Abstract
Endocrinologists are encountering patients with obesity-related complications such as metabolic syndrome (MetS) and type 2 diabetes mellitus (T2DM) on a daily basis. Nonalcoholic fatty liver disease (NAFLD) is a liver condition characterized by insulin resistance, hepatic steatosis and frequently T2DM. This is now the most common chronic liver condition in adults and is present in the majority of obese subjects. Liver fat accumulation may range from simple steatosis to severe steatohepatitis with hepatocyte necroinflammation (or nonalcoholic steatohepatitis [NASH]). Although the natural history is incompletely understood, NAFLD may lead to serious medical consequences ranging from cirrhosis and hepatocellular carcinoma to earlier onset of T2DM and cardiovascular disease (CVD). The diagnosis of NAFLD may be challenging because signs and symptoms are frequently absent or nonspecific, and thus easily missed. Liver aminotransferases may be helpful if elevated, but most times are normal in the presence of the disease. Liver imaging may assist in the diagnosis (ultrasound or MRI and spectroscopy) but a definitive diagnosis of NASH still requires a liver biopsy. This may change in the near future as novel biomarkers become available. Treatment of NAFLD includes aggressive management of associated cardiovascular risk factors and many times control of T2DM. Pioglitazone and vitamin E appear promising for patients with NASH, although long-term studies are unavailable. In summary, this review hopes to address the common clinical dilemmas that endocrinologists face in the diagnosis and management of NAFLD and increase awareness of a potentially serious medical condition.
Keywords: diabetes, fatty liver, insulin resistance, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH)
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease in Western countries. It is a liver condition characterized by insulin resistance, hepatic steatosis and frequently prediabetes or T2DM. Liver fat accumulation may range from simple triglyceride accumulation to severe steatohepatitis with lobular necroinflammation and variable degrees of fibrosis (nonalcoholic steatohepatitis [NASH]), cirrhosis and even hepatocellular carcinoma [Bugianesi et al. 2007]. In a recent report by Musso and colleagues [Musso et al. 2010], it was estimated that NAFLD increases healthcare costs by 26% and that it will be the leading cause of liver transplantation by 2020. This study did not take into account the public health burden associated with NAFLD-related conditions, such as diabetes and cardiovascular disease (CVD) [Targher et al. 2010; Marchesini et al. 2003]. On a daily basis, endocrinologists see patients who are obese and have the metabolic syndrome (MetS); yet, most are unaware of NAFLD. There are several reasons why NAFLD has not become a more widely recognized problem: the diagnosis may be difficult, the natural history and clinical implications remain poorly understood, and pharmacological treatment is not well established, although this is likely to change in the near future. NAFLD is a disease that requires unique considerations and it is likely that endocrinologists will play a larger role in the future in screening and treating these patients.
Magnitude of the problem: Prevalence and natural history
The precise prevalence of NAFLD remains unclear, depending on the methods used for screening, being lower when liver aminotransferases and/or liver ultrasound are used and higher with the gold-standard magnetic resonance spectroscopy (MRS). The prevalence of NAFLD in the industrialized countries is believed to be between 40% and 50%, with even higher prevalence rates in subjects with T2DM and as high as 90% in the morbidly obese [Chavez-Tapia et al. 2010; Musso et al. 2010; Pillai and Rinella, 2009]. In patients who have NAFLD, it is believed that about 40% may go on to develop NASH [Wieckowska et al. 2007; Browning et al. 2004b; Clark et al. 2002] although the true natural history of the disease is incompletely understood. The true prevalence of NASH in the general population is unknown because few studies have performed a liver biopsy in patients found to have NAFLD by liver aminotransferases or on liver imaging during routine screening [Leite et al. 2011; Williams et al. 2011]. However, it is clear that factors associated with disease progression include obesity and the cluster of factors associated with MetS, such as dyslipidemia, hypertension (HTN), insulin resistance and T2DM. For instance, in a recent analysis by our group, the presence of T2DM was associated with more insulin resistance and worse histology in patients with NASH [Ortiz-Lopez et al. 2010].
When comparing ethnicities, the Hispanic population has been reported to have a higher prevalence rate for NAFLD than the African American or White population [Williams et al. 2011; Neuschwander-Tetri et al. 2010; Mohanty et al. 2009; Browning et al. 2004b]. However, in these studies Hispanics had a higher prevalence of obesity, insulin resistance and T2DM, all established risk factors for NAFLD. A recent study in 152 subjects by Lomonaco and colleagues [Lomonaco et al. 2011a] has reported that Hispanics and Whites have similar severity of NASH if subjects are matched carefully for adiposity, and that previously described differences were more likely a reflection of the unfavorable metabolic risk of Hispanics. With the worldwide epidemic of obesity, the prevalence of NAFLD is increasing across the globe [Chitturi et al. 2011; Duseja, 2010]. Perhaps the most disturbing trend is the rise of NAFLD in the pediatric population, echoing the rise in childhood obesity [Mencin and Lavine, 2011; Schwimmer et al. 2006].
Simple steatosis can have a benign, nonprogressive course, but a number of studies suggest that approximately 30–40% of patients with NASH are at risk of developing fibrosis and potentially cirrhosis [Williams et al. 2011; Bugianesi et al. 2007; Ekstedt et al. 2006; Adams et al. 2005a; Browning et al. 2004a, 2004b; Fassio et al. 2004; Harrison et al. 2003]. Patients with NAFLD are also at an increased risk of endstage liver disease, CVD and diabetes, which explain their overall increased mortality rate [Ekstedt et al. 2006; Adams et al. 2005b]. NASH can progress to cirrhosis in up to 5–15% of patients and is now recognized as the most common cause of cryptogenic cirrhosis [Caldwell, 2010; Bugianesi et al. 2007; Harrison, 2006]. Obesity and T2DM are present in a large portion of patients who develop cryptogenic cirrhosis, an association not seen in hepatitis-C-related cirrhosis or primary biliary cirrhosis. Both NASH and cryptogenic cirrhosis share many similar risk factors, including T2DM, obesity, and the MetS [Bugianesi et al. 2007; Adams et al. 2005a]. When compared with viral-associated cirrhosis, cirrhosis linked to NASH has a similar liver-related mortality, but a significantly higher CVD-related death rate [Musso et al. 2010].
Diagnosis of NAFLD: An endocrinologist's challenge
The challenge endocrinologists face in the diagnosis of NAFLD is that signs and symptoms are frequently absent or nonspecific and thus easily missed. This requires a high degree of disease awareness. A complete history and physical examination are still useful tools that may offer clues about the disease. Some of the findings include general right upper quadrant pain, malaise, hepatomegaly or discomfort on exam, or evident signs of insulin resistance (i.e. acanthosis nigricans). While liver aminotransferases may be elevated (typically alanine aminotransferase [ALT] greater than aspartate aminotransferase [AST] levels), increases are usually mild to moderate and are normal in about two thirds of patients, making them an unreliable marker of NAFLD. Several medications (corticosteroids, HIV antiretroviral therapy, tamoxifen, others), viral hepatitis (i.e. hepatitis C genotype 3), autoimmune hepatitis and other conditions [Ali and Cusi, 2009; Vuppalanchi and Chalasani, 2009; Clark et al. 2003] should be ruled out. In addition, liver enzymes may even be normal in end-stage liver cirrhosis. Thus, the presence of elevated liver aminotransferases or fatty liver on imaging should prompt the physician to evaluate the patient for excessive alcohol intake and/or a number of liver-related medical illnesses (such as viral hepatitis, hemochromatosis, autoimmune liver disease, α-1 antitrypsin deficiency or Wilson's disease) before the diagnosis of NAFLD be made.
Noninvasive assessments of NAFLD
Typically the first test used in the evaluation of patients with suspected fatty liver (usually by history and elevated AST/ALT) is an ultrasound (US). Its advantages are that it is rather inexpensive, noninvasive, widely available, and there is no radiation exposure. Steatosis on a liver US appears as hyperechogenic when compared with the spleen or kidney. Other useful features on US include beyond increased parenchymal echogenicity are hepatic and portal vein blurring, far gain attenuation of the diaphragm, gallbladder blurring [Mazhar et al. 2009; Liang et al. 2007]. Liver US can detect changes in parenchyma but does not provide accurate quantification of the amount of fat present and does not allow the severity of histological disease to be established. The disadvantages are that it is highly operator dependent and it is less accurate in patients who have a large body mass. Also, there is a decrease in sensitivity when the liver fat content is less than 20–30% leaving many patients undiagnosed. Computed tomography (CT) is another possible imaging modality but it also fails to precisely quantify the degree of steatosis [Saadeh et al. 2002]. A fatty liver will have a characteristic decrease in liver attenuation compared with the spleen, making the liver appear darker than the spleen.
Magnetic resonance spectroscopy (MRS), the current gold-standard technique for diagnosing fatty liver, provides more sensitivity and reproducibility for determining the amount of liver fat. In a multiethnic group of 345 subjects without any risk factors for NAFLD (lean, no or low alcohol consumption, normal plasma glucose, no history of liver disease and normal liver amino-transferases) the median liver fat content was 1.9% with a 95% percentile of 5.6%. Therefore, the diagnosis of NAFLD is considered when the liver fat content by MRS is >5.6% (equivalent to 55.6 mg/g of liver tissue) [Szczepaniak et al. 2005]. MRS has been shown to have a good correlation with the amount of liver fat estimated by liver biopsy in small studies [Szczepaniak et al. 1999; Longo et al. 1995] and in our own experience [Lomonaco et al. 2011a; Belfort et al. 2006]. The disadvantages of MRS are cost and that it is only available at few academic centers. A few of the less commonly used tests include assessment of fibrosis using the BARD score, the NAFLD fibrosis score, or the fibroscan [Dowman et al. 2011; Musso et al. 2010; Wong et al. 2010]. These tests, however, have not been fully evaluated for widespread clinical use.
Liver biopsy
A liver biopsy is the only way to confirm the diagnosis of NASH and grade the severity of steatohepatitis and stage fibrosis. It is usually performed under US guidance and is generally a safe and well-tolerated procedure in experienced hands. The invasive nature of the test and the lack of established pharmacological treatments make it an option rarely chosen by physicians in patients with NAFLD. At the current time, it is best indicated for the diagnosis of NASH in patients with clinical risk factors (i.e. severe obesity, T2DM) and markedly elevated liver aminotransferases (i.e. greater than threefold the upper limit of normal [ULN]), when other causes of liver disease have been excluded or if a treatment decision will be made based on the results.
Future noninvasive diagnosis of NAFLD and NASH
The fact that at least 70–80% of obese subjects have NAFLD, and many with NASH, highlights the need for a noninvasive diagnosis. Current efforts include the use of a combination of clinical parameters (body mass index [BMI], presence of diabetes, HTN) and plasma biochemical measurements frequently associated with the disease (such as plasma ALT, bilirubin, glucose, triglycerides, other) [Angulo et al. 2007; Wieckowska et al. 2007; Poynard et al. 2006; Ratziu et al. 2006], use of imaging by means of transient elastography [Gaia et al. 2011; Wong et al. 2010; Friedrich-Rust et al. 2008] or the use of new plasma biomarkers of NASH [Pagadala et al. 2009; Wieckowska et al. 2007].
The most promising biomarker in NASH is measurement of plasma caspase-cleaved cytokeratin-18 (CK-18) fragment levels [Feldstein et al. 2009]. CK-18 is a major intermediate filament protein in the liver. In NASH there is significant caspase activation and hepatocyte cell death by apoptosis. It is believed that the increased caspase activity can be measured in plasma from the spillover of caspase-cleaved CK-18 fragments into the bloodstream. A number of studies have demonstrated significant elevation of this protein in NASH when compared to controls with a fatty liver, but not steatohepatitis [Feldstein et al. 2009; Yilmaz et al. 2009; Younossi et al. 2008; Diab et al. 2008]. In our hands, CK-18 fragments are clearly elevated in patients with NAFLD compared with those without a fatty liver and also when comparing those with ‘benign’ steatosis versus patients with NASH [Cusi et al. unpublished]. However, it had less accuracy in necroinflammation grading or fibrosis staging within subjects with NASH. Future studies will confirm the role of CK-18 and other emerging plasma biomarkers in the management of patients with NASH.
Metabolic consequences of NAFLD
NAFLD and T2DM
The most recent data published from the January 2011 national diabetes fact sheet show astounding statistics that 8.3% of the United States population (25.8 million people) are affected by diabetes [Centers for Disease Control and Prevention, 2011]. There is a close relationship between NAFLD and diabetes. For instance, in the general population, elevated liver aminotransferases are associated with a greater risk of having T2DM [Fraser et al. 2009; Sattar et al. 2004]. On the other hand, it is believed that the majority of patients with T2DM have a fatty liver and that as many as 50% or more may have NASH (see Table 1). NASH is often overlooked in patients with T2DM and no guidelines are available to assist clinicians on how to screen them for this condition. Elevated liver aminotransferases are a strong indicator of possible NASH and a greater risk of more advanced disease that should prompt a more aggressive diagnostic effort. Unfortunately, most patients with diabetes have normal liver aminotransferases and clinicians do not suspect the potential presence of NAFLD [Fracanzani et al. 2008; Kotronen et al. 2008; Mofrad et al. 2003]. Thus, normal ALT levels should not preclude a clinician from pursuing a diagnosis of fatty liver.
Table 1.
The link between NAFLD and type 2 diabetes.
|
In our experience, about 70% of patients with NASH have disordered glucose metabolism, either impaired fasting glucose, impaired glucose tolerance or T2DM, when systematically screened with an oral glucose tolerance test (OGTT) [Ortiz-Lopez et al. 2010]. Earlier screening and diagnosis for T2DM in NAFLD patients may allow for early intervention and prevention of diabetes complications. This is important because the presence of fatty liver in T2DM is associated with more difficult to control diabetes and higher insulin requirements [Ryysy et al. 2000]. In addition, patients with T2DM and NASH have more severe hepatic insulin resistance and progressive liver disease [Cusi, 2009a]. Also advanced fibrosis is associated with obesity, insulin resistance, hepatocyte lipo-toxicity, hyperinsulinemia, and abnormal glucose metabolism [Bataller et al. 2011; Neuschwander-Tetri et al. 2010].
NAFLD and CVD
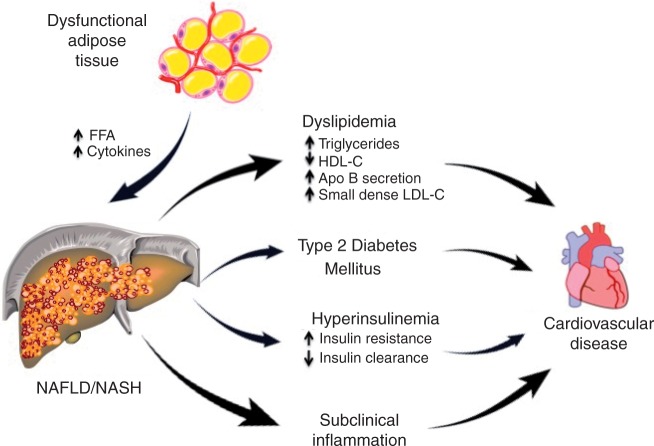
The association of NAFLD with MetS and obesity has led to the connection between NAFLD and the development and progression of CVD (Figure 1). Both adult and children patients with NAFLD typically meet the criteria for the MetS (HTN, abdominal obesity, atherogenic dyslipidemia, insulin resistance or glucose intolerance) and thus have multiple risk factors for CVD. As summarized in Table 2, patients with NAFLD/NASH more frequently have T2DM, more severe dyslipidemia, more insulin resistance, worse subclinical inflammation (i.e. high-sensitivity C-reactive protein [hsCRP], interleukin 6 [IL-6], tumor necrosis factor alpha [TNF-α]) and may be affected by myocardial lipotoxicity.
Figure 1.
Dysfunctional, insulin-resistant adipose tissue is common in overweight and obese subjects and leads to excessive free fatty acids (FFAs) in the liver. This promotes triglyceride accumulation, hepatocyte lipotoxicity with necrosis, inflammation and eventual fibrosis. The metabolic consequences are dyslipidemia, hyperglycemia, hyperinsulinemia and subclinical inflammation, all leading to premature cardiovascular disease (CVD). NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 2.
Risk factors for cardiovascular disease in nonalcoholic fatty liver disease.
|
Dyslipidemia in NAFLD is characterized by an increase in very-low-density lipoprotein (VLDL) secretion that leads to elevated plasma triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-C) [Adiels et al. 2006]. There is an association between the higher secretion of apolipoprotein B particles and the increase in the atherogenicity of VLDL secreted by the liver [Adiels et al. 2008]. Moreover, decreased lipoprotein lipase clearance promotes postprandial lipemia and is another contributor to vascular damage. Hypertriglyceridemia in NAFLD also leads to small, dense LDL-C and an added risk of atherogenesis. Patients with elevated ALT and high plasma TG and cholesterol have >80% chance of having NAFLD [Browning, 2006].
Dysfunctional fat releases excessive amounts of free fatty acids (FFAs) leading to ectopic fat deposition in tissues that are poorly adapted to TG accumulation such as muscle, liver, and pancreatic β-cells [Cusi, 2010]. In 187 middle-aged obese patients with biopsy-proven NASH, compared with well-matched obese controls without NAFLD, we observed that the severity of adipose tissue insulin resistance kept a close association with metabolic and histological damage in patients with NASH [Lomonaco et al. 2011b]. We also observed that hepatic insulin resistance and steatosis correlated closely with the severity of insulin resistance in adipose tissue [Ortiz-Lopez et al. 2011]. This was even as both groups had similar BMI and total adiposity as measured by dual X-ray absorptiometry (DXA), suggesting that it is not the total amount of fat but its degree of dysfunction and insulin resistance that account for the development of NASH.
Dysfunctional adipocytes secrete many inflammatory cytokines previously believed to be produced only by macrophages (i.e. TNF-α, IL-6, resistin, monocyte chemoattractant protein-1 [MCP-1], plasminogen activator inhibitor-1 [PAI-1], visfatin, angiotensinogen, retinol-binding protein-4 [RBP-4], etc.) [Gregor and Hotamisligil, 2007; Shoelson et al. 2006]. It is now accepted that adipokines promote insulin resistance by inhibiting key insulin signaling steps in liver and muscle, and are actively involved in the recruitment and ‘activation’ of local macrophages that play a role in the development of adipose tissue insulin resistance, increased plasma FFAs and ultimately lipotoxicity [Cusi, 2011; Muhlhausler and Smith, 2009]. Dysfunctional adipocyte function is also characterized by reduced plasma adiponectin levels as reported in NASH, and its increase during pioglitazone treatment closely associated with histological improvement [Gastaldelli et al. 2010]. There is a close relationship between the state of subclinical inflammation, insulin resistance and atherogenesis in obesity and likely this contributes to the CVD of patients with NAFLD
A chronic increase in plasma FFA levels is especially harmful to the heart and vascular beds, and it is now accepted that it plays an active role in the development of CVD [Cusi, 2009b; McGavock et al. 2006]. MRS has been used to show an association between hepatic and cardiac lipid accumulation [Reingold et al. 2005]. Myocardial TG content is elevated in subjects with either glucose intolerance or T2DM [Perseghin et al. 2008; Kankaanpaa et al. 2006]. It is believed that the prevalence of coronary, cerebrovascular, and peripheral vascular disease is higher among patients with NAFLD than those without NAFLD [Targher et al. 2010]. We have observed that just a mild elevation in plasma FFA to levels observed in T2DM for 48–72 hours by means of a lipid infusion is sufficient to increase blood pressure and induces the production of markers of systemic inflammation (i.e. soluble intercellular adhesion molecule [ICAM] and vascular adhesion molecule [VCAM], endothelin [ET]-1) in lean healthy subjects [Kashyap et al. 2008; Tay et al. 2006]. Moreover, we have recently expanded these observations by showing that a 48-hour increase in plasma FFA concentration also increases soluble E-Selectin (sE-Selectin), myeloperoxidase (MPO), and total plasminogen activator inhibitor-1 (tPAI-1), indicators of a procoagulant state and associated with abnormal vascular reactivity [Mathew et al. 2010]. Patients with NAFLD have impaired flow-mediated vasodilatation and increased carotid-artery intima-media thickness [Targher et al. 2010]. However, although many lines of evidence suggest that patients with NAFLD are at higher risk of CVD, this hypothesis requires more rigorous evaluation and confirmation in large, controlled studies.
Management of NAFLD: Practical considerations
A multifaceted approach should be taken for the management of patients with NAFLD (Table 3). First, physicians should make a distinction between diagnosing fatty liver and steatohepatitis (NASH). This is because the diagnosis of NAFLD (usually done by elevated liver amino-transferases and or imaging) carries metabolic consequences of insulin resistance and diabetes, while NASH implies liver inflammation with a risk of more severe disease and requires a liver biopsy to establish the diagnosis. Physicians must decide when to perform an US, and even a liver biopsy, in a patient suspected of having NAFLD. Currently, decision making is difficult because the natural history of the disease remains poorly understood and there is no pharmacological agent approved for the treatment of NAFLD. This may change in the near future. In any case, it should not prevent the clinician from efforts to aggressively treat the components of the MetS (i.e. obesity, dyslipidemia, and HTN) to avoid diabetes and CVD.
Table 3.
Management guidelines for patients with nonalcoholic fatty liver disease.
|
Lifestyle intervention: Diet and exercise
As awareness of the serious risks associated with NAFLD is increasing, there has been a greater effort to screen and implement combined lifestyle and pharmacological interventions. The most rational approach to weight reduction involves lifestyle modifications that incorporate diet and exercise [Cusi, 2009c]. Both, have proven effective to prevent T2DM [The Diabetes Prevention Program Research Group, 2005] and CVD [Fogelholm, 2010]. Many studies indicate that lifestyle [Haufe et al. 2011; Lazo et al. 2010; Kantartzis et al. 2009; Kirk et al. 2009; Viljanen et al. 2009] intervention may normalize liver aminotransferases and improve hepatic steatosis measured either by US [Sreenivasa Baba et al. 2006; Suzuki et al. 2005; Hickman et al. 2004; Kugelmas et al. 2003; Okita et al. 2001; Ueno et al. 1997; Andersen et al. 1991; Palmer and Schaffner, 1990] or MRS [Cowin et al. 2008; Larson-Meyer et al. 2008, 2006; Schafer et al. 2007; Thamer et al. 2007; Thomas et al. 2006; Petersen et al. 2005; Tamura et al. 2005; Westerbacka et al. 2005; Tiikkainen et al. 2003]. However, most of them are limited by small study size and short duration. Owing to the invasive nature of liver biopsy, numerous studies have used biochemical improvement as the primary endpoint but rather relied on surrogate markers, such as liver aminotransferases or imaging (US, CT, MRS). Histological improvement is proportional to the degree of total body weight loss. At least a total body weight loss of around 3–5% is necessary to improve liver steatosis, but a greater weight loss (7–10%) appears to be needed for improvements in necroinflammation in adult patients with NASH.
Because weight loss is challenging, approaches including medications or surgical procedures have been used in NAFLD. Medications such as orlistat [Harrison et al. 2009] or sibutramine [Zelber-Sagi et al. 2006] have not proved to be better than placebo if a comparable weight loss is achieved, suggesting that any benefit is strictly related to their potential to assist with weight loss. It is now accepted that bariatric surgery is associated with marked improvement or resolution of diabetes, HTN, and dyslipidemia [Chavez-Tapia et al. 2010; Pillai and Rinella, 2009]. In general, both Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB) have shown improvement in steatosis and a reduction in inflammation and ballooning. Fibrosis has been more inconsistent, with some studies reporting an increase in fibrosis [Csendes et al. 2006; Mathurin et al. 2009]. Procedures with a malabsorptive component, such as RYGB, lead to a greater weight loss and metabolic benefit than LAGB. However, LAGB appears to be becoming the procedure of choice in many centers as there is a clear trend favoring less invasive techniques (i.e. LAGB), although weight loss tends to be less. The best bariatric surgery for NASH is not known, as there are a number of limitations from the literature. Most studies are retrospective or uncontrolled and suffer from selection bias, poor standardization of presurgery and postsurgery dietary and follow-up procedures, and variable time of post-surgical follow up (from weeks to years). Of note, bariatric surgery as a method of weight loss is attractive as it has shown reductions in long-term mortality, but this has not been investigated specifically in patients with NAFLD [Sjostrom et al. 2007].
Treatment of dyslipidemia
As mentioned earlier, since NAFLD is strongly associated with the MetS and carries an elevated risk for CVD, patients would benefit from intensive medical intervention to control dyslipidemia. Asymptomatic elevations in AST or ALT three times the ULN have been reported with all statins in the general population. A threefold elevation of AST or ALT is seen in 1% of patients receiving initial and intermediate doses of statins and in 2–3% of patients at the maximal dose [McKenney et al. 2006]. The prescription of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, to patients with NAFLD is controversial, at best, due to an apparent risk of statin-related hepatotoxicity. However, several studies have now reported that statins can be safely used in patients with liver disease [Zamor and Russo, 2011; Argo et al. 2008; Riley et al. 2008; Ekstedt et al. 2007; Lewis et al. 2007; Chalasani, 2005]. Recent guidelines from the National Lipid Association (NLA) [McKenney et al. 2006], based at least in part on the assessment performed by the Expert Liver Panel [Cohen et al. 2006], concluded that statins could be given safely to patients with NAFLD or NASH. One should consider starting low-dose statin therapy, possibly of the more potent statins, and slowly titrate up with close monitoring of liver tests as needed to reach lipid targets. If an isolated asymptomatic aminotransferase level is found during a routine evaluation (up to three times the ULN), the NLA guidelines suggest that there is no need to discontinue the statin immediately but to have the test repeated. If still elevated, other causes should be ruled out and clinical judgment should be allowed to decide about continuing the statin or not. The appropriate frequency of monitoring liver function tests is unclear, but it is considered as appropriate to measure aminotransferase levels before starting therapy, 12 weeks after initiating therapy or after a dose increase, and periodically thereafter. However, the NLA did not believe that routine monitoring of liver aminotransferases was supported by the available evidence and suggested that the FDA reconsider this strategy. The statin should be discontinued at the first evidence of significant liver injury, the cause investigated and the patient referred to a hepatologist.
Because the most common dyslipidemia in NAFLD is an elevated plasma TG and a low HDL-C level, combination therapy with a lipid-lowering agent that specifically targets these defects is frequently needed. Fibrates can ameliorate atherogenic dyslipidemia in the MetS [Belfort et al. 2010] and improve dyslipidemia in NAFLD [Fernandez-Miranda et al. 2008; Basaranoglu et al. 1999]. Fenofibrate is preferred in combination therapy as it does not increase the levels of the statin [Bergman et al. 2004], in contrast to a twofold to threefold increase with gemfibrozil [Backman et al. 2000]. Based on results from recent clinical trials, combination therapy with fenofibrate should be targeted exclusively at patients with plasma TG concentrations greater than 200 mg/dl and a low HDL-C [Ginsberg et al. 2010; Keech et al. 2005; Tenenbaum et al. 2005]. Recently (25 May 2011), the AIM-HIGH study, a randomized, multicenter clinical trial sponsored by the National Heart, Lung and Blood Institute (NHLBI) in patients with low HDL-C and high TG, was discontinued due to the lack of efficacy in reducing CVD while examining the role of long-acting niacin (Niaspan®) in patients with a history of established CVD and well controlled LDL-C by simvastatin. There were 249 primary outcome events (15%) in the simvastatin plus placebo arm and 262 (15%) in the Niaspan plus simvastatin (p = 0.561). There were a total of 28 ischemic strokes (1.6%) in the Niaspan plus simvastatin arm and a total of 12 such events (0.7%) reported in the simvastatin arm.
Treatment of T2DM
Because insulin resistance is common in NAFLD, there has been significant interest in diabetes drugs with this mechanism of action, both for metformin and thiazolidinediones (TZDs). Metformin is a biguanide that ameliorates insulin resistance primarily at the level of the liver and to a lesser extent skeletal muscle [Cusi et al. 1996]. While several small trials have shown it can be used safely and that it reduces aminotransferase levels in NAFLD [Loomba et al. 2009; Duseja et al. 2007; Nair et al. 2004; Uygun et al. 2004; Marchesini et al. 2001], it should not be expected to improve histology [Haukeland et al. 2009; Loomba et al. 2009; Bugianesi et al. 2005]. In children and adolescents (aged 8–17 years), neither vitamin E nor metformin for 96 weeks significantly reduced plasma ALT levels (primary endpoint) or steatosis, lobular inflammation or fibrosis on individual scores, although the combined overall NAFLD Activity Score (NAS; NAS = combined steatosis, lobular inflammation, and ballooning) improved modestly with vitamin E compared with placebo [Lavine et al. 2011]. While the effect of metformin is rather modest to improve NASH, it remains as first-line therapy to treat hyperglycemia in T2DM and may have a modest beneficial effects on lipids and subclinical inflammation in this population [Cusi and DeFronzo, 1998].
Pioglitazone and rosiglitazone both belong to the thiazolidinedione (TZD) class of drugs that operate as ligands for the peroxisomal proliferators-activated receptor-γ(PPARγ)a class of nuclear transcription factors that are very abundant in adipose tissue. They are insulin-sensitizing agents approved for the treatment of T2DM only, but also effective in halting the progression of prediabetes to diabetes [DeFronzo et al. 2011; The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators, 2006]. Pioglitazone, but not rosiglitazone [Ratziu et al. 2010, 2008], has proven to be the most useful drug for the treatment of NASH. Therefore, a strong consideration should be given to its earlier use in this population. Other oral agents (sulfonylureas, insulin, dipeptidyl peptidase [DPP] IV inhibitors) may also be used safely in NAFLD. Incretin mimetics, such as exenatide (Byetta®) or liraglutide (Victoza®), have generated significant interest as they promote weight loss and activation of hepatic GLP-1 signaling causes reduction of hepatic steatosis in rodents [Ding et al. 2006]. In patients with well-controlled T2DM, 6 months of exenatide twice daily has been reported to lead to an around 20% reduction in hepatic steatosis by MRS [Orsi et al. 2009]. However, it does not have a significant effect on reversing NASH [Kenny et al. 2010].
Treatment of NASH
Only two pharmacological interventions are currently promising in NASH: pioglitazone and vitamin E. TZDs exert positive changes on adipocytes (i.e. restoring adipocyte insulin sensitivity, increasing plasma adiponectin levels, reducing excessive lipolysis and plasma FFA levels, among others) and improve hepatic and peripheral (muscle) insulin sensitivity. The first proof-of-concept controlled trial [Belfort et al. 2006] in patients with NASH and either with IGT or T2DM, demonstrated that pioglitazone significantly lowered liver aminotransferases, increased plasma adiponectin levels and improved adipose tissue, liver and muscle insulin sensitivity. Liver steatosis, ballooning necrosis and inflammation histology scores improved significantly with pioglitazone (combined necroinflammation score was reduced by 85%). Liver fibrosis improved versus baseline but did not reach statistical significance when compared with placebo. The NAFLD activity score improved with pioglitazone in 73% compared with 24% of placebo-treated patients. Since this early report, two studies have expanded this observation to subjects without diabetes [Sanyal et al. 2010; Aithal et al. 2008], the largest being the PIVENS trial [Sanyal et al. 2010], with histological improvement in steatosis and inflammation but not fibrosis after 2 years of pioglitazone treatment. Unfortunately these studies have been of relative short duration (6–24 months) and await confirmation about their long-term benefit. It should also be kept in mind that pioglitazone is only approved for the treatment of patients with T2DM. Therefore, at the present time, physicians should consider its use primarily for patients with NASH that also have T2DM after careful consideration of the treatment options and integrated proper lifestyle intervention. Finally, vitamin E at doses of 400 units twice daily was equally effective in patients without diabetes and NASH [Sanyal et al. 2010] and should be considered as another viable and inexpensive choice for patients with NASH.
Summary
NAFLD is the most common chronic liver condition in adults and is present in the majority of obese subjects that endocrinologists see in their daily practice. It may lead to serious medical consequences ranging from cryptogenic cirrhosis to hepatocellular carcinoma as well as T2DM and CVD. The diagnosis of NAFLD is challenging and liver aminotransferases may be helpful if elevated, but if normal the clinician must still suspect the presence of the disease based on patient's metabolic profile. Liver ultrasound may be of assistance in the diagnosis (MRI and spectroscopy is still a research tool) but a definitive diagnosis of NASH often requires ruling out other liver conditions and eventually a liver biopsy. Noninvasive approaches combining the clinical profile (obesity, T2DM, HTN, dyslipidemia) and novel biomarkers will change the management of the disease in the near future. Treatment of NAFLD includes lifestyle intervention and aggressive management of cardiovascular risk factors. Pioglitazone and vitamin E are currently the best pharmacological options for patients with NASH, although long-term studies are needed. Endocrinologists will likely be more often consulted and involved in the management of patients with NAFLD in the future.
Acknowledgements
Dr Kenneth Cusi is supported by the American Diabetes Association, the Burroughs Wellcome Fund, the Veterans Affairs Medical Research Fund, and the National Center for Research Resources (award number UL 1RR025767).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institutes of Health.
Footnotes
The authors declare no conflicts of interest in preparing this article.
References
- Adams L.A., Lymp J.F., St Sauver J., Sanderson S.O., Lindor K.D., Feldstein A., et al. (2005a) The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 129: 113–121 [DOI] [PubMed] [Google Scholar]
- Adams L.A., Sanderson S., Lindor K.D., Angulo P. (2005b) The histological course of nonalcoholic fatty liver disease: A longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 42: 132–138 [DOI] [PubMed] [Google Scholar]
- Adiels M., Taskinen M.-R., Boren J. (2008) Fatty liver, insulin resistance, and dyslipidemia. Curr Diab Rep 8: 60–64 [DOI] [PubMed] [Google Scholar]
- Adiels M., Taskinen M.-R., Packard C., Caslake M.J., Soro-Paavonen A., Westerbacka J., et al. (2006) Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 49: 755. [DOI] [PubMed] [Google Scholar]
- Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I., et al. (2008) Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135: 1176–1184 [DOI] [PubMed] [Google Scholar]
- Ali R., Cusi K. (2009) New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD). Ann Med 41: 265–278 [DOI] [PubMed] [Google Scholar]
- Andersen T., Gluud C., Franzmann M.B., Christoffersen P. (1991) Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol 12: 224–229 [DOI] [PubMed] [Google Scholar]
- Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C., et al. (2007) The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45: 846–854 [DOI] [PubMed] [Google Scholar]
- Argo C.K., Loria P., Caldwell S.H., Lonardo A. (2008) Statins in liver disease: A molehill, an iceberg, or neither? Hepatology 48: 662–669 [DOI] [PubMed] [Google Scholar]
- Backman J.T., Kyrklund C., Kivisto K.T., Wang J.S., Neuvonen P.J. (2000) Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther 68: 122–129 [DOI] [PubMed] [Google Scholar]
- Basaranoglu M., Acbay O., Sonsuz A. (1999) A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol 31: 384. [DOI] [PubMed] [Google Scholar]
- Bataller R., Rombouts K., Altamirano J., Marra F. (2011) Fibrosis in alcoholic and nonalcoholic steatohepatitis. Best Pract Res Clin Gastroenterol 25: 231–244 [DOI] [PubMed] [Google Scholar]
- Belfort R., Berria R., Cornell J., Cusi K. (2010) Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J Clin Endocrinol Metab 95: 829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J., et al. (2006) A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307 [DOI] [PubMed] [Google Scholar]
- Bergman A.J., Murphy G., Burke J., Zhao J.J., Valesky R., Liu L., et al. (2004) Simvastatin does not have a clinically significant pharmacokinetic interaction with fenofibrate in humans. J Clin Pharmacol 44: 1054–1062 [DOI] [PubMed] [Google Scholar]
- Browning J.D. (2006) Statins and hepatic steatosis: Perspectives from the Dallas Heart Study. Hepatology 44: 466–471 [DOI] [PubMed] [Google Scholar]
- Browning J.D., Kumar K.S., Saboorian M.H., Thiele D.L. (2004a) Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol 99: 292–298 [DOI] [PubMed] [Google Scholar]
- Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C., et al. (2004b) Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 40: 1387–1395 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Gentilcore E., Manini R., Natale S., Vanni E., Villanova N., et al. (2005) A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 100: 1082–1090 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Vanni E., Marchesini G. (2007) NASH and the risk of cirrhosis and hepatocellular carcinoma in type 2 diabetes. Curr Diab Rep 7: 175–180 [DOI] [PubMed] [Google Scholar]
- Caldwell S. (2010) Cryptogenic cirrhosis: What are we missing? Curr Gastroenterol Rep 12: 40–18 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2011) National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011, US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- Chalasani N. (2005) Statins and hepatotoxicity: Focus on patients with fatty liver. Hepatology 41: 690–695 [DOI] [PubMed] [Google Scholar]
- Chavez-Tapia N.C., Tellez-Avila F.I., Barrientos-Gutierrez T., Mendez-Sanchez N., Lizardi-Cervera J., Uribe M. (2010) Bariatric surgery for nonalcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev 1: CD007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitturi S., Wong V.W., Farrell G. (2011) Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol 26(Suppl. 1): 163–172 [DOI] [PubMed] [Google Scholar]
- Clark J.M., Brancati F.L., Diehl A.M. (2002) Nonalcoholic fatty liver disease. Gastroenterology 122: 1649–1657 [DOI] [PubMed] [Google Scholar]
- Clark J.M., Brancati F.L., Diehl A.M. (2003) The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 98: 960–967 [DOI] [PubMed] [Google Scholar]
- Cohen D.E., Anania F.A., Chalasani N. (2006) An assessment of statin safety by hepatologists. Am J Cardiol 97(8A): 77C–81C [DOI] [PubMed] [Google Scholar]
- Cowin G.J., Jonsson J.R., Bauer J.D., Ash S., Ali A., Osland E.J., et al. (2008) Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging 28: 937–945 [DOI] [PubMed] [Google Scholar]
- Csendes A., Smok G., Burgos A.M. (2006) Histological findings in the liver before and after gastric bypass. Obes Surg 16(5): 607–611 [DOI] [PubMed] [Google Scholar]
- Cusi K. (2009a) Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 16: 141–149 [DOI] [PubMed] [Google Scholar]
- Cusi K. (2009b) Role of insulin resistance and lipo-toxicity in non-alcoholic steatohepatitis. Clin Liver Dis 13: 545–563 [DOI] [PubMed] [Google Scholar]
- Cusi K. (2009c) The epidemic of type 2 diabetes mellitus: Its links to obesity, insulin resistance and lipotoxicity. In Regensteiner Judith, Reusch Jane, Stewart Kerry, Veves Aris. (eds.) Textbook of Diabetes and Exercise, Humana Press Inc; pp 3–54 [Google Scholar]
- Cusi K. (2010) The role of adipose tissue and lipo-toxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep 10: 306–315 [DOI] [PubMed] [Google Scholar]
- Cusi K. (2011) Clinical implications of obesity and lipotoxicity for the treatment of nonalcoholic steato-hepatitis (NASH). Gastroenterology, in press. [Google Scholar]
- Cusi K., Consoli A., DeFronzo R.A. (1996) Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 81: 4059–4067 [DOI] [PubMed] [Google Scholar]
- Cusi K., DeFronzo R. (1998) Metformin: A review of its metabolic effects. Diabetes Reviews 6: 89–131 [Google Scholar]
- DeFronzo R.A., Tripathy D., Schwenke D.C., Banerji M., Bray G.A., Buchanan T.A., et al. (2011) Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 364: 1104–1115 [DOI] [PubMed] [Google Scholar]
- Diab D.L., Yerian L., Schauer P., Kashyap S.R., Lopez R., Hazen S.L., et al. (2008) Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol 6: 1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Saxena N.K., Lin S., Gupta N.A., Anania F.A. (2006) Exendin-4, a glucagon-like pro-tein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowman J.K., Tomlinson J.W., Newsome P.N. (2011) Systematic review: The diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther 33: 525–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duseja A. (2010) Nonalcoholic fatty liver disease in India—a lot done, yet more required! Indian J Gastroenterol 29: 217–225 [DOI] [PubMed] [Google Scholar]
- Duseja A., Das A., Dhiman R.K., Chawla Y.K., Thumburu K.T., Bhadada S., et al. (2007) Metformin is effective in achieving biochemical response in patients with nonalcoholic fatty liver disease (NAFLD) not responding to lifestyle interventions. Ann Hepatol 6: 222–226 [PubMed] [Google Scholar]
- Ekstedt M., Franzen L.E., Mathiesen U.L., Holmqvist M., Bodemar G., Kechagias S. (2007) Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: A histopathological follow-up study. J Hepatol 47: 135–141 [DOI] [PubMed] [Google Scholar]
- Ekstedt M., Franzen L.E., Mathiesen U.L., Thorelius L., Holmqvist M., Bodemar G., et al. (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873 [DOI] [PubMed] [Google Scholar]
- Fassio E., Alvarez E., Dominguez N., Landeira G., Longo C. (2004) Natural history of nonalcoholic steatohepatitis: A longitudinal study of repeat liver biopsies. Hepatology 40: 820–826 [DOI] [PubMed] [Google Scholar]
- Feldstein A.E., Wieckowska A., Lopez A.R., Liu Y.C., Zein N.N., McCullough A.J. (2009) Cytokeratin-18 fragment levels as noninvasive bio-markers for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology 50: 1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Miranda C., Perez-Carreras M., Colina F., Lopez-Alonso G., Vargas C., Solis-Herruzo J.A. (2008) A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis 40: 200–205 [DOI] [PubMed] [Google Scholar]
- Fogelholm M. (2010) Physical activity, fitness and fatness: Relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev 11: 202–221 [DOI] [PubMed] [Google Scholar]
- Fracanzani A.L., Valenti L., Bugianesi E., Andreoletti M., Colli A., Vanni E., et al. (2008) Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 48: 792–798 [DOI] [PubMed] [Google Scholar]
- Fraser A., Thinggaard M., Christensen K., Lawlor D.A. (2009) Alanine aminotransferase, gamma-glutamyltransferase (GGT) and all-cause mortality: Results from a population-based Danish twins study alanine aminotransferase, GGT and mortality in elderly twins. Liver Int 29: 1494–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich-Rust M., Ong M.F., Martens S., Sarrazin C., Bojunga J., Zeuzem S., et al. (2008) Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology 134: 960–974 [DOI] [PubMed] [Google Scholar]
- Gaia S., Carenzi S., Barilli A.L., Bugianesi E., Smedile A., Brunello F., et al. (2011) Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol 54: 64–71 [DOI] [PubMed] [Google Scholar]
- Gastaldelli A., Harrison S., Belfort-Aguiar R., Hardies J., Balas B., Schenker S., et al. (2010) Pioglitazone in the treatment of NASH: The role of adiponectin. Aliment Pharmacol Ther 32: 769–775 [DOI] [PubMed] [Google Scholar]
- Ginsberg H.N., Elam M.B., Lovato L.C., Crouse J.R., III, Leiter L.A., Linz P., et al. (2010) Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 362: 1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M., Hotamisligil G. (2007) Thematic review series: Adipocyte Biology. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J Lipid Res 48: 1905–1914 [DOI] [PubMed] [Google Scholar]
- Harrison S.A. (2006) Liver disease in patients with diabetes mellitus. J Clin Gastroenterol 40: 68–76 [DOI] [PubMed] [Google Scholar]
- Harrison S.A., Fecht W., Brunt E.M., Neuschwander-Tetri B.A. (2009) Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology 49: 80–86 [DOI] [PubMed] [Google Scholar]
- Harrison S.A., Torgerson S., Hayashi P.H. (2003) The natural history of nonalcoholic fatty liver disease: A clinical histopathological study. Am J Gastroenterol 98: 2042–2047 [DOI] [PubMed] [Google Scholar]
- Haufe S., Engeli S., Kast P., Bohnke J., Utz W., Haas V., et al. (2011) Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 53: 1504–1514 [DOI] [PubMed] [Google Scholar]
- Haukeland J.W., Konopski Z., Eggesbo H. B., von Volkmann H.L., Raschpichler G., Bjoro K., et al. (2009) Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand J Gastroenterol 44: 853–860 [DOI] [PubMed] [Google Scholar]
- Hickman I.J., Jonsson J.R., Prins J.B., Ash S., Purdie D.M., Clouston A.D., et al. (2004) Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 53: 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankaanpaa M., Lehto H.-R., Parkka J., Komu M., Viljanen A. (2006) Myocardial triglyceride content and epicardial fat mass in human obesity: Relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol 91: 4689–4695 [DOI] [PubMed] [Google Scholar]
- Kantartzis K., Thamer C., Peter A., Machann J., Schick F., Schraml C., et al. (2009) High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 58: 1281–1288 [DOI] [PubMed] [Google Scholar]
- Kashyap, Belfort R., Cersosimo E., Lee S., Cusi K. (2008) Chronic low-dose lipid infusion in healthy patients induces markers of endothelial activation independent of its metabolic effects. J Cardiometabol Syndrome 3: 141–146 [DOI] [PubMed] [Google Scholar]
- Keech A., Simes R.J., Barter P., Best J., Scott R., Taskinen M.R., et al. (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 366: 1849–1861 [DOI] [PubMed] [Google Scholar]
- Kenny P.R., Brady D.E., Torres D.M., Ragozzino L., Chalasani N., Harrison S.A. (2010) Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: A case series. Am J Gastroenterol 105: 2707–2709 [DOI] [PubMed] [Google Scholar]
- Kirk E., Reeds D.N., Finck B.N., Mayurranjan S.M., Patterson B.W., Klein S. (2009) Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 136: 1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotronen A., Juurinen L., Hakkarainen A., Westerbacka J., Corner A., Bergholm R., et al. (2008) Liver fat Is Increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care 31: 165–169 [DOI] [PubMed] [Google Scholar]
- Kugelmas M., Hill D.B., Vivian B., Marsano L., McClain C.J. (2003) Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology 38: 413–419 [DOI] [PubMed] [Google Scholar]
- Larson-Meyer D.E., Heilbronn L.K., Redman L.M., Newcomer B.R., Frisard M.I., Anton S., et al. (2006) Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer D.E., Newcomer B.R., Heilbronn L.K., Volaufova J., Smith S.R., Alfonso A.J., et al. (2008) Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 16: 1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine J.E., Schwimmer J.B., Van Natta M.L., Molleston J.P., Murray K.F., Rosenthal P., et al. (2011) Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 305: 1659–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo M., Solga S.F., Horska A., Bonekamp S., Diehl A.M., Brancati F.L., et al. (2010) Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 33: 2156–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite N.C., Villela-Nogueira C.A., Pannain V.L., Bottino A.C., Rezende G.F., Cardoso C.R., et al. (2011) Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: Prevalences and correlated factors. Liver Int 31: 700–706 [DOI] [PubMed] [Google Scholar]
- Lewis J.H., Mortensen M.E., Zweig S., Fusco M.J., Medoff J.R., Belder R. (2007) Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 46: 1453–1463 [DOI] [PubMed] [Google Scholar]
- Liang R.J., Wang H.H., Lee W.J., Liew P.L., Lin J.T., Wu M.S. (2007) Diagnostic value of ultrasonographic examination for nonalcoholic steatohepatitis in morbidly obese patients undergoing laparoscopic bariatric surgery. Obes Surg 17: 45–56 [DOI] [PubMed] [Google Scholar]
- Lomonaco R., Ortiz-Lopez C., Orsak B., Finch J., Webb A., Bril F., et al. (2011a) Role of ethnicity in overweight and obese subjects with nonalcoholic steatohepatitis (NASH). Hepatology, in press doi: 10.1002/hep.24483. [DOI] [PubMed] [Google Scholar]
- Lomonaco R., Orsak B., Ortiz-Lopez C., Chen J., Webb A., Cusi K. (2011b) Effect of liver fat accumulation on insulin resistance and severity of NASH in obese patients with T2DM. Diabetes 60: A1668 [Google Scholar]
- Longo R., Pollesello P., Ricci C., Masutti F., Kvam B.J., Bercich L., et al. (1995) Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging 5: 281–285 [DOI] [PubMed] [Google Scholar]
- Loomba R., Lutchman G., Kleiner D.E., Ricks M., Feld J.J., Borg B.B., et al. (2009) Clinical trial: Pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 29: 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Zoli M., Melchionda N. (2001) Metformin in non-alcoholic steatohepatitis. Lancet 358: 893–894 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., et al. (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917–923 [DOI] [PubMed] [Google Scholar]
- Matthew M., Tay E., Cusi K. (2010) Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P., Hollebecque A., Arnalsteen L., Buob D., Leteurtre E., Caiazzo R., et al. (2009) Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 137: 532–540 [DOI] [PubMed] [Google Scholar]
- Mazhar S.M., Shiehmorteza M., Sirlin C.B. (2009) Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol 7: 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavock J., Victor R., Unger R., Szczepaniak L. (2006) Adiposity of the heart, revisited. Ann Intern Med 144: 517–524 [DOI] [PubMed] [Google Scholar]
- McKenney J.M., Davidson M.H., Jacobson T.A., Guyton J.R. (2006) Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 97(8A): 89C–94C [DOI] [PubMed] [Google Scholar]
- Mencin A.A., Lavine J.E. (2011) Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care 14: 151–157 [DOI] [PubMed] [Google Scholar]
- Mofrad P., Contos M.J., Haque M., Sargeant C., Fisher R.A., Luketic V.A., et al. (2003) Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37: 1286–1292 [DOI] [PubMed] [Google Scholar]
- Mohanty S.R., Troy T.N., Huo D., O'Brien B.L., Jensen D.M., Hart J. (2009) Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol 50: 797–804 [DOI] [PubMed] [Google Scholar]
- Muhlhausler B., Smith S.R. (2009) Early-life origins of metabolic dysfunction: Role of the adipocyte. Trends Endocrinol Metab 20: 51–57 [DOI] [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M., Pagano G. (2010) Meta-analysis: Natural history of nonalcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med, in press. [DOI] [PubMed] [Google Scholar]
- Nair S., Diehl A.M., Wiseman M., Farr G.H., Jr., Perrillo R.P. (2004) Metformin in the treatment of non-alcoholic steatohepatitis: A pilot open label trial. Aliment Pharmacol Ther 20: 23–28 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A., Clark J.M., Bass N.M., Van Natta M.L., Unalp-Arida A., Tonascia J., et al. (2010) Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 52: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita M., Hayashi M., Sasagawa T., Takagi K., Suzuki K., Kinoyama S., et al. (2001) Effect of a moderately energy-restricted diet on obese patients with fatty liver. Nutrition 17: 542–547 [DOI] [PubMed] [Google Scholar]
- Orsi C., Matthew M., Chen J., Darland J., Gastaldelli A., Ali R., et al. (2009) Adding exenatide to bedtime insulin detemir promotes weight loss and improves hepatic steatosis in insulin-treated patients with T2DM. Diabetologia 52(Suppl. 1): A248 [Google Scholar]
- Ortiz-Lopez C., Orsak B., Darland C., Finch J., Lomonaco R., Cusi K. (2010) Abnormal glucose metabolism is common in NASH patients and associated with more severe hepatic and adipose tissue insulin resistance and hepatocyte necroinflammation. Diabetes 59(Suppl. 1): A88 [Google Scholar]
- Ortiz-Lopez C., Orsak B., Lomonaco R., Finch J., Darland C., Cusi K. (2011) Insulin resistance but not hyperglycemia, plays a key role in the severity of NAFLD in T2DM patients. Diabetes 60(Suppl. 1): A1665 [Google Scholar]
- Pagadala M., Zein C.O., McCullough A.J. (2009) Predictors of steatohepatitis and advanced fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis 13: 591–606 [DOI] [PubMed] [Google Scholar]
- Palmer M., Schaffner F. (1990) Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 99: 1408–1413 [DOI] [PubMed] [Google Scholar]
- Perseghin G., Lattuada G., De Cobelli F., Esposito A., Belloni E., Ntali G., et al. (2008) Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology 47: 51–58 [DOI] [PubMed] [Google Scholar]
- Petersen K.F., Dufour S., Befroy D., Lehrke M., Hendler R.E., Shulman G.I. (2005) Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54: 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A.A., Rinella M.E. (2009) Non-alcoholic fatty liver disease: Is bariatric surgery the answer? Clin Liver Dis 13: 689–710 [DOI] [PubMed] [Google Scholar]
- Poynard T., Ratziu V., Charlotte F., Messous D., Munteanu M., Imbert-Bismut F., et al. (2006) Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V., Charlotte F., Bernhardt C., Giral P., Halbron M., Lenaour G., et al. (2010) Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: Results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 51: 445–453 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Giral P., Jacqueminet S., Charlotte F., Hartemann-Heurtier A., Serfaty L., et al. (2008) Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135: 100–110 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Massard J., Charlotte F., Messous D., Imbert-Bismut F., Bonyhay L., et al. (2006) Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold J.S., McGavock J.M., Kaka S., Tillery T., Victor R.G., Szczepaniak L.S. (2005) Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: Reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab 289: E935–E939 [DOI] [PubMed] [Google Scholar]
- Riley P., Sudarshi D., Johal M., Benedict A., Panteli J., Crook M., et al. (2008) Weight loss, dietary advice and statin therapy in non-alcoholic fatty liver disease: A retrospective study. Int J Clin Pract 62: 374–381 [DOI] [PubMed] [Google Scholar]
- Ryysy L., Hakkinen A.M., Goto T., Vehkavaara S., Westerbacka J., Halavaara J., et al. (2000) Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 49: 749–758 [DOI] [PubMed] [Google Scholar]
- Saadeh S., Younossi Z.M., Remer E.M., Gramlich T., Ong J.P., Hurley M., et al. (2002) The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123: 745–750 [DOI] [PubMed] [Google Scholar]
- Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., et al. (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362: 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., Scherbakova O., Ford I., O'Reilly D.S., Stanley A., Forrest E., et al. (2004) Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes 53: 2855–2860 [DOI] [PubMed] [Google Scholar]
- Schafer S., Kantartzis K., Machann J., Venter C., Niess A., Schick F., et al. (2007) Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur J Clin Invest 37: 535–543 [DOI] [PubMed] [Google Scholar]
- Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. (2006) Prevalence of fatty liver in children and adolescents. Pediatrics 118: 1388–1393 [DOI] [PubMed] [Google Scholar]
- Shoelson S., Lee J., Goldfine A. (2006) Inflammation and insulin resistance. J Clin Invest 116: 1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom L., Narbro K., Sjostrom C.D., Karason K., Larsson B., Wedel H., et al. (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752 [DOI] [PubMed] [Google Scholar]
- Baba C. Sreenivasa, Alexander G., Kalyani B., Pandey R., Rastogi S., Pandey A., et al. (2006) Effect of exercise and dietary modification on serum amino-transferase levels in patients with nonalcoholic steato-hepatitis. J Gastroenterol Hepatol 21(1 Pt 1): 191–198 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Lindor K., St Saver J., Lymp J., Mendes F., Muto A., et al. (2005) Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 43: 1060–1066 [DOI] [PubMed] [Google Scholar]
- Szczepaniak L.S., Babcock E.E., Schick F., Dobbins R.L., Garg A., Burns D.K., et al. (1999) Measurement of intracellular triglyceride stores by H spectroscopy: Validation in vivo. Am JPhysiol 276(5 Pt 1): E977–E989 [DOI] [PubMed] [Google Scholar]
- Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S., et al. (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: E462–E468 [DOI] [PubMed] [Google Scholar]
- Tamura Y., Tanaka Y., Sato F., Choi J.B., Watada H., Niwa M., et al. (2005) Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 90: 3191–3196 [DOI] [PubMed] [Google Scholar]
- Targher G., Day C.P., Bonora E. (2010) Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 363: 1341–1350 [DOI] [PubMed] [Google Scholar]
- Tay C., Belfort R., Mathew M., Cusi K. (2006) A 2-day lipid or combined lipid-glucose infusion reproduce in healthy subjects the metabolic abnormalities seen in the metabolic syndrome. Diabetes 55(Suppl. 1): A66 [Google Scholar]
- Tenenbaum A., Motro M., Fisman E.Z., Tanne D., Boyko V., Behar S. (2005) Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med 165: 1154–1160 [DOI] [PubMed] [Google Scholar]
- Thamer C., Machann J., Stefan N., Haap M., Schafer S., Brenner S., et al. (2007) High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 15: 531–538 [DOI] [PubMed] [Google Scholar]
- The Diabetes Prevention Program Research Group (2005) Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: Effects of lifestyle intervention and metformin. Diabetes 54: 2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. The Lancet 368: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Thomas E.L., Brynes A.E., Hamilton G., Patel N., Spong A., Goldin R.D., et al. (2006) Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol 12: 5813–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiikkainen M., Bergholm R., Vehkavaara S., Rissanen A., Hakkinen A.M., Tamminen M., et al. (2003) Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes 52: 701–707 [DOI] [PubMed] [Google Scholar]
- Ueno T., Sugawara H., Sujaku K., Hashimoto O., Tsuji R., Tamaki S., et al. (1997) Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27: 103–107 [DOI] [PubMed] [Google Scholar]
- Uygun A., Kadayifci A., Isik A.T., Ozgurtas T., Deveci S., Tuzun A., et al. (2004) Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 19: 537–544 [DOI] [PubMed] [Google Scholar]
- Viljanen A.P., Iozzo P., Borra R., Kankaanpaa M., Karmi A., Lautamaki R., et al. (2009) Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab 94: 50–55 [DOI] [PubMed] [Google Scholar]
- Vuppalanchi R., Chalasani N. (2009) Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 49: 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerbacka J., Lammi K., Hakkinen A.M., Rissanen A., Salminen I., Aro A., et al. (2005) Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab 90: 2804–2809 [DOI] [PubMed] [Google Scholar]
- Wieckowska A., McCullough A.J., Feldstein A.E. (2007) Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and future. Hepatology 46: 582–589 [DOI] [PubMed] [Google Scholar]
- Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M., et al. (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 140: 124–131 [DOI] [PubMed] [Google Scholar]
- Wong V.W., Vergniol J., Wong G.L., Foucher J., Chan H. L., Le Bail B., et al. (2010) Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51: 454–462 [DOI] [PubMed] [Google Scholar]
- Yilmaz Y., Kedrah A.E., Ozdogan O. (2009) Cytokeratin-18 fragments and biomarkers of the metabolic syndrome in nonalcoholic steatohepatitis. World J Gastroenterol 15: 4387–4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z.M., Jarrar M., Nugent C., Randhawa M., Afendy M., Stepanova M., et al. (2008) A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH). Obes Surg 18: 1430–1437 [DOI] [PubMed] [Google Scholar]
- Zamor P.J., Russo M.W. (2011) Liver function tests and statins. Curr Opin Cardiol 4: 338–341 [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Kessler A., Brazowsky E., Webb M., Lurie Y., Santo M., et al. (2006) A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 4: 639–644 [DOI] [PubMed] [Google Scholar]