on the promoter of genes involved in the regulation of self-renewal and pluripotency state, thus making the genes actively expressed in iPS cells [Okita et al. 2007; Takahashi et al. 2007]. iPS cells also expressed bivalent histone protein H3K27/H3K4me3 (i.e. H3 lysine 27/H3 lysine 4 trimethylation) in their chromatin [Mikkelsen et al. 2007]. H3K27/H3K4me3 are proteins specifically expressed in human ESCs and act to suppress the expression of lineage-specific or differentiation-associated genes [Maherali et al. 2008; Mikkelsen et al. 2008, 2007; Bernstein et al. 2007].
Thus, the ability of iPS cells to fulfill the most stringent criteria of pluripotency has ensured its quality and authenticity to an undeniable position. In this era of regenerative medicine, iPS cells bring a new hope as a potential source of stem cells for cell replacement therapy and would probably replacing human ESCs in the future.
Generation of iPS cell-derived insulin-producing pancreatic β cells
Owing to its unlimited replicative capacity (i.e. self-renewal) and pluripotency, the differentiation potential of iPS cells into pancreatic endocrine lineage cells (i.e. the functional insulin-producing pancreatic β cells) has been thoroughly investigated. Indeed, positive results have been reported consistently among several in vitro studies that used protocols that mimic the mechanism of in vivo pancreas development to guide the differentiation of iPS cells into functional β cells [Alipio et al. 2010; Maehr et al. 2009; Zhang et al. 2009a; Tateishi et al. 2008].
The generation of iPS cell-derived functional β cells was first demonstrated in one study by Tateishi and colleagues [Tateishi et al. 2008]. They reported that when subjected to a four-stage serum-free in vitro differentiation procedure, human dermal fibroblast-derived iPS cells can differentiate into functional (i.e. insulin-producing) islet-like clusters (ILCs) composed of mixed C-peptide+ and glucagon+ cells. During the differentiation process, iPS cells underwent stage-specific morphological changes in a manner similar to those of human ES cells. The functional analysis by quantitative reverse transcriptase polymerase chain reaction (RT-PCR) and immunostaining had revealed that during the differentiation towards insulin-producing ILCs, the differentiated iPS cells positively expressed stage-specific genes and antigen markers for each developmental stage (i.e. definitive endoderm [Foxa2 and Sox17], pancreatic endoderm [Pdx1], exocrine/endocrine cells [NKX6.1, Ptf1, and Insulin], and insulin-producing cells [Insulin, C-peptide, and glucagon]) in a manner similar to those of human ESCs. Remarkably, the iPS cell-derived ILCs were able to secrete insulin in response to glucose stimulation and the pattern of secretion appeared to run in a dose-dependent manner. However, Tateishi and colleagues reported that clonal variability exists in the potential of iPS cells to differentiate into pancreatic endocrine lineage cells. Also, the efficiency of differentiation is still limited [Tateishi et al. 2008].
On the other hand, Maehr and colleagues have successfully demonstrated that iPS cells can be generated from skin fibroblasts of patient with T1DM and further differentiate into insulin-producing/glucose-responsive cells [Maehr et al. 2009]. T1DM patient-derived iPS cells, further called DiPS, can provide a patient-specific or autologous source of stem cells. Thus, it can solve the problems with immune rejection that classically hamper the transplantation of allogenic stem cells. In addition, it can also capture the genotypic abnormalities that underlie T1DM and thus, can be used as a disease model to study the pathologic process involved in the development of T1DM. DiPS cells can further differentiate under a specific in vitro differentiation protocol into cells of pancreatic endocrine lineage that stained positively for somatostatin, glucagon, insulin, C-peptide, and also express specific gene markers of pancreatic endocrine lineage (i.e. insulin, Pdx1, Nkx2.2, glucagon, and somatostatin). Moreover, DiPS cell-derived pancreatic endocrine cells also secrete insulin upon glucose stimulation in a dose-dependent manner.
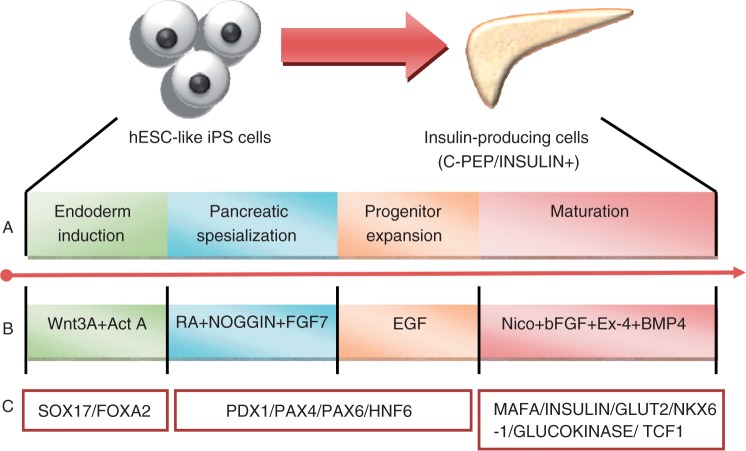
However, most of the differentiation protocols that induce differentiation of iPS cells into pancreatic endocrine lineage have a low efficiency and yield pancreatic islet cells with immature islet characteristics, such as co-expression of insulin/C-peptide and glucagon, and a low insulin/C-peptide secreting level in the differentiated cells. Zhang and colleagues had successfully developed a highly efficient differentiation protocols (with 25% efficiency) for human ESCs that also yield functionally β-like cells resembling those of adult human pancreatic β cells (i.e. co-express specific adult β cell transcription factors and functional markers in similar pattern to adult β cells in vivo [C-peptide, Pdx1, Glut2, MafA, Nkx6-1, Isl-1, and NeuroD], do not co-express somatostatin and glucagon, secrete insulin upon glucose stimulation in a comparable manner to that of adult human islet cells [levels of secretion and dose-dependent response]) [Zhang et al. 2009a]. Moreover, quantitative PCR-based gene expression profiling demonstrated that the pancreatic differentiation induced by this approach closely parallels the key gene expression pattern of in vivo pancreas development (see Figure 1). Most importantly, when this protocol is adapted to iPS cells, most of iPS cells are induced to differentiate into Pdx1+ pancreatic progenitor cells and further differentiate into functional islet cells expressing most of the crucial β cell transcription factors and functional markers (i.e. Pdx1, MafA, Glut2 and insulin or C-peptide) in a manner comparable to those of human ESC-derived functional β cells. The co-expression of PDX1 and C-peptide indicates the final mature stage of iPS cells differentiation toward pancreatic β cells.
Figure 1.
In vitro differentiation protocol that guaides the differentiation of induced pluripotent stem (iPS) cells into insulin-producing β cells.(A) Stepwise differentiation of iPs cells onto insulin-producing cells.(B) combination of factors used to guide the differentiation, (C) specific markes used ot evaluate the corresponding stage of differentiation. (Adapted from Zhang et al. [2009a]).
The proof of principle for the potential clinical application of iPS cells in the treatment of T1DM and T2DM also comes from one in vivo study by Alipio and colleagues [Alipio et al. 2010]. Following engraftment into the liver parenchyma of a mouse model of T2DM (n = 30) via intraportal vein injection, iPS cell-derived insulin-producing β-like cell transplants were able to normalize the blood glucose levels for more than 3 months and significantly improved the levels of HbA1C. They also found that the correction of hyperglycemia was conferred in a long-term period since the normal glycemic state could be maintained for about 20–30% of the life expectancy of those mouse models. Moreover, the amelioration of hyperglycemia even occurred concomitantly with an increase in in vivo insulin concentration as measured by mouse insulin enzyme-linked immunosorbent assays (ELISAs). During 21 −56 days of posttransplantation, β-cell-transplanted mice had an ˜4.35-fold increase in insulin levels as compared with untreated mice (p < 0.05).
Most importantly, the iPS-derived insulin-producing β-like cells engrafted stably and evenly distributed into the liver parenchyma of the diabetic mouse models as evidenced from the immunohistochemical and immunofluorescence analysis of the liver parenchyma at 7 days and 4 weeks posttransplantation. Alipio and colleagues considered that the possibility of graft rejection following transplantation was minimal since iPS cells were derived from fibroblasts of C57BL6 background mice, which were close relatives of the diabetic mouse models used in their study [Alipio et al. 2010]. Thus, the transplanted iPS-derived β-like cells were able to survive within the tissue environment where they were engrafted. Moreover, preliminary data from a small cohort of T1DM mouse model (STZ-treated mice, n = 6) also demonstrated a promising result in which correction of hyperglycemia was achieved following transplantation of iPS-derived β cells into the liver parenchyma and normoglycemia was maintained over the 4-month period of follow up.
Thus, iPS cell-derived insulin-producing β-like cells have been proved able to normalize the blood glucose level in vivo in both T1DM and T2DM mouse models. Normoglycemia is also maintained in the long term given that the iPS-derived β-like cells engraft and survive within the host tissue. However, future studies that investigate more closely the survival times and long-term complications following transplantation of iPS cell-derived insulin-producing β-like cells are highly demanded to validate the true clinical potential of iPS cells for the treatment of diabetes mellitus.
Current challenges and future research directions
There are several crucial challenges that must be addressed prior to the translation of iPS cell technology in the clinical settings, i.e. in the treatment of diabetes mellitus. These are illustrated in Figure 2.
Figure 2.
Multifaceted problems of generating induced pluripotent stem (iPS) cells as the source for pancreatic β-like cells transplantation and the potential strategies to overcome.
Generation of safe iPS cells
The risks of insertional mutagenesis and tumor formation have been attributed to the use of retroviral vector and the integration of transgenes (i.e. genes encoding the reprogramming factors) into the host genome during the generation of iPS cells (Figure 2). However, there is plenty of evidence to date that iPS cells can be generated by using a safer vector that is devoid of the integration of foreign genetic materials into the host genome, thus minimizing the risk of mutagenesis and tumorigenesis. For example, the application of nonintegrating adenovirus, transient plasmid vectors [Okita et al. 2010, 2008], episomal plasmid [Yu et al. 2009], and transposon [Kaji et al. 2009; Woltjen et al. 2009] in the generation of iPS cells have been investigated. Moreover, convincing evidence for the use of recombinant proteins [Zhou et al. 2009] or certain pharmacologic agents [Anastasia et al. 2010] to induce the reprogramming process that is devoid of host genome manipulation have also been made available. In addition, there is an alternative emerging effort of using a nonviral minicircle vector to carry the pluripotent transcription factors [Jia et al. 2010]. Minicircle vectors are described as super-coiled DNA molecules that are free of bacterial DNA and lack the antibiotic resistance gene, therefore composed of eukaryotic expression cassette [Jia et al. 2010; Chen et al. 2003]. Minicircle vectors maintain a longer expression of the carried transcription factors due to their lower degree of activating exogenous silencing mechanisms when compared with plasmids [Chen et al. 2003], in addition to yielding a higher reprogramming efficiency (0.005% versus <0.003%) when compared with other safe methods for integrating iPS cells (i.e. nonviral and/or nonintegrating viral methods) [Jia et al. 2010]. Their repeated transfection to human adipose stromal cells have successfully generated transgene-free human iPS cells [Narsinh et al. 2011]. Most importantly, iPS cells generated by such safer methods still maintain high similarities with those of ESCs despite the lower reprogramming efficiency (e.g. transient plasmid vectors yield lower reprogramming efficiency of one to two orders of magnitude compared with using retro-viral or lentiviral vectors) [Hochedlinger and Plath, 2009].
The use of oncogenic reprogramming factors (i.e. cMyc and Klf4) must be strictly avoided. It has been found that the reactivation of cMyc induces tumor formation in animal models [Yamanaka, 2009]. However, generation of iPS cells without involving the use of cMyc and Klf4 have been demonstrated in several studies [Nakagawa et al. 2008; Yu et al. 2007]. cMyc and Klf4 can be replaced by using Lin28 and Nanog as the reprogramming factors accompanying the indispensable Oct4 and Sox2, and still yield iPS cell clones resembling ESCs [Yu et al. 2007].
Low reprogramming efficiency
Despite the high similarity with ESCs, it is known that the reprogramming efficiency (i.e. generation of iPS cells) is low (Figure 2). It is known that a typical reprogramming event only occurs in 0.01–0.1% of the cell population being cultured [Hochedlinger and Plath, 2009]. Several direct measures have been investigated to increase the reprogramming efficiency. Olmer and colleagues [Olmer et al. 2010] applied a long-term expansion of human iPS cells in a specific culture medium and successfully increased the iPS cell numbers up to sixfold, whereas Mali and colleagues [Mali et al. 2008] inserted SV40 large T antigen to one of the four delivered transcription factors and subsequently achieved a 23- to 70-fold increase in reprogramming efficiency. However, the real problem perhaps lies in the epigenetic profile of the corresponding cells. The pluripotent genes are consistently kept in an inactive state through DNA methylation at the promoter regions. This is especially crucial for Oct4 since its reactivation is compulsory for reprogramming success [Nichols et al. 1998].
In addition, it has been reported that low-passage iPS cells still retain their epigenetic memories from their lineage-specific origin in the form of residual DNA methylation that matched to the previous differentiated state [Kim et al. 2010]. This epigenetic memory could be reset by differentiation, serial reprogramming, or administration of epigenetic drugs such as valproic acid and 5-AZA [Anastasia et al. 2010]. Therefore, through inhibiting the methylation activity of Oct4 by blocking DNMT1 (DNA methyltrans-ferase 1) certainly would accelerate the reprogramming process as well as to prevent the cell to become partially reprogrammed (Figure 2). However, inhibiting DNMT1 by using epigenetic drugs should be investigated deliberately since it directly modifies the cellular epigenetic architectures. These intricate mechanisms provide new insights to devise new culture protocol strategies that are more than just transduction with transcription factors associated with pluripotency to achieve the ultimate outcome of ideal iPS cells that are safe, functional, and easily scalable for clinical purposes.
Low differentiation efficiency and immature β cell phenotypes
It is noted in several studies that the general efficiency of in vitro iPS cell differentiation into functional insulin-producing β-like cells is low (Figure 2). Thus, it is highly essential to develop a safe, efficient, and easily scalable differentiation protocol before its clinical application. In addition, it is also important that insulin-producing β-like cells generated from the differentiation of iPS cells have an identical phenotype resembling that of adult human pancreatic β cells in vivo before the transplantation can take place. Thus, the quality must be ensured by using stringent criteria. In order to be said to resemble the original pancreatic β cells, the iPS-derived β-like cells must have several characteristics, such as expressing specific adult β cell transcription factors and functional markers in vivo (e.g. C-peptide, Pdx1, Glut2, MafA, Nkx6-1, Isl-1, and NeuroD), do not co-express somatostatin or glucagon (i.e. pure β cell population), and secrete insulin upon glucose stimulation in a comparable manner to that of adult human islet cells in vivo (i.e. levels of secretion and dose-dependent response).
As discussed earlier, an in vitro differentiation protocol developed by Zhang and colleagues is one example of the ideal protocol with a high efficiency (˜25%) [Zhang et al. 2009a]. The protocol involves a stage of pancreatic progenitor expansion through the administration of EGF (epidermal growth factor) which is followed by maturation through the administration of various growth hormones (bFGF, Exendin-4, nicotinamide, and BMP4). This protocol also yields insulin-producing β-like cells. However, taking into considerations that technologies for differentiation are growing fast nowadays, it can be stated with great optimism that the problem with the low efficiency and the immature phenotype of the resulting β-like cells would not be a major obstacle hampering the future clinical translation of iPS cell technology to the treatment of diabetes mellitus.
Autoimmunity and β cell destruction
The problem of immune rejection of the transplanted cells is another obstacle which hampers the use of cellular therapy (Figure 2). Even with autologous cell replacement, the corresponding immune system of a T1DM patient exhibits an autoimmunity that easily obliterates the novel generated β cells [Aguayo-Mazzucato and Bonner-Weir, 2010]. One way to reduce this immunoreactivity is through using immunosuppressive agents. Although significant progress has been made in this therapeutic field, there are still major limitations, mainly related to the safety aspects. Currently, there is no ideal immunosuppressor with regard to selective efficacy to suppress immune-mediated graft rejection while still allowing the immune system to protect the host from infection, without exerting serious long-term side effects. Several agents have been used, each with its own limitations. Glucocorticoids have systemic side effects and reduce the survival of the implanted cells when compared with the glucocorticoid-free regimen. Cyclosporin A is well known to exacerbate the diabetic state through destruction of the remaining islets. On the other hand, macrocyclic antibiotic-like sirolimus and tacrolimus, despite their effective cytostatic immune cell inhibition, are proven to cause dyslipidemia, thrombocytopenia, leucopenia, thus increasing the risk of infection and lymphoma (for sirolimus), as well as inducing systemic hypertension, altering glucose metabolism, and causing neurotoxicity and nephrotoxicity.
Two alternative solutions to get over this immune rejection problem are emerging and have drawn intensive attention from scientific investigators. These are cell-based microencapsulation technologies and induction of immune tolerance by antigen introduction, which are both elucidated in brief below.
Cell-based microencapsulation technology
A microencapsulation technology is an effort to protect the transplanted tissue or cells from immune attack by packaging these products into semipermeable membranes (Figure 2). The membrane in use usually composed of a synthetic biopolymer that isolates the corresponding β cell transplant yet still allows the diffusion of nutrients and oxygen from external environment as well as facilitating insulin secretion to the blood stream from internal capsules [de Vos et al. 2006]. There are various sources that can be applied to fabricate the biomaterial capsule, but the most commonly used is derived from alginate [Soon-Shiong et al. 1992]. This product has major advantages, i.e. it is found not to interpose with the β cell metabolism and insulin-secreting function [de Haan et al. 2003; Fritschy et al. 1991], easy material processing because it can be done at physiological conditions [de Vos et al. 2006], and assurance of adequate cell survival during long-term encapsulation due to its ability to provide a microenvironment favoring β cells survival [Lopez-Avalos et al. 2001; Sandler et al. 1997]. Alginate is isolated from seaweed and constituted by β-D-mannuronic (M) and α-L-guluronic acids (G), respectively [Donati et al. 2005]. The β-cells are entrapped in an alginate droplet and further be solidified by gelification in a divalent cation solution such as barium or calcium. These crosslinking ions will bind to the alginate M or G group, thus providing stronger gels [de Vos et al. 2006].
In order to sustain optimal access of nutrients and oxygen to the islets inside the microcapsules, the islets can be enveloped with vascular prostheses which anastomose in a direct manner with blood vessels [Petruzzo et al. 1991]. However, with this method, there is an increased risk of thrombosis due to partial occlusion of the corresponding blood vessels [de Vos et al. 2002]. Therefore, an alternative strategy is to apply an extravascular system that allows for nutrient diffusion without direct contact with the blood vessel. Obviously, the latter strategy requires a close contact (if not connection) between the capsules and blood vessels that normally are difficult to attain [Roche et al. 2005]. The peritoneal cavity can be a candidate owing to its capacity to bear a large graft volume, despite the relatively low degree of vascularization [de Vos et al. 2006]. In order to generate a sufficient blood supply to the capsule, there is an urgent need to reduce the corresponding size and volume [Halle et al. 1994].
Indeed, the implantation of alginate microcapsules into nonobese autoimmune diabetic mice has been shown to neutralize hyperglycemia for a year [Omer et al. 2005]. Giving the life span of a β cell of approximately 3 months, the study suggested that islet regeneration has occurred, thus confirming the successful diffusion of either insulin into the bloodstream or nutrients and oxygen into the capsule [Finegood et al. 1995]. The spherical shape of alginate microcapsules can facilitate insulin release in a similar fashion with nonencapsulated islets at 800 μm size [de Haan et al. 2003]. Furthermore, alginate microcapsule has been reported to confer an effective protection to the islets for more than a year, indicating a good endurance and quality of the biomaterial [Duvivier-Kali et al. 2001].
Despite its potential capacity to protect the islets against immune attack, the clinical use of microcapsule is hampered by several problems. These are intrinsic immune activation with subsequent inflammatory leakage and fibrosis overgrowth. Crude alginate contains endotoxins, potent immunostimulators, which in turn are able to trigger an inflammatory reaction in the microcapsule vicinity [Dusseault et al. 2006]. When the permeability of the capsule is impaired (e.g. owing to the relatively small size of cytokines with large pore size), the inflammatory cells and mediators can easily penetrate the capsule and induce β cells death [de Vos et al. 2006]. Whereas fibrosis may occur when there are excessive attachments of the host cells on the capsule surface due to electrochemical bonds. These attachments will decrease the nutrient and oxygen diffusion into the capsule and consequently kill the islets gradually [de Vos et al. 2006].
Several solutions to the former problem are through alginate purification until it reaches the endotoxin level below 100 EU/g [Dusseault et al. 2006; de Vos et al. 1997] or decreasing the pore size by coating alginate beads with polycation (alginate—PLL system) [Kulseng et al. 1997; Vandenbossche et al. 1993; Halle et al. 1992]. This method has been shown to increase the mechanical stability and results in a more restricted permeability of the corresponding capsules. Fibrosis overgrowth is evitable if we use alginate-derived microcapsules, since they are negatively charged, therefore reducing cell attachment because the common cell surface also expresses negative ions [de Vos et al. 2006].
The use of microcapsules to protect the reprogrammed β-like cells should be taken into account for further therapeutic human applications.
Immune tolerance induction through antigenic stimulation
This method introduces antigens (in this case, the novel generated β cells) into the thymus to induce self-tolerance and subsequent acceptance of the implanted autologous cells. As it is well known, there are two phases of immunological self-tolerance, i.e. central and peripheral [Miyara and Sakaguchi, 2007]. The central tolerance comprises deletion of T cells showing high-affinity receptor binding with self-antigens presented by dendritic cells in the thymus (so-called negative selection) and the generation of regulatory T (Treg) cells [Chidgey et al. 2008]. Whereas peripheral tolerance induces T cell anergy (lack of immunological response upon antigen binding) or Treg cell activation which occur outside the thymus [Miyara and Sakaguchi, 2007].
The β cell antigens were administrated in the thymus, concomitantly with the engraftment of recipient's enriched hematopoietic stem cells (HSCs) in the bone marrow [Kaufman et al. 2001]. The objective of this effort is to boost the proliferation and migration of the progenitor cells which subsequently migrate to the thymus (at corticomedullary junction) and differentiate into dendritic or T (either CD4+ or CD8+) cells [Verda et al. 2008]. Dendritic cells will go directly into the medulla, while T cell precursors will move to the cortex where they undergo positive selection (i.e. survival of the cells which produce receptors that are able to interact with self-peptide presented by cortical thymic epithelial cells [cTECs]). Later, the survived cells continue their way to the medulla where they pass through negative selection (i.e. deletion of cells which demonstrate strong binding affinity to the self-antigen presented by dendritic cells) [Anderson et al. 2007]. The resulting cells still contain partially autoreactive cells because not all of the self-antigens are presented by the dendritic cells [Chidgey et al. 2008]. Thus, further exclusion is made by differentiated CD80+ medullary TECs (mTECs) [Gabler et al. 2007; Derbinski et al. 2001].
The remaining cells leave the thymus and migrate into the systemic blood circulation. In order to ensure that there are no autoreactive T cells that escape thymic deletion, the thymus increases Treg cell numbers [Spence and Green, 2008; Sakaguchi et al. 2006] which are able to suppress the remaining autoreactive T cells through inter-leukin-10 (IL-10) and transforming growth factor β (TGF-β) secretion, which are known to act as immunosuppressors [Joffre et al. 2008; King et al. 1998].
This emerging concept has been proven clinically in a young patient (9 years of age) receiving a liver allograft which later exhibited a hematopoetic chimerism (i.e. mixtures between the differentiated donor's and recipient's dendritic and T cells) as well as tolerance to the donor graft [Alexander et al. 2008]. It has been suggested that the liver contained large amounts of HSCs which in turn migrated to the thymus and induced T cell anergy due to antigenic (liver cells) presentation to the corresponding immune cells.
The apparent major problem of this concept is a reduced thymic function and capacity in older people (a thymus of a 40–50-year-old patient has less than 10% of its original volume before puberty) [Flores et al. 1999] while the highly active thymus is a major prerequisite of T cell maturation and antigenic presentation. Several strategies are thus proposed to rejuvenate the thymus, i.e. activation of the endocrine-immune axis by luteinizing hormone-releasing hormone (LHRH) administration to inhibit the sexual hormone production in adults (because sex hormones are the major thymic suppressor) [Sutherland et al. 2005]. The other strategy is to provide a microenvironment that is suitable for thymus regeneration. This can be achieved through thymic growth factor administration (e.g. IL-7, KGF [FGF7]) [Chidgey et al. 2008; Okamoto et al. 2002].
More intensive and specified studies should be conducted, mainly related to the actual effectiveness of the self-tolerance induction in clinical settings, correlation between the degree of thymus regeneration and self-tolerance intensity, finding the most effective agent with its appropriate dose for rejuvenating the thymus with fewest side effects, and other considered essential fields to be defined.
Conclusion
Diabetes mellitus has no permanent cure to date. One of the plausible therapeutic strategies to achieve this goal is through pancreatic stem cell transplantation. There are various stem cell sources and manipulation techniques that can be used to generate functional β-like cells in a safe and efficient manner. Cellular reprogramming of iPS cell technology could be one of the most promising solutions. It involves the administration of transcription factors in order to transduce multiple terminally differentiated cell types into insulin-producing β-like cells. However, its application in the clinical setting is limited by several factors, including the risk of tumor formation resulted from the use of retroviral/lentiviral vectors and/or the administration of cMyc oncogene, low reprogramming efficiency associated with epigenetic profiles of the corresponding cells, and the autoimmune response generated by the host that will destroy the transplanted cells. Further research is therefore required to further clarify the molecular events occurring during reprogramming which are essential to improving the safety and efficiency of culture protocols, and also to define the state-of-the-art differentiation steps for generating a safe, effective, and scalable insulin-secreting pancreatic β-like cells, as well as to protect the successfully transplanted cells from the autoimmune process.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest in preparing this article.
References
- Aasen T., Raya A., Barrero M.J., Garreta E., Consiglio A., Gonzalez F., et al. (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26: 1276–1284 [DOI] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C., Bonner-Weir S. (2010) Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol 6: 139–148 [DOI] [PubMed] [Google Scholar]
- Alexander S.I., Smith N., Hu M., Verran D., Shun A., Dorney S., et al. (2008) Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med 358: 369–374 [DOI] [PubMed] [Google Scholar]
- Ali M.A., Dayan C.M. (2009) The importance of residual endogenous beta-cell preservation in type 1 diabetes. Br J Diabetes Vasc Dis 9: 6 [Google Scholar]
- Alipio Z., Liao W., Roemer E.J., Waner M., Fink L.M., Ward D.C., et al. (2010) Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic β-like cells. Proc Natl Acad Sci 107: 13426–13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasia L., Pelissero G., Venerando B., Tettamanti G. (2010) Cell reprogramming: Expectations and challenges for chemistry in stem cell biology and regenerative medicine. Cell Death Differ 17: 1230–1237 [DOI] [PubMed] [Google Scholar]
- Anderson G., Lane P.J., Jenkinson E.J. (2007) Generating intrathymic microenvironments to establish T-cell tolerance. Nat Rev Immunol 7: 954–963 [DOI] [PubMed] [Google Scholar]
- Assady S., Maor G., Amit M., Itskovitz-Eldor J., Skorecki K.L., Tzukerman M. (2001) Insulin production by human embryonic stem cells. Diabetes 50: 1691–1697 [DOI] [PubMed] [Google Scholar]
- Bernstein B.E., Meissner A., Lander E.S. (2007) The mammalian epigenome. Cell 128: 669–681 [DOI] [PubMed] [Google Scholar]
- Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., et al. (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326 [DOI] [PubMed] [Google Scholar]
- Chen Z.Y., He C.Y., Ehrhardt A., Kay M.A. (2003) Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther 8: 495–500 [DOI] [PubMed] [Google Scholar]
- Chidgey A.P., Layton D., Trounson A., Boyd R.L. (2008) Tolerance strategies for stem-cell-based therapies. Nature 453: 330–337 [DOI] [PubMed] [Google Scholar]
- de Haan B.J., Faas M.M., de Vos P. (2003) Factors influencing insulin secretion from encapsulated islets. Cell Transplant 12: 617–625 [DOI] [PubMed] [Google Scholar]
- de Vos P., De Haan B.J., Wolters G.H., Strubbe J.H., Van Schilfgaarde R. (1997) Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 40: 262–270 [DOI] [PubMed] [Google Scholar]
- de Vos P., Faas M.M., Strand B., Calafiore R. (2006) Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 27: 5603–5617 [DOI] [PubMed] [Google Scholar]
- de Vos P., Hamel A.F., Tatarkiewicz K. (2002) Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia 45: 159–173 [DOI] [PubMed] [Google Scholar]
- Derbinski J., Schulte A., Kyewski B., Klein L. (2001) Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2: 1032–1039 [DOI] [PubMed] [Google Scholar]
- Donati I., Holtan S., Morch Y.A., Borgogna M., Dentini M., Skjak-Braek G. (2005) New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules 6: 1031–1040 [DOI] [PubMed] [Google Scholar]
- Dusseault J., Tam S.K., Menard M., Polizu S., Jourdan G., Yahia L., et al. (2006) Evaluation of alginate purification methods: Effect on polyphenol, endotoxin, and protein contamination. J Biomed Mater Res A 76: 243–251 [DOI] [PubMed] [Google Scholar]
- Duvivier-Kali V.F., Omer A., Parent R.J., O'Neil J.J., Weir G.C. (2001) Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 50: 1698–1705 [DOI] [PubMed] [Google Scholar]
- Efrat S. (2008) Beta-cell replacement for insulin-dependent diabetes mellitus. Adv Drug Deliv Rev 60: 114–123 [DOI] [PubMed] [Google Scholar]
- Eisenbarth G.S. (2007) Update in type 1 diabetes. J Clin Endocrinol Metab 92: 2403–2407 [DOI] [PubMed] [Google Scholar]
- Finegood D.T., Scaglia L., Bonner-Weir S. (1995) Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44: 249–256 [DOI] [PubMed] [Google Scholar]
- Flores K.G., Li J., Sempowski G.D., Haynes B.F., Hale L.P. (1999) Analysis of the human thymic perivascular space during aging. J Clin Invest 104: 1031–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy W.M., Wolters G.H., van Schilfgaarde R. (1991) Effect of alginate-polylysine-alginate microencapsulation on in vitro insulin release from rat pancreatic islets. Diabetes 40: 37–43 [DOI] [PubMed] [Google Scholar]
- Gabler J., Arnold J., Kyewski B. (2007) Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur J Immunol 37: 3363–3372 [DOI] [PubMed] [Google Scholar]
- Gallwitz B. (2008) Managing the β-cell with GLP-1 in type 2 diabetes. Br J Diabetes Vasc Dis 8(Suppl 2): 7 [Google Scholar]
- Gillespie K.M. (2006) Type 1 diabetes: Pathogenesis and prevention. CMAJ 175: 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle J.P., Leblond F.A., Landry D., Fournier A., Chevalier S. (1992) Studies of 300-microns microcapsules: I. Use of arginine esterase release by microencapsulated prostatic cells as a measure of membrane permeability. Transplant Proc 24: 2930–2932 [PubMed] [Google Scholar]
- Halle J.P., Leblond F.A., Pariseau J.F., Jutras P., Brabant M.J., Lepage Y. (1994) Studies on small (< 300 microns) microcapsules: II–Parameters governing the production of alginate beads by high voltage electrostatic pulses. Cell Transplant 3: 365–372 [DOI] [PubMed] [Google Scholar]
- Hochedlinger K., Plath K. (2009) Epigenetic reprogramming and induced pluripotency. Development 136: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.Y., Weick J.P., Yu J., Ma L.X., Zhang X.Q., Thomson J.A., et al. (2010) Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 107: 4335–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F., Wilson K.D., Sun N., Gupta D.M., Huang M., Li Z., et al. (2010) A nonviral minicircle vector for deriving human iPS cells. Nat Methods 7: 197–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O., Santolaria T., Calise D., Al Saati T., Hudrisier D., Romagnoli P., et al. (2008) Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 14: 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K., Norrby K., Paca A., Mileikovsky M., Mohseni P., Woltjen K. (2009) Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458: 771–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D.S., Hanson E.T., Lewis R.L., Auerbach R., Thomson J.A. (2001) Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 98: 10716–10721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Doi A., Wen B., Ng K., Zhao R., Cahan P., et al. (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467: 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C., Davies J., Mueller R., Lee M.S., Krahl T., Yeung B., et al. (1998) TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity 8: 601–613 [DOI] [PubMed] [Google Scholar]
- Kulseng B., Thu B., Espevik T., Skjak-Braek G. (1997) Alginate polylysine microcapsules as immune barrier: Permeability of cytokines and immunoglobulins over the capsule membrane. Cell Transplant 6: 387–394 [DOI] [PubMed] [Google Scholar]
- Limbert C., Path G., Jakob F., Seufert J. (2008) Beta-cell replacement and regeneration: Strategies of cell-based therapy for type 1 diabetes mellitus. Diabetes Res Clin Pract 79: 389–399 [DOI] [PubMed] [Google Scholar]
- Lopez-Avalos M.D., Tatarkiewicz K., Sharma A., Bonner-Weir S., Weir G.C. (2001) Enhanced maturation of porcine neonatal pancreatic cell clusters with growth factors fails to improve transplantation outcome. Transplantation 71: 1154–1162 [DOI] [PubMed] [Google Scholar]
- Lowry W.E., Richter L., Yachechko R., Pyle A.D., Tchieu J., Sridharan R., et al. (2008) Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A 105: 2883–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R., et al. (2009) Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A 106: 15768–15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N., Ahfeldt T., Rigamonti A., Utikal J., Cowan C., Hochedlinger K. (2008) A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 3: 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N., Hochedlinger K. (2008) Induced pluripotency of mouse and human somatic cells. Cold Spring Harb Symp Quant Biol 73: 157–162 [DOI] [PubMed] [Google Scholar]
- Mali P., Ye Z., Hommond H.H., Yu X., Lin J., Chen G., et al. (2008) Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells 26: 1998–2005 [DOI] [PubMed] [Google Scholar]
- Martinez-Fernandez A., Nelson T.J., Yamada S., Reyes S., Alekseev A.E., Perez-Terzic C., et al. (2009) iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ Res 105: 648–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T.S., Hanna J., Zhang X., Ku M., Wernig M., Schorderet P., et al. (2008) Dissecting direct reprogramming through integrative genomic analysis. Nature 454: 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T.S., Ku M., Jaffe D.B., Issac B., Lieberman E., Giannoukos G., et al. (2007) Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448: 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M., Sakaguchi S. (2007) Natural regulatory T cells: Mechanisms of suppression. Trends Mol Med 13: 108–116 [DOI] [PubMed] [Google Scholar]
- Moretti A., Bellin M., Jung C.B., Thies T.-M., Takashima Y., Bernshausen A., et al. (2010) Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors. FASEB J 24: 700–711 [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., et al. (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotech 26: 101–106 [DOI] [PubMed] [Google Scholar]
- Narsinh K.H., Jia F., Robbins R.C., Kay M.A., Longaker M.T., Wu J.C. (2011) Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc 6: 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T.J., Martinez-Fernandez A., Yamada S., Perez-Terzic C., Ikeda Y., Terzic A. (2009) Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 120: 408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., et al. (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95: 379–391 [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Douek D.C., McFarland R.D., Koup R.A. (2002) Effects of exogenous interleukin-7 on human thymus function. Blood 99: 2851–2858 [DOI] [PubMed] [Google Scholar]
- Okita K., Hong H., Takahashi K., Yamanaka S. (2010) Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nat Protoc 5: 418–428 [DOI] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., Yamanaka S. (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448: 313–317 [DOI] [PubMed] [Google Scholar]
- Okita K., Nakagawa M., Hyenjong H., Ichisaka T., Yamanaka S. (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322: 949–953 [DOI] [PubMed] [Google Scholar]
- Olmer R., Haase A., Merkert S., Cui W., Palecek J., Ran C., et al. (2010) Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res 5: 51–64 [DOI] [PubMed] [Google Scholar]
- Omer A., Duvivier-Kali V., Fernandes J., Tchipashvili V., Colton C.K., Weir G.C. (2005) Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation 79: 52–58 [DOI] [PubMed] [Google Scholar]
- Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., et al. (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451: 141–146 [DOI] [PubMed] [Google Scholar]
- Petruzzo P., Pibiri L., De Giudici M.A., Basta G., Calafiore R., Falorni A., et al. (1991) Xenotransplantation of microencapsulated pancreatic islets contained in a vascular prosthesis: Preliminary results. Transpl Int 4: 200–204 [DOI] [PubMed] [Google Scholar]
- Reik W. (2007) Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447: 425–432 [DOI] [PubMed] [Google Scholar]
- Roche E., Reig J.A., Campos A., Paredes B., Isaac J.R., Lim S., et al. (2005) Insulin-secreting cells derived from stem cells: Clinical perspectives, hypes and hopes. Transpl Immunol 15: 113–129 [DOI] [PubMed] [Google Scholar]
- Ryan E.A., Paty B.W., Senior P.A., Bigam D., Alfadhli E., Kneteman N.M., et al. (2005) Five-year follow-up after clinical islet transplantation. Diabetes 54: 2060–2069 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., et al. (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212: 8–27 [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A., Eizirik D.L., Hellerstrom C., Espevik T., Kulseng B., et al. (1997) Assessment of insulin secretion in vitro from microencapsulated fetal porcine islet-like cell clusters and rat, mouse, and human pancreatic islets. Transplantation 63: 1712–1718 [DOI] [PubMed] [Google Scholar]
- Soon-Shiong P., Feldman E., Nelson R., Heintz R., Merideth N., Sandford P., et al. (1992) Long-term reversal of diabetes in the large animal model by encapsulated islet transplantation. Transplant Proc 24: 2946–2947 [PubMed] [Google Scholar]
- Soria B., Roche E., Berna G., Leon-Quinto T., Reig J.A., Martin F. (2000) Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49: 157–162 [DOI] [PubMed] [Google Scholar]
- Spence P.J., Green E.A. (2008) Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A 105: 973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M., Nagaya M., Utikal J., Weir G., Hochedlinger K. (2008) Induced pluripotent stem cells generated without viral integration. Science 322: 945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J.S., Goldberg G.L., Hammett M.V., Uldrich A.P., Berzins S.P., Heng T.S., et al. (2005) Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 175: 2741–2753 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Tateishi K., He J., Taranova O., Liang G., D'Alessio A.C., Zhang Y. (2008) Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem 283: 31601–31607 [DOI] [PubMed] [Google Scholar]
- Vandenbossche G.M., Bracke M.E., Cuvelier C.A., Bortier H.E., Mareel M.M., Remon J.P. (1993) Host reaction against empty alginate-polylysine microcapsules. Influence of preparation procedure. J Pharm Pharmacol 45: 115–120 [DOI] [PubMed] [Google Scholar]
- Verda L., Kim D.A., Ikehara S., Statkute L., Bronesky D., Petrenko Y., et al. (2008) Hematopoietic mixed chimerism derived from allogeneic embryonic stem cells prevents autoimmune diabetes mellitus in NOD mice. Stem Cells 26: 381–386 [DOI] [PubMed] [Google Scholar]
- Wernig M., Meissner A., Foreman R., Brambrink T., Ku M., Hochedlinger K., et al. (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448: 318–324 [DOI] [PubMed] [Google Scholar]
- Wernig M., Zhao J.-P., Pruszak J., Hedlund E., Fu D., Soldner F., et al. (2008) Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci 105: 5856–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S., Roglic G., Green A., Sicree R., King H. (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053 [DOI] [PubMed] [Google Scholar]
- Woltjen K., Michael I.P., Mohseni P., Desai R., Mileikovsky M., Hamalainen R., et al. (2009) piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458: 766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. (2009) A Fresh Look at iPS Cells. Cell 137: 13–17 [DOI] [PubMed] [Google Scholar]
- Yao S., Chen S., Clark J., Hao E., Beattie G.M., Hayek A., et al. (2006) Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A 103: 6907–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Chang J.C., Lin C., Sun X., Yu J., Kan Y.W. (2009) Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc Natl Acad Sci U S A 106: 9826–9830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin II, et al. (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324: 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- Zhang D., Jiang W., Liu M., Sui X., Yin X., Chen S., et al. (2009a) Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19: 429–438 [DOI] [PubMed] [Google Scholar]
- Zhang J., Wilson G.F., Soerens A.G., Koonce C.H., Yu J., Palecek S.P., et al. (2009b) Functional cardio-myocytes derived from human induced pluripotent stem cells. Circ Res 104: e30–e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.-y., Li W., Lv Z., Liu L., Tong M., Hai T., et al. (2009) iPS cells produce viable mice through tetraploid complementation. Nature 461: 86–90 [DOI] [PubMed] [Google Scholar]
- Zhou H., Wu S., Joo J.Y., Zhu S., Han D.W., Lin T., et al. (2009) Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4: 381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwi L., Caspi O., Arbel G., Huber I., Gepstein A., Park I.H., et al. (2009) Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 120: 1513–1523 [DOI] [PubMed] [Google Scholar]