Introduction
Nonhealing diabetic foot ulcers are a major complication in patients with diabetes. In an unacceptably high number of cases, the final treatment result is lower extremity amputation, which might have been avoided if an aggressive therapy had been carried out earlier. Emerging cellular therapies such as platelet-rich plasma gel provide completely new ulcer management options that might help to avoid limb loss [Driver, 2006].
Case report
A 78-year-old patient with a 26-year history of type 2 diabetes mellitus and known macro- and microvascular complications was admitted to our interdisciplinary diabetic foot unit with a nonhealing diabetic foot ulcer, which had existed for 8 months (Figure 1).
Figure 1.
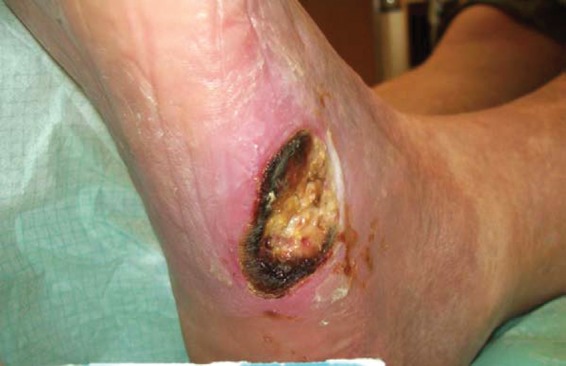
Initial presentation of the diabetic ulcer at the left lateral foot.
Mechanical relief by offloading pressure carried out on an outpatient basis was only partially successful and infection control of the forefoot failed despite antibiotic treatment. The ulcer ground extended to the metatarsal bone (os metatarsale V).
The patient’s initial management included measures to optimize glycaemic control and medical treatment of comorbidities (i.e. chronic cardiac and renal failure), as well as extensive debridement, antibiotic treatment based on wound pathogen cultures (intravenous application of tazobactam 4.5 g three times daily over 2 weeks in combination with clindamycin 600 mg three times daily orally; clindamycin treatment was continued as a monotherapy until complete wound healing was accomplished), the use of moisture dressings and offloading pressure from the wound.
Glycaemic control was accomplished by titrating blood glucose levels with insulin analogues (insulin lispro and detemir) in combination with metformin (2 × 1 g twice daily).
The residual perfusion of the affected foot was evaluated by measurement of ankle-brachial pressure index (ABPI) as well as colour-flow duplex ultrasonography. The ABPI of the affected side (0.5), the flow spectrum and the clinical picture indicated critical limb ischaemia. Therefore, we performed a percutaneous transluminal angioplasty. We found that arterial blood supply of the lower leg was provided only by the arteria tibialis posterior with collaterals from the plantar arch. Dilatation of stenoses of the tibiofibular outflow was successful but without peripheral arterial supply from the arteria tibialis anterior and arteria fibularis. Therefore, the arterial supply of the foot was insufficient for wound healing.
After exhaustive measures of conservative therapy remained unsuccessful, we performed surgical resection of the metatarsal bone (Os metatarsale V) and the cuboid bone (Figure 2). Thereafter, moisture wound dressings were used and vacuum-assisted therapy was performed. With this treatment, a clean wound situation was achieved but without sufficient granulation tissue and partially visible bone.
Figure 2.
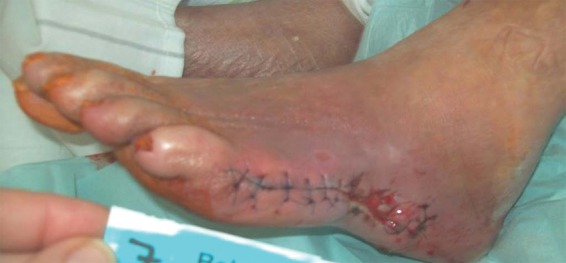
Intermediate state after surgical resection of metatarsale V and cuboid bone.
In this situation we decided to administer autologous platelet-rich plasma (PRP) gel into the ulcer. We used the GPS® III (Gravitational Platelet Separation) system (Biomet, Warsaw, IN, USA). With the GPS® III system circulating autologous platelets can be highly concentrated. After activation with autologous thrombin, these platelets can be applied to the wound and a PRP gel is formed locally.
In a two-step process, a whole blood sample of the patient is first centrifuged to separate red cells from platelet-rich and platelet poor plasma. PRP is then activated and mixed with autologous thrombin, resulting in a gelatinous platelet gel.
First, we performed surgical debridement of the large ulcer (length 10 cm, width 2 cm, depth 1.5 cm). Consecutively, the ulcer was completely filled with the autologous PRP gel (Figure 3). Initiation of granulation and closure of the wound is thought to be based on the local release of growth factors from the platelet gel.
Figure 3.
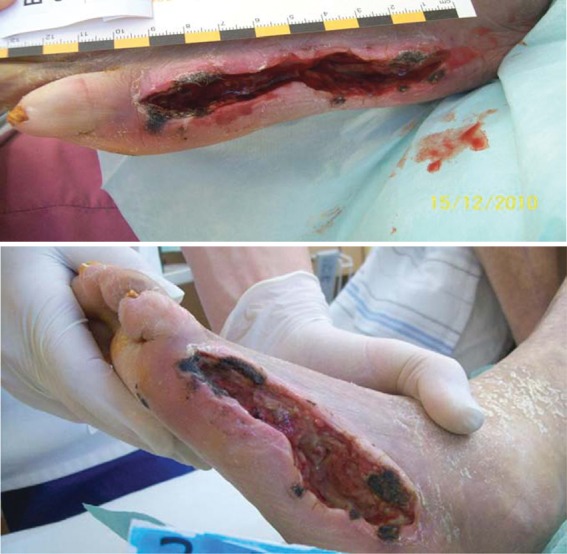
Prior to and immediately after application of platelet-rich plasma into the wound.
One week after application of the PRP gel we could not observe sufficient wound healing but an infection and increasing dehiscence of the distal wound region (Figure 4).
Figure 4.
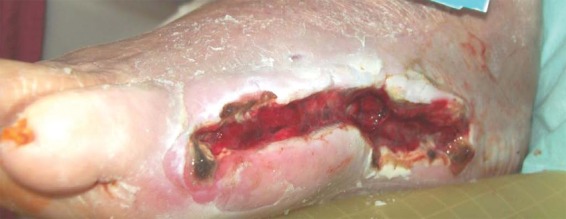
Infected wound 1 week after application of platelet-rich plasma.
Therefore, we decided to resect surgically the rest of the fifth toe and combined this procedure with an advanced flap on the distal wound region. One week after these procedures we observed a sufficient granulation of the whole wound and the beginning of wound retraction. At 3 weeks following application of the PRP gel the complete wound showed granulation and an advanced wound retraction (Figure 5). The wound was completely epithelialized after 6 weeks (Figure 6).
Figure 5.
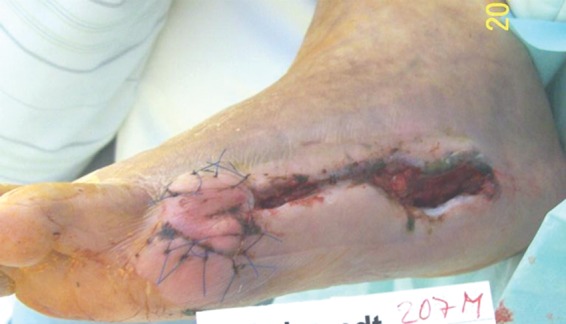
Granulation and wound healing after the second surgical treatment and 3 weeks after application of platelet rich plasma.
Figure 6.
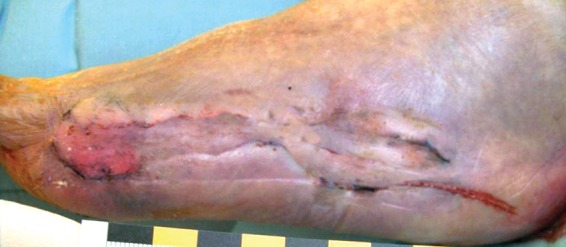
Wound healing 6 weeks after treatment.
For the first 3 weeks after GPS treatment the patient was under bed rest in order to secure adequate mechanical protection of the wound and clot. Thereafter, the patient was subsequently mobilized successfully and discharged after 4 weeks. During mobilization, the patient used custom-moulded orthopaedic shoes.
Discussion
Diabetic foot complications with critical ischaemia and ulcers reaching the bone or tendon are often limb threatening. In the present case report, we describe the case of a patient with a long-lasting, nonhealing, infected diabetic foot ulcer combined with critical limb ischaemia. In this patient, lower extremity amputation could be avoided by the application of autologous PRP gel.
PRP is defined as the plasma fraction of autologous blood with a platelet count concentrated above the baseline [Mehta and Watson, 2008]. It contains a high level of platelets and a full complement of clotting and growth factors. The secretory proteins contained in the α-granules of platelets include platelet-derived growth factor, transforming growth factor β, platelet factor 4, interleukin-1, platelet-derived angiogenesis factor, vascular endothelial growth factor, epidermal growth factor, platelet-derived endothelial growth factor, epithelial cell growth factor, insulin-like growth factor, osteocalcin, osteonectin, fibrinogen, vitronectin, fibronectin and thrombospondin-1 [Lacci and Dardik, 2010]. These growth factors may contribute to the healing process of chronic wounds by attracting undifferentiated cells in the newly formed matrix and triggering cell–cell division. PRP may suppress cytokine release and limit the amount of inflammation, interacting with macrophages to improve tissue healing and regeneration, promote new capillary growth and accelerate epithelialization [Lacci and Dardik, 2010]. Moreover, the activated platelets apparently have potential antibacterial effects and may thereby support wound healing [Bielecki et al. 2007; Tang et al. 2002].
Currently, only a few reports have been published on the use of autologous PRP for the treatment of diabetic foot ulcers [Akingboye et al. 2010; Driver et al. 2006; Frykberg et al. 2010; Margolis et al. 2001; Mc Aleer et al. 2006; Scimeca et al. 2010; Villela and Santos, 2010]. Most of these studies excluded ulcers with ‘challenging presentations’ such as critical limb ischaemia and exposed tendon or bone.
De Leon and colleagues recently published one of the most comprehensive studies on the clinical relevance of treating chronic wounds with PRP gel. In this multicentre study, 200 patients with 285 wounds were included. The authors stated that these ‘wounds are representative of real-world wound care populations’ [de Leon et al. 2011]. On the other hand, the number of ulcers with peripheral arterial disease (as is common in diabetic ulcers) was very small. Data on angiological observation were not available. Wounds with infection were included. Therefore, it is not possible to compare these data with our case report of a chronic nonhealing diabetic foot ulcer with limb-threatening ischaemia. A noninfected chronic wound without critical limb ischaemia should heal with appropriate standard wound care without the use of PRP gel. Most recently Carter and colleagues provided a meta-analysis on the use of PRP gel on wound healing [Carter et al. 2011]. This meta-analysis has the same limitations as the paper of de Leon and colleagues as the studies on well defined groups of patients with diabetic foot ulcer were scarce and again no data on angiological examinations were available [de Leon et al. 2011]. Nevertheless, our report supports the conclusion that autologous PRP gel shows promise as an effective treatment option in severe diabetic foot ulcers.
We used this treatment option in diabetic patients with nonhealing diabetic foot ulcers and critical limb ischaemia where all other options of standard and extended wound care (such as vacuum-assisted closure, extensive surgical debridement, aggressive treatment of peripheral arterial disease) were utilised, that means in the ‘challenging situation’ where limb loss is threatening.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest in preparing this article.
References
- Akingboye A.A., Giddins S., Gamston P., Tucker A., Navsaria H., Kyriakides C. (2010) Application of autologous derived-platelet-rich plasma gel in the treatment of chronic wound ulcer: diabetic foot ulcer. J Extra Corpor Technol 42: 20–29 [PMC free article] [PubMed] [Google Scholar]
- Bielecki T.M., Arendt J., Szczepanski T., Krol W., Wielkoszynski T. (2007) Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br 89: 417–420 [DOI] [PubMed] [Google Scholar]
- Carter M.J., Fylling C.P., Parnell L.K.S. (2011) Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty 11: 382–410 [PMC free article] [PubMed] [Google Scholar]
- de Leon J.M., Driver V.R., Fylling C.P., Carter M.J., Anderson C., Wilson J., et al. (2011) The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv Skin Wound Care 24: 357–368 [DOI] [PubMed] [Google Scholar]
- Driver V.R., Hanft J., Fylling C.P., Beriou J.M. (2006) Autologel Diabetic Foot Ulcer Study Group: a prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage 52: 68–70 [PubMed] [Google Scholar]
- Frykberg R.G., Driver V.R., Carman D., Lucero B., Borris-Hale C., Fylling C.P., et al. (2010). Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: a prospective case series. Ostomy Wound Manage 56: 36–44 [PubMed] [Google Scholar]
- Lacci K.M., Dardik A. (2010) Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med 83: 1–9 [PMC free article] [PubMed] [Google Scholar]
- Margolis D.J., Kantor J., Santanna J., Strom B.L., Berlin J.A. (2001) Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 24: 483–488 [DOI] [PubMed] [Google Scholar]
- McAleer J.P., Sharma S., Kaplan E.M., Persich G. (2006) Use of autologous platelet concentrate in a nonhealing lower extremity wound. Adv Skin Wound Care 19: 354–363 [DOI] [PubMed] [Google Scholar]
- Mehta S., Watson J.T. (2008) Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma 22: 432–438 [DOI] [PubMed] [Google Scholar]
- Scimeca C.L., Bharara M., Fisher T.K., Kimbriel H., Armstrong D.G. (2010) Novel use of platelet-rich plasma to augment curative diabetic foot surgery. J Diabetes Sci Technol 4: 1121–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.Q., Yeaman M.R., Selsted M.E. (2002) Antimicrobial peptides from human platelets. Infect Immun 70: 6524–6533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villela D., Santos V.L. (2010) Evidence on the use of platelet-rich plasma for diabetic ulcer: a systematic review. Growth Factors 28: 111–116 [DOI] [PubMed] [Google Scholar]


