Abstract
Background:
Even though patients with class 3 obesity (body mass index ≥ 40 kg/m2) are prone to arterial hypertension and respond less to antihypertensive drugs, they are not considered in hypertension treatment guidelines and data from prospective clinical trials are lacking.
Methods:
In a post hoc analysis of a clinical trial, we compared patients with class 3 obesity with patients with class 1/2 obesity.
Results and Conclusions:
Blood pressure control in class 3 obesity was less likely to be achieved with hydrochlorothiazide monotherapy. While addition of amlodipine, irbesartan, or aliskiren to hydrochlorothiazide improved the blood pressure response, amlodipine was less effective and induced peripheral edema in 19% of patients with class 3 obesity.
Keywords: obesity, hypertension, renin, aliskiren, irbesartan, placebo, morbid obesity
Introduction
The prevalence of class 3 obesity [body mass index (BMI) ≥40 kg/m2] in the USA increased fourfold between 1986 and 2000 [Sturm, 2003]. The 2007-2008 National Health and Nutrition Examination Survey showed that 4% of men and 7% of women had class 3 obesity [Flegal et al. 2010]. Class 3 obesity is associated with increased mortality compared with class 1/2 obesity, largely caused by increases in hypertension, diabetes, and hyperlipidemia [McTigue et al. 2006]. Blood pressure control is particularly difficult to achieve in patients with class 3 obesity because the need for multiple antihypertensive drugs increases with rising body mass index [Bramlage et al. 2004]. Yet, clinical trials on antihypertensive treatment in patients with class 3 obesity have not been conducted. We previously reported data from a 12-week, randomized, double-blind trial in 489 patients with obesity and arterial hypertension who were non-responders to hydrochlorothiazide (HCT) [Jordan et al. 2007]. We now report the results of a post hoc analysis of blood pressure control at week 12 in the subgroup of patients with class 3 obesity compared with patients with class 1/2 obesity.
Methods
We included men and women aged at least 18 years with hypertension and BMI of 30 kg/m2 or higher. Patients with diastolic blood pressure of 110 mmHg or higher or systolic blood pressure of 180 mmHg or higher were excluded, as were patients with secondary hypertension, diabetes mellitus, history of severe cardiovascular or cerebrovascular disease, or other severe diseases. Patients provided written informed consent, and the study protocol was approved by local ethical committee review boards. Following screening, antihypertensive medications were discontinued for 2–4 weeks. Then, a single-blind run-in period with HCT 25 mg once daily for 4 weeks was begun. Patients whose blood pressure was controlled with HCT were discontinued from the study. Non-responders to single-blind HCT were randomized to double-blind, once-daily treatment with aliskiren 150 mg, irbesartan 150 mg, amlodipine 5 mg, or placebo in addition to HCT 25 mg. After 4 weeks, aliskiren, irbesartan, and amlodipine doses were doubled and treatment continued for an additional 8 weeks. Changes from baseline in blood pressure at week 12 were analyzed separately for the subgroups of patients (intent-to-treat population) with class 1/2 obesity or class 3 obesity at baseline using a two-way analysis of covariance model. Blood pressure control rates were analyzed for each subgroup using a logistic regression model. All statistical tests were performed at a two-sided significance level of 0.05, and 95% confidence intervals were provided for differences between treatment groups.
Results
The baseline characteristics of patients with class 1/2 and patients with class 3 obesity are given in Table 1. The class 3 obesity subgroup was younger overall, and comprised a higher proportion of women and patients with metabolic syndrome compared with the class 1/2 obesity subgroup. Blood pressure was not different between both obesity groups.
Table 1.
Patient baseline and demographic characteristics.
| Parameter | Class 1/2 obesity (BMI 30 to <40 kg/m2) (n = 435) |
Class 3 obesity (BMI ≥ 40 kg/m2) (n=54) |
Totals | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aliskiren/HCT (n = 106) | Irbesartan/HCT (n = 109) | Amlodipine/HCT (n = 110) | HCT alone (n = 110) | Aliskiren/HCT (n = 16) | Irbesartan/HCT (n = 10) | Amlodipine/HCT (n = 16) | HCT alone (n = 12) | Class 1/2 obesity (n = 435) | Class 3 obesity (n = 54) | |
| Age, years | 54.2 ± 11.7 | 53.3 ± 10.9 | 55.9 ± 12.0 | 56.0 ± 12.0 | 45.2 ± 10.5 | 50.0 ± 12.5 | 50.2 ± 10.3 | 47.3 ± 12.8 | 54.9 ± 11.7 | 48.0 ± 11.3 |
| Men, n (%) | 55 (52) | 46 (42) | 46 (42) | 49 (45) | 5 (31) | 2 (20) | 7 (44) | 3 (25) | 196 (45) | 17 (32) |
| Weight, kg | 94.2 ± 13.3 | 93.6 ± 12.2 | 92.8 ± 12.8 | 93.1 ± 12.6 | 128.5 ± 16.5 | 122.3 ± 17.0 | 123.2 ± 18.0 | 117.3 ± 10.0 | 93.4 ± 12.7 | 123.3 ± 16.0 |
| BMI, kg/m2 | 33.2 ± 2.7 | 33.2 ± 2.6 | 33.3 ± 2.6 | 33.0 ± 2.7 | 45.5 ± 5.1 | 46.6 ± 4.8 | 42.9 ± 2.6 | 43.3 ± 3.0 | 33.2 ± 2.6 | 44.4 ± 4.2 |
| Waist circumference, cm | 109 ± 10 | 107 ± 12 | 109 ± 11 | 108 ± 11 | 127 ± 12 | 129 ± 16 | 125 ± 12 | 124 ± 13 | 108 ± 11 | 126 ± 13 |
| Duration of hypertension, years | 8.8 ± 8.4 | 8.3 ± 8.0 | 9.9 ± 9.0 | 8.5 ± 8.1 | 6.3 ± 5.3 | 7.3 ± 3.2 | 6.0 ± 5.0 | 10.5 ± 11.5 | 8.9 ± 8.4 | 7.3 ± 6.9 |
| Metabolic syndrome*, n (%) | 75 (71) | 76 (70) | 68 (62) | 67 (61) | 14 (88) | 9 (90) | 14 (88) | 8 (67) | 286 (66) | 45 (83) |
| Current smoker, n (%) | 15 (14) | 19 (17) | 28 (26) | 24 (22) | 3 (19) | 1 (10) | 2 (13) | 0 | 86 (20) | 6 (11) |
| msDBP, mmHg | 96.7 ± 5.0 | 96.5 ± 4.1 | 96.7 ± 5.0 | 97.2 ± 4.6 | 97.4 ± 4.4 | 98.0 ± 7.2 | 96.1 ± 5.4 | 97.3 ± 4.7 | 96.8 ± 4.7 | 97.1 ± 5.2 |
| msSBP, mmHg | 149.4 ± 11.9 | 149.1 ± 13.4 | 150.4 ± 11.5 | 149.1 ± 10.9 | 149.3 ± 10.1 | 148.4 ± 13.6 | 145.6 ± 10.8 | 152.7 ± 14.7 | 149.5 ± 11.9 | 148.8 ± 12.0 |
Baseline msDBP and msSBP were evaluated at baseline of the double-blind treatment period after the single-blind HCT run-in period. Data are expressed as mean ± standard deviation unless otherwise stated.
Metabolic syndrome was defined as any three of the following, according to National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) diagnostic criteria: waist circumference >102 cm for men or >88 cm for women; triglycerides ≥150 mg/dl (≥1.69 mmol/liter); high-density lipoprotein cholesterol <40 mg/dl (<1.04 mmol/liter) for men or <50 mg/dl (<1.29 mmol/liter) for women; BP ≥130/85 mmHg; fasting glucose ≥110 mg/dl (≥6.1 mmol/liter).
BMI, body mass index; msDBP, mean sitting diastolic blood pressure; msSBP, mean sitting systolic blood pressure.
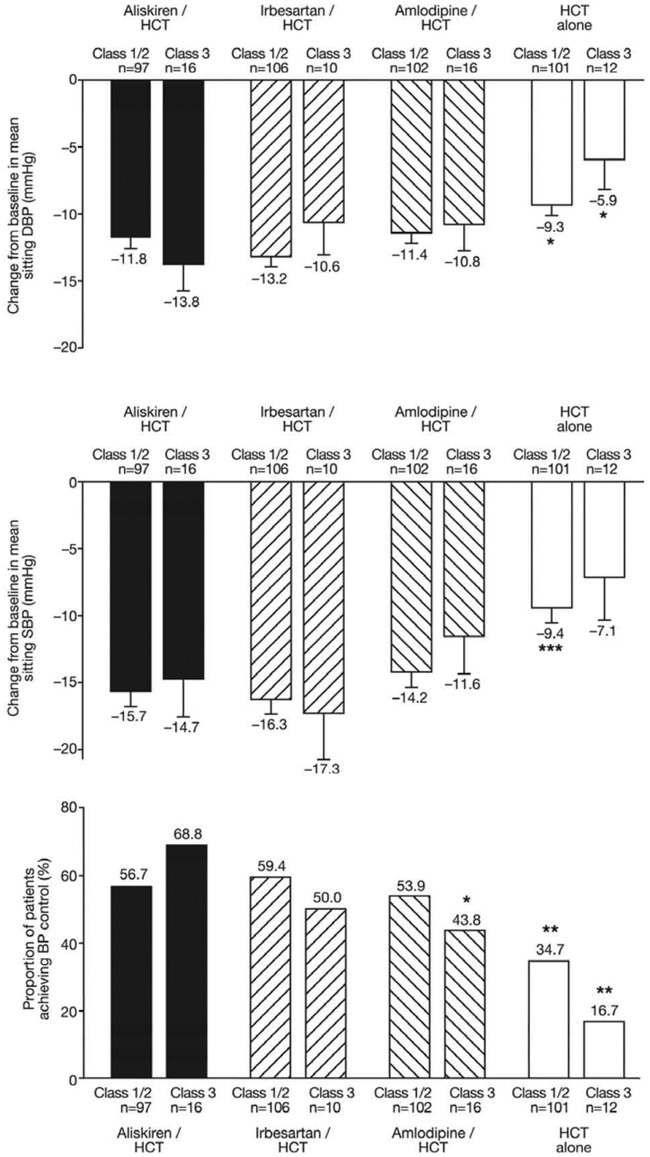
At week 12, 34.7% of patients with class 1/2 obesity and 16.7% of patients with class 3 obesity on placebo/HCT had their blood pressure controlled to up to 140/90 mm Hg (Figure 1). In class 3 obesity, aliskiren/HCT treatment achieved blood pressure control in an additional 52% of patients compared with placebo/HCT (p = 0.004), 18.8% compared with irbesartan/HCT [p = nonsignificant (NS)], and 25.0% compared with amlodipine/HCT (p = 0.036). In class 3 obesity, aliskiren/HCT combination treatment lowered blood pressure by 14.7/13.8 mm Hg, equating to an additional reduction of 7.6/7.8 mm Hg compared with continuing HCT 25 mg alone (p = 0.086 for systolic and p = 0.013 for diastolic blood pressure, compared with placebo/HCT). Irbesartan/HCT and amlodipine/HCT lowered BP by 17.3/10.6 and 11.6/10.8 mmHg respectively (both p = NS versus aliskiren/HCT).
Figure 1.
Changes from baseline in mean sitting diastolic blood pressure (DBP, upper panel) and mean sitting systolic blood pressure (SBP, middle panel) at week 12 endpoint in patients according to body mass index (BMI) subgroup [intent-to-treat (ITT) population]. Blood pressure data are presented as the least-squares mean ± standard error of the mean (SEM). *p < 0.05, ***p < 0.0001 versus aliskiren/hydrochlorothiazide (HCT) in pairwise comparisons. The lower panel shows the percentage of patients with blood pressure control (<140/90 mmHg). *p < 0.05, **p < 0.01 versus aliskiren/HCT in a logistic regression model with treatment and region as factors and centered baseline as covariate.
Selected adverse events (AEs) and safety laboratory data are given in Table 2. Treatment with amlodipine/HCT was associated with the highest incidence of AEs in both BMI subgroups due to a higher rate of peripheral edema with amlodipine. The only serious AE suspected to be related to study treatment was a case of peripheral edema in a patient in the class 1/2 obesity subgroup receiving amlodipine/HCT. There were no deaths during the study. Mean potassium levels tended to increase with aliskiren/HCT and irbesartan/HCT, as did the incidence of potassium elevations to greater than 5.5 mmol/liter (5/103 and 3/109 patients in the class 1/2 obesity subgroup). Two patients with class 1/2 obesity exhibited serum potassium greater than 6.0 mmol/liter with aliskiren/HCT. Serum potassium reductions to less than 3.5 mmol/liter were most common with amlodipine/HCT (12/109 patients versus 5/103, 3/109, and 5/107 patients with aliskiren/HCT, irbesartan/HCT, and HCT, respectively). Two cases of serum creatinine elevation to higher than 177 μmol/liter were seen in patients with class 1/2 obesity on HCT alone. The only notable laboratory abnormalities in the class 3 obesity subgroup were two cases of potassium less than 3.5 mmol/liter (one each with aliskiren/HCT and amlodipine/HCT).
Table 2.
Safety and tolerability.
| Adverse event | Aliskiren/ HCT | Irbesartan/ HCT | Amlodipine/ HCT | HCT | Total |
|---|---|---|---|---|---|
| Class 1/2 obesity (BMI 30 to <40 kg/m2) | n = 106 | n = 109 | n = 110 | n = 110 | n = 435 |
| Any AE | 41 (38.7) | 40 (36.7) | 49 (44.5) | 42 (38.2) | 172 (39.5) |
| Nasopharyngitis | 8 (7.5) | 5 (4.6) | 6 (5.5) | 3 (2.7) | 22 (5.1) |
| Headache | 5 (4.7) | 2 (1.8) | 6 (5.5) | 4 (3.6) | 17 (3.9) |
| Back pain | 1 (0.9) | 2 (1.8) | 5 (4.5) | 5 (4.5) | 13 (3.0) |
| Peripheral edema | 0 | 0 | 11 (10.0) | 2 (1.8) | 13 (3.0) |
| Dizziness | 3 (2.8) | 3 (2.8) | 1 (0.9) | 2 (1.8) | 9 (2.1) |
| Discontinuations due to AEs | 1 (0.9) | 4 (3.7) | 5 (4.5) | 4 (3.6) | 14 (3.2) |
| SAEs | 1 (0.9) | 3 (2.8) | 4 (3.6) | 4 (3.6) | 12 (2.8) |
| Laboratory values (mean ± SD)* | |||||
| Potassium, mmol/liter | |||||
| Baseline | 4.22 ± 0.45 | 4.15 ± 0.40 | 4.20 ± 0.38 | 4.24 ± 0.43 | |
| Week 12 | 4.33 ± 0.60 | 4.30 ± 0.41 | 4.14 ± 0.51 | 4.16 ± 0.42 | |
| Change | 0.11 ± 0.59 | 0.15 ± 0.44 | −0.06 ± 0.50 | −0.08 ± 0.49 | |
| Creatinine, μmol/liter | |||||
| Baseline | 81.8 ± 16.1 | 78.3 ± 16.0 | 78.2 ± 14.2 | 81.9 ± 19.7 | |
| Week 12 | 82.0 ± 17.0 | 81.1 ± 19.6 | 79.2 ± 15.9 | 83.0 ± 22.9 | |
| Change | 0.2 ± 11.7 | 2.8 ± 11.2 | 1.1 ± 10.4 | 1.1 ± 14.7 | |
| Class 3 obesity (BMI ≥40 kg/m2) | n = 16 | n = 10 | n = 16 | n = 12 | n = 54 |
| Any AE | 7 (43.8) | 3 (30.0) | 8 (50.0) | 5 (41.7) | 23 (42.6) |
| Nasopharyngitis | 2 (12.5) | 1 (10.0) | 1 (6.3) | 2 (16.7) | 6 (11.1) |
| Peripheral edema | 1 (6.3) | 1 (10.0) | 3 (18.8) | 0 | 5 (9.3) |
| Gastroenteritis | 1 (6.3) | 2 (20.0) | 0 | 1 (8.3) | 4 (7.4) |
| Headache | 0 | 1 (10.0) | 3 (18.8) | 0 | 4 (7.4) |
| Dry mouth | 0 | 0 | 2 (12.5) | 0 | 2 (3.7) |
| Discontinuations due to AEs | 1 (6.3) | 0 | 1 (6.3) | 0 | 2 (3.7) |
| SAEs | 1 (6.3) | 0 | 0 | 0 | 1 (1.9) |
| Laboratory values (mean ± SD)$ | |||||
| Potassium, mmol/liter | |||||
| Baseline | 4.08 ± 0.40 | 3.95 ± 0.31 | 4.13 ± 0.27 | 4.11 ± 0.41 | |
| Week 12 | 4.29 ± 0.47 | 4.17 ± 0.34 | 4.10 ± 0.28 | 4.31 ± 0.16 | |
| Change | 0.21 ± 0.39 | 0.22 ± 0.48 | −0.03 ± 0.38 | 0.20 ± 0.45 | |
| Creatinine, μmol/liter | |||||
| Baseline | 69.9 ± 11.0 | 67.1 ± 10.1 | 72.3 ± 9.7 | 70.4 ± 21.3 | |
| Week 12 | 65.9 ± 10.3 | 68.3 ± 9.8 | >68.5 ± 11.7 | 73.4 ± 21.3 | |
| Change | –4.0 ± 10.7 | 1.2 ± 6.6 | −3.7 ± 8.7 | 3.0 ± 6.4 | |
Values are presented as the number (%) of patients unless otherwise stated.
Values at baseline and week 12 not available for all patients; n = 100–102 (aliskiren/HCT), n = 104–107 (irbesartan/HCT), n = 104–106 (amlodipine/HCT) and n = 102–104 (HCT alone).
Values at baseline and week 12 not available for all patients; n = 15 (amlodipine/HCT) and n = 11 (HCT alone).
AE, adverse event; BMI, body mass index; HCT, hydrochlorothiazide; SAE, serious adverse event.
Discussion
Patients with class 3 obesity represent a hard-to-treat group prone to hypertension and associated cardiovascular complications. Treatment guidelines advocate weight loss in this patient group as a means to lower blood pressure. A recent scientific statement by the European Society of Hypertension Working Group on Obesity reviewed the evidence for blood pressure influences of weight loss [Straznicky et al. 2010]. Even with profound weight loss induced by bariatric surgery, long-term blood pressure control is not achieved in many patients [Sjostrom et al. 2000]. Most patients with hypertension and class 3 obesity ultimately require antihypertensive therapy.
In the majority of patients with class 3 obesity in this study, low-dose HCT monotherapy did not control blood pressure, thus extending and confirming previous observations. In a German survey conducted in the primary care setting, patients with obesity and hypertension were on more antihypertensive medications while blood pressure was less well controlled compared with normal weight patients with hypertension [Bramlage et al. 2004]. In 7357 high-risk vascular outpatients a BMI of at least 30 kg/m2 decreased the likelihood of having blood pressure controlled [Bhan et al. 2010]. Similarly, patients with a BMI of at least 30 kg/m2 in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) required more antihypertensive medications but were nevertheless less likely to attain target blood pressure [Cushman et al. 2002].
Renin–angiotensin system inhibitors may be particularly useful in a combination regimen in patients with severe obesity in terms of efficacy and they are well tolerated. From a pathophysiological point of view, renin–angiotensin system inhibition is a sensible treatment approach. Studies applying the norepinephrine spillover technique showed excessive renal sympathetic activation in obesity-associated arterial hypertension [Rumantir et al. 1999]. Renal sympathetic activation may contribute to renin–angiotensin system activation in patients with obesity and hypertension [Engeli and Sharma, 2001]. Consequently, sodium retention, volume expansion, and increases in cardiac output may ensue [Messerli et al. 1981; Stelfox et al. 2006; Strazzullo et al. 2001]. Renin–angiotensin system inhibitors do not promote diabetes mellitus. One potential advantage of aliskiren is that it achieves relatively high concentrations in the adipose tissue of patients with obesity and hypertension [Boschmann et al. 2012]. Moreover, relevant aliskiren tissue concentrations can still be measured 8 weeks after treatment discontinuation [Boschmann et al. 2012].
Our analysis supports previous observations suggesting that obesity modulates the efficacy of dihydropyridine calcium channel blockade [Schmieder et al. 1993]. Moreover, in a relatively large proportion of patients with class 3 obesity, the clinical utility of dihydropyridine calcium channel blockers is limited by occurrence of peripheral edema.
Although further studies are required to confirm our post hoc analysis on a small subpopulation of patients with class 3 obesity and hypertension, direct renin inhibition with aliskiren is another therapeutic option for the hard-to-treat group of patients with severe obesity.
Footnotes
This study was supported by Novartis.
SWB, SL, DLK, and MFP are Novartis employees. JJ served as a consultant and lecturer for Novartis.
Contributor Information
Jens Jordan, Institute of Clinical Pharmacology, Hannover Medical School, Carl-Neuberg-Strasse 1, D-30625 Hannover, Germany.
Sam W. Boye, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
Stephanie Le Breton, Novartis Pharma AG, Basel, Switzerland.
Deborah L. Keefe, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
Stefan Engeli, Institute of Clinical Pharmacology, Hannover Medical School, Hannover, Germany.
Margaret Forney Prescott, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
References
- Bhan V., Yan R., Leiter L., Fitchett D., Langer A., Lonn E., et al. (2010) Relation between obesity and the attainment of optimal blood pressure and lipid targets in high vascular risk outpatients. Am J Cardiol 106: 1270–1276 [DOI] [PubMed] [Google Scholar]
- Boschmann M., Nussberger J., Engeli S., Danser A., Yeh C., Prescott M., et al. (2012) Aliskiren penetrates adipose and skeletal muscle tissue and reduces renin-angiotensin system activity in obese hypertensive patients. J Hypertens 30: 561–566 [DOI] [PubMed] [Google Scholar]
- Bramlage P., Pittrow D., Wittchen H., Kirch W., Boehler S., Lehnert H., et al. (2004) Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 17: 904–910 [DOI] [PubMed] [Google Scholar]
- Cushman W., Ford C., Cutler J., Margolis K., Davis B., Grimm R., et al. (2002) Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich ) 4: 393–404 [DOI] [PubMed] [Google Scholar]
- Engeli S., Sharma A. (2001) The renin–angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med 79: 21–29 [DOI] [PubMed] [Google Scholar]
- Flegal K., Carroll M., Ogden C., Curtin L. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303: 235–241 [DOI] [PubMed] [Google Scholar]
- Jordan J., Engeli S., Boye S., Le Breton S., Keefe D. (2007) Direct renin inhibition with aliskiren in obese patients with arterial hypertension. Hypertension 49: 1047–1055 [DOI] [PubMed] [Google Scholar]
- McTigue K., Larson J., Valoski A., Burke G., Kotchen J., Lewis C., et al. (2006) Mortality and cardiac and vascular outcomes in extremely obese women. JAMA 296: 79–86 [DOI] [PubMed] [Google Scholar]
- Messerli F., Christie B., DeCarvalho J.G., Aristimuno G.G., Suarez D.H., Dreslinski G., et al. (1981) Obesity and essential hypertension. Hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med 141: 81–85 [DOI] [PubMed] [Google Scholar]
- Rumantir M., Vaz M., Jennings G., Collier G., Kaye D., Seals D., et al. (1999) Neural mechanisms in human obesity-related hypertension. J Hypertens 17: 1125–1133 [DOI] [PubMed] [Google Scholar]
- Schmieder R., Gatzka C., Schachinger H., Schobel H., Ruddel H. (1993) Obesity as a determinant for response to antihypertensive treatment. BMJ 307: 537–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom C., Peltonen M., Wedel H., Sjostrom L. (2000) Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 36: 20–25 [DOI] [PubMed] [Google Scholar]
- Stelfox H., Ahmed S., Ribeiro R., Gettings E., Pomerantsev E., Schmidt U. (2006) Hemodynamic monitoring in obese patients: the impact of body mass index on cardiac output and stroke volume. Crit Care Med 34: 1243–1246 [DOI] [PubMed] [Google Scholar]
- Straznicky N., Grassi G., Esler M., Lambert G., Dixon J., Lambert E., et al. (2010) European Society of Hypertension Working Group on Obesity antihypertensive effects of weight loss: myth or reality? J Hypertens 28: 637–643 [DOI] [PubMed] [Google Scholar]
- Strazzullo P., Barba G., Cappuccio F., Siani A., Trevisan M., Farinaro E., et al. (2001) Altered renal sodium handling in men with abdominal adiposity: a link to hypertension. J Hypertens 19: 2157–2164 [DOI] [PubMed] [Google Scholar]
- Sturm R. (2003) Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med 163: 2146–2148 [DOI] [PubMed] [Google Scholar]