Abstract
Objective:
To describe the rationale and design of PATRO Adults, a postmarketing surveillance study of the long-term efficacy and safety of somatropin (Omnitrope®) for the treatment of adult patients with growth hormone deficiency (GHD).
Methods:
PATRO Adults is an observational, multicentre, open, longitudinal, noninterventional study being conducted in hospitals and specialized endocrinology clinics across several European countries. The primary objective is to assess the safety and efficacy of Omnitrope® in adults treated in routine clinical practice. Eligible patients are male or female adults who are receiving treatment with Omnitrope® and who have provided informed consent. Patients who have been treated with another human growth hormone (hGH) product before starting Omnitrope® therapy will also be eligible for inclusion. Efficacy assessments will be based on the analysis of the following: insulin-like growth factor-1 levels within age- and gender-adjusted normal ranges; anthropometric measures (weight, waist circumference, total fat mass, lean body mass, total body water); bone mineral density; lipids; effects on cardiovascular risk factors such as glucose metabolism, blood pressure and inflammatory markers (e.g. C-reactive protein); and quality of life. All adverse events will be monitored and recorded. Particular emphasis will be placed on long-term safety, the recording of malignancies, the occurrence and clinical impact of antirecombinant hGH antibodies, the incidence, severity and duration of hyperglycaemia, and the development of diabetes during treatment with Omnitrope®.
Conclusions:
PATRO Adults is a large, long-term, postmarketing surveillance study that will extend the safety database for Omnitrope®, as well as contributing to the available data for all recombinant hGH products. Of particular interest, the study will provide important data on the impact of long-term GH replacement therapy on the development of diabetes mellitus, the recurrence/regrowth of hypothalamic–pituitary tumours, and de novo malignancy or recurrence of other (non-hypothalamic–pituitary) tumours.
Keywords: adults, growth hormone deficiency, human growth hormone, somatropin
Introduction
Growth hormone (GH) deficiency (GHD) is a well-recognized clinical entity in adults that results in abnormalities in substrate metabolism, body composition, physical and psychosocial function [Ho, 2007]. Adult GHD can persist from childhood or be newly acquired [Molitch et al. 2011]. Newly acquired GHD in adults is most often a result of structural lesions or trauma, or can in very rare cases be idiopathic.
All adults with documented severe GHD are eligible for GH replacement therapy, and the goals of treatment are to correct the metabolic, functional and psychological abnormalities associated with adult GHD [Ho, 2007; Molitch et al. 2011]. Demonstrated benefits of GH replacement therapy include improvements in body composition, exercise capacity, skeletal integrity, blood lipid profile and quality of life [Molitch et al. 2011].
Clinical practice guidelines are available for the diagnosis and treatment of adults with GHD [Ho, 2007; Molitch et al. 2011]. Both sets of guidelines recommend individualized rather than weight-based GH dosing regimens, with doses guided by serum insulin-like growth factor-1 (IGF-1) levels and the presence or absence of adverse effects. Monitoring of biochemistry, body composition and quality of life is also advised in order to assess response to treatment [Ho, 2007].
The assessment of clinical practice guidelines is that the risks associated with GH therapy are low [Molitch et al. 2011]. Nevertheless, extended clinical studies are needed to confirm the long-term safety of this therapy in routine clinical practice, particularly with regard to diabetogenic potential. A number of recombinant human growth hormone (hGH) products are approved for the treatment of adult GHD, including Omnitrope® (somatropin, Sandoz Biopharmaceuticals). Omnitrope® is a biosimilar medicine, approved by the European Medicines Agency (EMA) in 2007 on the basis of comparable quality, safety and efficacy to the reference product, Genotropin® (Pfizer) [EMA, 2008a]. This manuscript describes the rationale and design of PATRO Adults, a postmarketing surveillance study of the long-term efficacy and safety of somatropin (Omnitrope®) for the treatment of adult patients with GHD. The study is part of the postapproval pharmacovigilance plan for Omnitrope®, and therefore fulfils the regulatory obligations of the study sponsor. It will also extend the safety database for Omnitrope®, as well as contributing to the available data for all recombinant hGH products.
Study design
PATRO Adults is an observational, multicentre, open, longitudinal, noninterventional study. The study is being conducted in hospitals and specialised endocrinology clinics across several European countries. All physicians prescribing Omnitrope® in participating countries will be invited to enrol patients into the study. The primary objective is to collect and analyse data on the safety and efficacy of Omnitrope® in adults treated in routine clinical practice, with particular emphasis on the risk of glucose intolerance or diabetes and normalization of IGF-1 levels.
The treated population will also be monitored for other safety and efficacy issues related to Omnitrope® treatment, including: occurrence of malignancies and recurrence of pituitary/ hypothalamic tumours; effects on cardiovascular risk factors related to GHD; effects on body composition; effects on parameters of bone metabolism related to GHD; quality of life; characteristics of adult patients treated with Omnitrope® (e.g. age, gender); and characteristics of Omnitrope® treatment in adults (e.g. posology).
Study population
Eligible patients will be male or female (minimum age 15 years) who are receiving treatment with Omnitrope® and who have provided informed consent. Patients who have been treated with another hGH product before starting Omnitrope® therapy will also be eligible for inclusion.
The minimum enrolment target is 1500 patients. As GHD in adults is a rare disease, it will not be possible to include thousands of patients in a few years. Therefore, this target is based on the minimum recommended size of a premarketing safety database for products intended for long-term treatment of non-life-threatening conditions [FDA, 2005], using the rule of three in order to be able to estimate an incidence of adverse events (AEs) of 4/10,000 people-years [Eypasch et al. 1995].
Treatment
Included patients will receive Omnitrope® treatment in accordance with the recommendations in the Summary of Product Characteristics, including contraindications, special warnings and precautions [EMA, 2008b]. If treatment is interrupted, all data generated before and after the interruption will be included in the analysis, and the reason(s) for the interruption will be recorded. Should treatment be discontinued, all data generated up to the time of discontinuation will be included in the analysis, and the reason(s) for discontinuation will be recorded. All concomitant therapy (dose, date of administration and reason for use) will also be recorded in the case report form (CRF).
Visit schedule and assessments
All visits, tests and assessments will be performed as part of routine clinical practice; no additional or specific visits, tests or assessments are required as part of the study. Data will be collected during each routine visit during treatment with Omnitrope®; in some countries data will be collected after treatment with Omnitrope® has been stopped. For all patients included in the study, all available data (visits, laboratory data, findings from examinations, etc.) will be recorded in a CRF twice a year. Table 1 lists the tests and assessments that will be performed at visit 1 (the start of Omnitrope® treatment), and at subsequent visits if performed as part of routine clinical practice.
Table 1.
Visit schedule and assessments.
| Parameter (if appropriate / performed) | Visit 1 (baseline / start) | Subsequent visits (intervals corresponding to routine clinical practice) |
|---|---|---|
| Informed consent | x | |
| Date of visit | x | x |
| Patient data (sex, DOB) | x | DOB for identification purposes |
| Diagnosis (date, aetiology) | x | |
| Additional hormone deficiencies | x | |
| Anamnesis and medical history | x | |
| Family history | x | |
| Puberty (date of onset, menarche) | x | |
| Smoking habits | x | x |
| Radiotherapy | x | |
| Glucose homeostasis | x | x |
| Scoliosis | x | x |
| Osteoporosis | x | x |
| Cardiovascular examination | x | x |
| Previous, current and chronic relevant illnesses | x | |
| Current drug treatment / concomitant medication | x | x |
| Previous GH treatment | x | |
| Height | x | |
| Weight | x | x |
| Waist and hip circumference | x | x |
| Physical examination | x | x |
| Vital signs | x | x |
| Body composition | x | x |
| Bone densitometry | x | x |
| Ophthalmologic examinations | x | x |
| Electrocardiogram | x | x |
| CT, MRI and sonography results | x | x |
| Haematology | x | x |
| Blood chemistry | x | x |
| Glucose metabolism / OGTT | x | x |
| HOMA | x | x |
| Fasting lipid profile | x | x |
| Urinalysis | x | x |
| Hormones (thyroid, gonadal, adrenal function) | x | x |
| IGF-1, IGFBP-3 determinations | x | x |
| Anti-hGH antibody determination | x | x |
| GH stimulation test(s) | x | |
| Spontaneous GH secretion | x | |
| Omnitrope® dose | x | x |
| Adverse events | x | |
| Discontinuation (reasons) | x | |
| QoL assessment* | x | x |
In certain countries. CT, computerized tomography; DOB, date of birth; GH, growth hormone; hGH, human growth hormone; HOMA, homeostatic model assessment; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; MRI, magnetic resonance imaging; OGTT, oral glucose tolerance test; QoL, quality of life.
Efficacy assessments
Adult GHD typically presents with alterations in body composition, including reduced lean body mass and bone mineral density, and increased fat mass with a preponderance of abdominal adiposity. Muscle strength and exercise capacity is reduced, and an impaired sense of well-being and other psychological complaints are common. Efficacy assessments will therefore be based on analysis of the following: IGF-1 levels within age- and gender-adjusted normal ranges; anthropometric measures (weight, waist circumference, total fat mass, lean body mass, total body water); bone mineral density; lipids; effects on cardiovascular risk factors such as glucose metabolism, blood pressure and inflammatory markers (e.g. C-reactive protein); and quality of life (in particular participating countries). In addition, collected data will be evaluated for information on compliance with Omnitrope® treatment and patient characteristics associated with a good response to treatment.
Safety assessments
All AEs, including serious AEs, will be monitored and recorded. Particular emphasis will be placed on long-term safety, the recording of malignancies and recurrence of hypothalamic–pituitary tumours, the occurrence and clinical impact of antirecombinant hGH antibodies, the incidence, severity and duration of hyperglycaemia, and the development of diabetes during treatment with Omnitrope®.
Data collection and analysis
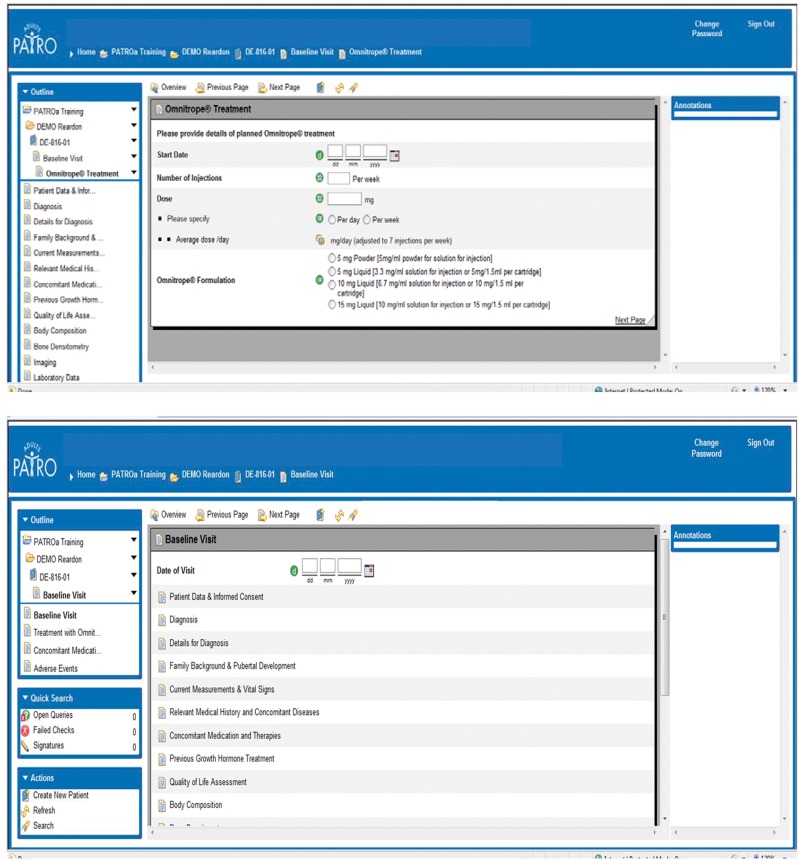
Investigators will enter data from source documents into a web-based electronic data collection (EDC) system (Figure 1). All eCRFs will be reviewed by the responsible physician, and centralized monitoring will be performed by a contract research organization. The state-of-the-art EDC system is easy to use and navigate, with entered data saved automatically. The system also includes automatic data checks to assure the quality of collected data; any data entered that is outside of the expected range for a particular category is automatically queried by the system. Other features of the system include automatic recognition of the presence of the metabolic syndrome based on entered values for the different components (waist circumference, blood lipids, blood pressure and fasting plasma glucose). In addition, if the indication for administration of a concomitant medication is a possible AE, the system automatically requests information on the event.
Figure 1.
Examples of web pages for electronic data collection.
All enrolled patients who have received at least one dose of Omnitrope® will be included in the safety and efficacy analyses. Descriptive statistics will be used to describe continuous parameters (e.g. age, weight, waist circumference) and categorical parameters (e.g. gender). Poisson distribution will be used to assess the diabetogenic potential of GH, the occurrence of malignancies, and the incidence of other relevant AEs.
Discussion
PATRO Adults is a postmarketing surveillance study designed to prospectively record the safety and efficacy of long-term Omnitrope® treatment in adults in the setting of routine clinical practice. The possible relationship between GH treatment and the development of diabetes mellitus (DM) is an important safety aspect of replacement therapy. A sixfold increase in the incidence of type 2 DM (T2DM) has been reported in a large study of paediatric patients receiving GH treatment, with the authors proposing that GH treatment may accelerate the onset of diabetes in predisposed individuals [Cutfield et al. 2000]. Adults with GHD also present with metabolic abnormalities that resemble those of the metabolic syndrome [Johansson and Bengtsson, 1999], which itself is a strong predictor of T2DM risk. Data from other surveillance studies of patients treated with recombinant hGH indicate that the prevalence of the metabolic syndrome is higher in GHD patients (40–50%) than in the general population (20–30%) [Attanasio et al. 2010; Verhelst et al. 2011]. In one study, while prevalence was unaffected by GH replacement therapy, baseline metabolic syndrome status or obesity were strong predictors of the metabolic syndrome after 3 years of GH treatment [Attanasio et al. 2010]. Conversely, GH replacement reduces abdominal fat mass and may therefore improve insulin resistance and glucose homeostasis in adult patients with GHD [Monson et al. 2000]. This has been documented in some studies [Svensson et al. 2002] but not in others [Arwert et al. 2005]. More recently, an increased risk of T2DM has been reported in adult GHD patients receiving GH replacement therapy [Attanasio et al. 2011]; consistent with the data from paediatric patients, the increased risk appeared to be related to a predisposition to diabetes (e.g. presence of the metabolic syndrome or obesity) rather than GH therapy per se. The PATRO Adults study should provide additional insight into the relationship between GH therapy in adults with GHD and the development of diabetes.
Both GH and IGF-1 have mitogenic properties. Therefore, another potential concern with GH therapy is the risk of malignant disease, either recurrence/regrowth of hypothalamic–pituitary tumours responsible for most of the hypopituitarism seen in patients with adult-onset GHD, or the development of de novo malignancies. Data from several relatively small studies suggest that GH replacement therapy does not increase the risk of recurrence or regrowth of hypothalamic–pituitary tumours [Hatrick et al. 2002; Frajese et al. 2001; Jostel et al. 2005; Arnold et al. 2009]. A case–control study of 55 matched pairs followed for at least 5 years also found no evidence of tumour recurrence or regrowth in patients receiving GH therapy [Buchfelder et al. 2007]. PATRO Adults, with its larger patient cohort and longer duration of follow up, will provide additional important information on the risk of tumour regrowth and recurrence.
Similarly, there is no evidence that GH replacement therapy in adults increases the risk of de novo malignancy or recurrence of other (non-hypothalamic–pituitary) tumours [Svensson and Bengtsson, 2009; Child et al. 2011]. Again, data from studies with longer-term follow up are needed before firmer conclusions about the long-term risk can be made, and PATRO Adults will provide important information in this regard.
In summary, PATRO Adults is a large, long-term, postmarketing surveillance study that will extend the safety database for Omnitrope®, as well as contributing to the available data for all recombinant hGH products. Of particular interest, the study will provide important data on the impact of long-term GH replacement therapy on the development of DM, the recurrence/regrowth of hypothalamic–pituitary tumours, and de novo malignancy or recurrence of other (non-hypothalamic–pituitary) tumours.
Acknowledgments
Medical writing assistance in the preparation of this paper was provided by Tony Reardon of Spirit Medical Communications Ltd and funded by Sandoz Biopharmaceuticals (Sandoz International GmbH).
Footnotes
This study is funded by Sandoz Biopharmaceuticals.
PBP has received research grants from Pfizer and lecture fees from Eli Lilly and Sandoz. FM has received research grants from Sandoz, Ipsen and Pfizer. ALC and GS have no conflicts to declare. MZ is an employee of Sandoz.
Contributor Information
Paolo Beck-Peccoz, Endocrinology and Diabetology Unit, Medical Sciences Department, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Cà Granda Ospedale Maggiore Policlinico, Milan, Italy.
Francesco Minuto, Department of Internal Medicine, University of Genova, Italy.
Alfonso Leal-Cerro, Instituto de Biomedicina de Sevilla (IBiS), Hospital Universitario Virgen del Rocío Seville, Spain.
Markus Zabransky, Sandoz International GmbH, Industriestr. 25, D-83607 Holzkirchen, Germany.
Günter Stalla, Max Planck Institute of Psychiatry, Department of Internal Medicine, Endocrinology and Clinical Chemistry, Munich, Germany.
References
- Arnold J., Arnold D., Marland A., Karavitaki N., Wass J. (2009) Growth hormone replacement in patients with non-functioning pituitary adenoma treated solely by surgery is not associated with increased risk of tumour recurrence. Clin Endocrinol 70: 435–438 [DOI] [PubMed] [Google Scholar]
- Arwert L., Roos J.C., Lips P., Twisk J.W., Manoliu R.A., Drent M.L. (2005) Effects of 10 years of growth hormone (GH) replacement therapy in adult GH-deficient men. Clin Endocrinol 63: 310–316 [DOI] [PubMed] [Google Scholar]
- Attanasio A.F., Jung H., Mo D., Chanson P., Bouillon R., Ho K.K., et al. (2011) Prevalence and incidence of diabetes mellitus in adult patients on growth hormone replacement for growth hormone deficiency: a surveillance database analysis. J Clin Endocrinol Metab 96: 2255–2261 [DOI] [PubMed] [Google Scholar]
- Attanasio A.F., Mo D., Erfurth E.M., Tan M., Ho K.K., Kleinberg D., et al. (2010) Prevalence of metabolic syndrome in adult hypopituitary growth hormone (GH)-deficient patients before and after GH replacement. J Clin Endocrinol Metab 95: 74–81 [DOI] [PubMed] [Google Scholar]
- Buchfelder M., Kann P.H., Wüster C., Tuschy U., Saller B., Brabant G., et al. (2007) Influence of GH substitution therapy in deficient adults on the recurrence rate of hormonally inactive pituitary adenomas: a case control study. Eur J Endocrinol 157: 149–156 [DOI] [PubMed] [Google Scholar]
- Child C.J., Zimmerman A.G., Woodmansee W.W., Green D.M., Li J.J., Jung H., et al. (2011) Assessment of primary cancers in GH-treated adult hypopituitary patients: an analysis from the Hypopituitary Control and Complications Study. Eur J Endocrinol 165: 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutfield W.S., Wilton P., Bennmarker H., Albertsson-Wikland K., Chatelain P., Ranke M.B., et al. (2000) Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet 355: 610–613 [DOI] [PubMed] [Google Scholar]
- EMA (2008a) Omnitrope® European Public Assessment Report. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000607/WC500043692.pdf [accessed 3rd February 2012].
- EMA (2008b) Omnitrope® Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000607/WC500043695.pdf [accessed 3rd February 2012].
- Eypasch E., Lefering R., Kum C.K., Troidl H. (1995) Probability of adverse events that have not yet occurred: a statistical reminder. BMJ 311: 619–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2005) Guidance for industry. Premarketing risk assessment. March 2005. Available from: http://www.pharmacoepi.org/riskmgmt/fda_premarketing_ra_050324.pdf [accessed 3rd February 2012].
- Frajese G., Drake M.M., Loureiro R.A., Evanson J., Coyte D., Wood D.F., et al. (2001) Hypothalamo–pituitary surveillance imaging in hypopituitary patients receiving long-term GH replacement therapy. J Clin Endocrinol Metab 86: 5172–5175 [DOI] [PubMed] [Google Scholar]
- Hatrick A.G., Boghalo P., Bingham J.B., Ayres A.B., Sonksen P.H., Russell-Jones D.L. (2002) Does GH replacement therapy in adult GH-deficient patients result in recurrence or increase in size of pituitary tumours? Eur J Endocrinol 146: 807–811 [DOI] [PubMed] [Google Scholar]
- Ho K.K. for the GH Deficiency Consensus Workshop Participants (2007) Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 157: 695–700 [DOI] [PubMed] [Google Scholar]
- Johansson G., Bengtsson B.A. (1999) Growth hormone and the metabolic syndrome. J Endocrinol Invest 22(5 Suppl.): 41–46 [PubMed] [Google Scholar]
- Jostel A., Mukherjee A., Hulse P.A., Shalet S.M. (2005) Adult growth hormone replacement therapy and neuroimaging surveillance in brain tumour survivors. Clin Endocrinol 62: 698–705 [DOI] [PubMed] [Google Scholar]
- Molitch M.E., Clemmons D.R., Malozowski S., Marriam G.R., Vance M.L. (2011) Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1587–1609 [DOI] [PubMed] [Google Scholar]
- Monson J.P., Bengtsson B.A., Abs R., Feldt-Rasmussen U., Wüster C. (2000) Can growth hormone therapy cause diabetes? Lancet 355: 1728–1729 [DOI] [PubMed] [Google Scholar]
- Svensson J., Bengtsson B.A. (2009) Safety aspects of GH replacement. Eur J Endocrinol 161: S65–S74 [DOI] [PubMed] [Google Scholar]
- Svensson J., Fowelin J., Landin K., Bengtsson B.A., Johansson J.O. (2002) Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab 87: 2121–2127 [DOI] [PubMed] [Google Scholar]
- Verhelst J., Mattsson A.F., Luger A., Thunander M., Góth M.I., Koltowska-Häggström M., et al. (2011) Prevalence and characteristics of the metabolic syndrome in 2479 hypopituitary patients with adult-onset GH deficiency before GH replacement: a KIMS analysis. Eur J Endocrinol 165: 881–889 [DOI] [PubMed] [Google Scholar]