Abstract
The gaseous plant hormone ethylene is perceived by a family of five ethylene receptor members in the dicotyledonous model plant Arabidopsis. Genetic and biochemical studies suggest that the ethylene response is suppressed by ethylene receptor complexes, but the biochemical nature of the receptor signal is unknown. Without appropriate biochemical measures to trace the ethylene receptor signal and quantify the signal strength, the biological significance of the modulation of ethylene responses by multiple ethylene receptors has yet to be fully addressed. Nevertheless, the ethylene receptor signal strength can be reflected by degrees in alteration of various ethylene response phenotypes and in expression levels of ethylene-inducible genes. This mini-review highlights studies that have advanced our understanding of cooperative ethylene receptor signaling.
Keywords: emergent function, ethylene, ethylene receptor, ethylene signaling, receptor cooperation
Ethylene is a gaseous plant hormone playing important roles in many aspects of plant growth and development such as fruiting ripening, seedling growth, leaf senescence, and responses to stresses, pathogens, and flooding.1-5 Ethylene is perceived by the ethylene receptor. Five Arabidopsis ethylene receptor genes [Ethylene Response1 (ETR1), Ethylene Response Sensor1 (ERS1), ETR2, Ethylene Insensitive4 (EIN4), and ERS2)] have been isolated from mutants showing dominant ethylene insensitivity or from homologous genes with artificial mutations conferring ethylene insensitivity.6-8 An intragenic suppressor screen for the dominant ethylene-insensitive etr1–1, etr2–1, and ein4–1 mutants isolated the corresponding loss-of-function alleles. etr1–9, ers1–2, ers1–3, and ers2–3 are the loss-of-function mutations with a T-DNA insertion at the corresponding loci.9-11
Hua and Meyerowitz have shown that among the ethylene receptor mutants, single mutants do not produce a prominent phenotypic alteration, except that etr1 and ein4 loss-of-function mutants have a marginally shorter seedling hypocotyl than the wild-type seedlings.9 Mutants defective in multiple ethylene receptor genes show various degrees of the constitutive ethylene response phenotype, which indicates negative regulation of the ethylene response by the receptor genes.9,10,12,13
The work by Hua and Meyerowitz9 is profound to the study of ethylene receptor signaling. The authors propose that ethylene receptors or receptor complexes are signaling active and actively repress the constitutive ethylene response in the absence of ethylene treatment. With ethylene treatment, the receptors or receptor complexes are signaling inactive and fail to repress ethylene responses. In contrast to nullified ethylene receptor proteins, with abolished receptor signaling, the dominant mutant receptor proteins are signaling active and prevent the constitutive ethylene response. To explain why there are several ethylene receptors or receptor complexes and how these genes are maintained during evolution in Arabidopsis, Hua and Meyerowitz propose that these receptor genes might have been selected for their emergent and divergent functions. One emergent function of the ethylene receptor genes might enable a plant to sense ethylene over a wide range of concentrations and another emergent function could obtain tissue-specific ethylene sensitivity.9
Genetic and biochemical studies have provided evidence to support the hypothesis by Hua and Meyerowitz and have shed light on the biological significance of cooperation of multiple receptor members in ethylene signaling.
Ethylene Receptors Function as Clusters
The ETR1 histidine-kinase (HK) domain mediates the receptor signal via its physical interaction with the downstream component CTR1, a mitogen-activated protein kinase kinase kinase. ETR1 can also mediate the receptor signal output via its N terminus (residues 1–349), which contains the transmembrane helixes and GAF domain, with the deletion of the C-terminal HK and receiver domains.14-17
Lines of evidence from genetic and biochemical studies suggest that the ethylene receptors function as a cluster and cooperatively regulate the ethylene response. Arabidopsis defective in both ETR1 and ERS1 shows a strong constitutive ethylene response phenotype, with extreme growth retardation, throughout development.10 Expression of the ETR1 N-terminal portion etr11–349 or the dominant ethylene-insensitive etr1–11–349 moderately suppresses the etr1–7 ers1–2 mutant phenotype. The (ETR1 ERS1) etr2 ein4 ers2 mutant phenotype is rescued largely by expression of etr1–11–349. Thus, ETR1 N-terminal signaling may primarily depend on ETR1 and ERS1.14 Yeast two-hybrid assay revealed that the GAF domain mediates heteromeric receptor interactions.14,18 The co-purification of ETR1 with other ethylene receptors biochemically suggests heteromeric interactions among the receptors.18 The ctr1–1 loss-of-function mutation suppresses ethylene insensitivity conferred by dominant ethylene-insensitive receptor alleles. Expression of etr11–349 restores ethylene insensitivity conferred by dominant receptor alleles in ctr1–1 17. The GAF domain is thus likely responsible for the non-covalent ethylene receptor complex formation, whereby the receptor signal can be mediated cooperatively. Evidence for the formation of homomeric and hetermeric ethylene receptor complexes in vivo was revealed from a membrane recruitment assay in leaf epidermal cells of tobacco (Nicotiana benthamiana).19
Gel filtration assay demonstrated that Arabidopsis ethylene receptors form qualitatively different protein complexes of various high-molecular weights. The protein complex formation of an ethylene receptor is independent of other receptors because the molecular mass of ETR1 complexes is not altered in mutants defective in other receptor members and the molecular sizes of ETR1 and ERS1 complexes differ.20 These observations are somehow inconsistent with results showing the presence of higher-order receptor interactions18; however, the higher-order interactions may not be preserved during protein solubilization.
Therefore, each ethylene receptor may form high-molecular-mass complexes, and protein complexes of different receptors may form higher-order interactions, whereby the receptors may function cooperatively to suppress the constitutive ethylene response.
Ethylene Receptor Genes May Have Distinct Roles in Ethylene Signaling
Ethylene receptor genes are functionally redundant, and to be stably maintained in the genome, they may have evolved and acquired divergent functions.9,13 Driven by the native ETR1 promoter, ectopic expression of ETR2, EIN4, and ERS2 does not rescue the etr1 ers1 mutant phenotype, so the functions of ETR1 and ERS1 cannot be replaced by these ethylene receptor genes.10 ERS1 overexpression enhances the constitutive ethylene response phenotype of (ETR1 ERS1) etr2 ein4 ers2, which suggests negative regulation of ETR1 receptor signaling by ERS1.12 Thus, receptors may have distinct roles that are not replaceable by the others in ethylene signaling, although a common function of the receptors is to repress the constitutive ethylene response.
With little knowledge of the biochemical nature of the receptor signal and the limitations of genetic redundancy, investigating the distinct roles of receptors in ethylene signaling is challenging. Hua and Meyeriwiz showed that the (ERS1) etr1 etr2 ein4 ers2 quadruple mutant [designated (ERS1)4LOF] has an extremely strong constitutive ethylene response phenotype, so ERS1 alone cannot effectively suppress the constitutive ethylene response. In contrast, the ethylene receptor triple mutants (ETR1 ERS1) etr2 ein4 ers2 and (ERS1 ERS2) etr1 etr2 ein4 have a weaker constitutive ethylene response phenotype than (ERS1)4LOF. Therefore, each ethylene receptor alone may be insufficient to suppress the constitutive ethylene response and ethylene receptors may act additively or cooperatively as complexes.9
Unexpectedly, ETR1 is the only ethylene receptor in (ETR1) ers1 etr2 ein4 ers2 [designated (ETR1)4LOF], and the quadruple mutant shows a moderate constitutive ethylene response phenotype throughout development, so ETR1 alone may be sufficient to suppress the constitutive ethylene response to a great extent. ETR1 receptor signaling can be largely independent of positive cooperation with other receptor members.13 These results are not in agreement with the degree of ethylene response being associated with number of receptor members. Interestingly, the (ETR1 ERS1) etr2 ein4 ers2 triple mutant, with ETR1 and ERS1 the wild-type receptors, shows a stronger constitutive ethylene-response phenotype than (ETR1)4LOF, which lends support to the argument for negative regulation of the ETR1 receptor signaling by ERS1.12 These results reveal the negative cooperation of ERS1 with ETR1 and the divergent functions of ETR1 and ERS1 in ethylene signaling. The synergistic rather than additive function of ETR1 and ERS1 in receptor signaling is inferred from the etr1 ers1 double mutant phenotype, which shows extremely strong constitutive ethylene responses exceeding the additive effects caused by etr1 and ers1.10,11,14 The strong constitutive ethylene response phenotype of etr1 ers1 could imply synergistic functions of ETR1 and ERS1 in mediating the receptor signal by members of subfamily II (ETR2, EIN4, and ERS2). Without the synergistic function, members of subfamily II cannot mediate their receptor signal to suppress the constitutive ethylene response.
A distinct role derived from functional divergence of the receptor genes in ethylene signaling could facilitate receptor cooperativity. Without such collaborations or functional divergence, the loss-of-function mutation in the ethylene receptor genes would not have resulted in various degrees of the ethylene response.
ETR1 and ERS1 Differentially Collaborate with Other Ethylene Receptors to Modulate Ethylene Responses
ETR1 and ERS1 alone suppress constitutive ethylene responses differentially. The constitutive ethylene-response phenotype is weak for (ETR1)4LOF but strong for (ERS1)4LOF.12,13 This argument is supported by results showing that expression of the dominant ethylene-insensitive etr1–1 suppresses the constitutive ethylene response phenotype and confers ethylene insensitivity in receptor quintuple mutants, which lack wild-type ethylene receptors. In contrast, expression of the dominant ethylene-insensitive ers1–1 has little effect on reversing the quintuple mutant growth defect and conferring ethylene insensitivity.13 Because the ers1–1 mutation confers ethylene insensitivity in the presence of other receptor genes, ers1–1 signaling is supported differentially by other receptors. ETR1 and EIN4 alone are sufficient to support ers1–1 signaling to a great extent, and co-expression of ers1–1 with ETR1 or EIN4 [i.e., in (ETR1)4LOF or (EIN4)4LOF that expresses the ers1–1 transgene] substantially suppresses the constitutive ethylene response and confers ethylene insensitivity. The effect of ERS2 is greater than that of ETR2 in supporting ers1–1 signaling, and ERS1 has the least effect. Measurement of the receptor gene expression does not favor the scenario that the differential receptor signaling of ers1–1 supported by the other receptors is due to different levels of receptor gene expression. Of note, signaling by the dominant ethylene-insensitive ers1–1 and ers1C65Y isoforms is substantially alleviated in etr1 ein4, so ETR1 and EIN4 may have synergistic rather than additive effects on ERS1 signaling.13
Differential Ethylene Receptor Cooperation May Modulate Responses Induced by Ethylene in a Wide Range of Concentrations
Hua and Meyerowitz proposed that members of the ethylene receptor family would have different affinities to ethylene so that a plant could sense a wide range of ethylene concentrations.9 However, this hypothesis is not supported by ethylene binding experiments showing all members of the ethylene receptor family with similar affinities to ethylene.21 Gao and Schaller propose that an ethylene-bound, inactivated receptor may inactivate the un-bound isoforms within the same receptor cluster, which amplifies the ethylene signal and elevates ethylene sensitivity. This model provides an explanation for how a plant can sense ethylene of very low concentrations.22 However, how a plant can sense ethylene over a wide range of concentrations and why a plant senses ethylene with multiple ethylene receptors have to be addressed.
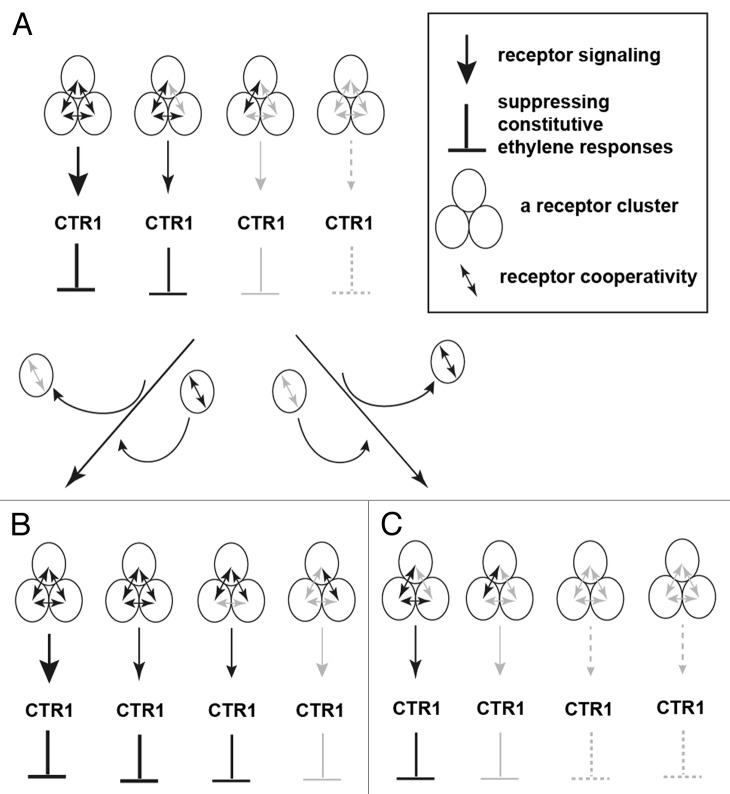
The genetic study by Liu and Wen indicate the presence of differential cooperation of individual ethylene receptors with other members.13 Conceivably, an ethylene-receptor member could preferentially or randomly form clusters with other members. The presence of the five ethylene receptor members in different amounts within a cell may facilitate the formation of receptor clusters of various combinations. The cooperation of ethylene receptors within a receptor cluster determines the receptor signal strength. A receptor cluster with strong positive cooperation may mediate strong ethylene receptor signaling, whereas a cluster with weak or negative cooperation may mediate weak signaling. The various receptor clusters within a cell thus mediate a gradient from weak to strong of ethylene receptor signal strength. The constitutive ethylene response is suppressed to a greater extent with strong than weak receptor signaling. With the various strengths of receptor signaling, the constitutive ethylene response is differentially suppressed, and therefore, a plant can respond to ethylene in a wide range of concentrations (Fig. 1).
Figure 1. A model for the modulation of ethylene responses by formation of a dynamic ethylene receptor cluster. (A) Ethylene receptor clusters may mediate a gradient of receptor signal with strength from strong to weak, by which the constitutive ethylene response is suppressed differentially. Ethylene binding to the receptor prevents the suppression of constitutive ethylene responses by receptor clusters. As a result, the corresponding cells or tissues can respond to ethylene over a wide range of concentration. With the alteration in receptor composition, the total ethylene receptor signal strength can be increased or decreased. The increase in receptor members with strong positive cooperation and decrease in those with weak or negative cooperation elevates the total receptor signal strength (B) The total receptor signal strength can be reduced by an increase in receptor members with weak or negative cooperation and decrease in those with strong positive cooperation (C) With ethylene treatment, the receptor clusters that bind ethylene are signaling inactive and the remaining unbound receptor clusters are still signaling active. With clusters predominantly mediating strong ethylene receptor signal output, high but not low levels of ethylene are sufficient to weaken the total receptor signal output, and the corresponding cells or tissues respond to ethylene of higher concentrations. With clusters predominantly mediating weak signal output, low levels of ethylene are sufficient to weaken the total receptor signal output, and the corresponding cells or tissues respond to low ethylene concentrations. Shading differences indicate different levels of strength in receptor signal output (arrow heads), inhibition of constitutive ethylene responses (lines), or receptor cooperation (double arrow heads).
A change in the relative amount of these ethylene receptor members will alter the composition of receptor clusters: the total receptor signal strength mediated by the receptor clusters will be changed, and the suppressed constitutive ethylene response will change accordingly. Plant tissues may modulate ethylene sensitivity by changing the ethylene receptor composition. If the ethylene receptor clusters within a plant tissue are predominantly capable of strong signal output, the tissue may show responses to ethylene of higher concentrations. In contrast, if the receptor clusters within a plant tissue are predominantly capable of weak signal output, the tissue may show responses to ethylene of lower concentrations or exhibit constitutive ethylene responses. Thus, ethylene responses of a plant tissue can be induced by ethylene, as well as by the modulation of the ethylene receptor composition (Fig. 1). If plants sense ethylene with a single ethylene receptor or ethylene receptors with little cooperation, plants may sense ethylene of a narrow concentration range due to lack of receptor signaling of various strength.
Summary and Perspectives
With the lack of knowledge in the nature of the ethylene receptor signal, the receptor signal strength is unlikely to be quantitatively measurable. Even if the receptor signal can be biochemically measured, qualitatively distinguishing the signal output by individual receptors is unlikely because of the nature of redundancy. Thus, addressing the functional significance of ethylene receptor cooperation in ethylene signaling has been challenging. With the availability of loss-of-function and dominant ethylene-insensitive receptor mutants, the functional significance of the receptor complex and mediation of the ethylene signal by multiple receptor members can be addressed in vivo. CTR1 is a downstream component directly mediating the receptor signal, and the possible modulation of the signal mediation via CTR1 needs to be addressed.
Although the present data do not support Arabidopsis ethylene receptor genes being functionally exchangeable in ethylene signaling, the tomato ethylene receptor gene Never Ripe (NR) can be functionally compensated by LeETR4.23 The possibility that some Arabidopsis ethylene receptor genes may be functionally compensated in part by another gene should not be excluded. Alternatively, because of the respective natural evolution and selective breeding of Arabidopsis and tomato, the divergent roles of receptors in ethylene signaling between the two plant species become partly distinct. The latter scenario is in agreement with the distinct roles of Arabidopsis Reversion-To-Ethylene Sensitivity1 (RTE1) and tomato RTE1 homolog Green Ripe (GR) in ethylene signaling: elevated levels of RTE1 promotes ETR1 receptor signaling and confers whole-plant ethylene insensitivity, but elevated levels of GR delays fruit ripening.2,13,24,25
The degradation of ligand-associated receptors can be a mechanism for de-sensitization. ETR2 protein level is reduced with elevated ethylene concentrations in Arabidopsis seedlings, which indicates that the degradation of the ethylene-bound, inactivated ETR2 can be replaced by unbound ETR2 to activate CTR1.26 In contrast, ERS1 accumulation in the rosette is not attenuated with elevated ethylene dose (up to 100 μL L−1).12 The dynamic change in ethylene receptor amount and signaling activity of different members in response to ethylene could also modulate the cooperative signaling of a receptor complex (Fig. 1).
Ethylene signaling machinery may be highly conserved across higher plants, but little is known about lower plants.5 Evolutionary evidence for when plants would have acquired genes to sense the ethylene signal and functional divergence of the ethylene receptor genes in ethylene signaling will further support the hypothesis for the biological significance of the perception of the ethylene signal by multiple receptors in plants.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (grant N0s. 3112306 and 31100212) to C-K Wen and Q Liu.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20937
References
- 1.Hattori Y, Nagai K, Furukawa S, Song X-J, Kawano R, Sakakibara H, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–30. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- 2.Barry CS, Giovannoni JJ. Ripening in the tomato Green-ripe mutant is inhibited by ectopic expression of a protein that disrupts ethylene signaling. Proc Natl Acad Sci U S A. 2006;103:7923–8. doi: 10.1073/pnas.0602319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–23. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ku HS, Suge H, Rappaport L, Pratt HK. Stimulation of rice coleoptile growth by ethylene. Planta. 1970;90:333–9. doi: 10.1007/BF00386385. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Zhou X, Wen C-K. Modulation of ethylene responses by OsRTH1 overexpression reveals the biological significance of ethylene in rice seedling growth and development. J Exp Bot. 2012;••• doi: 10.1093/jxb/ers098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, et al. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–32. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, et al. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci U S A. 1998;95:5812–7. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science. 1995;269:1712–4. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- 9.Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–71. doi: 10.1016/S0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Hall AE, O’Malley R, Bleecker AB. Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci U S A. 2003;100:352–7. doi: 10.1073/pnas.0237085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu X, Hall BP, Gao Z, Schaller GE. A strong constitutive ethylene-response phenotype conferred on Arabidopsis plants containing null mutations in the ethylene receptors ETR1 and ERS1. BMC Plant Biol. 2007;7:3. doi: 10.1186/1471-2229-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Xu C, Wen C-K. Genetic and transformation studies reveal negative regulation of ERS1 ethylene receptor signaling in Arabidopsis. BMC Plant Biol. 2010;10:60. doi: 10.1186/1471-2229-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Wen C-K. Arabidopsis ETR1 and ERS1 differentially repress the ethylene response in combination with other ethylene receptor genes. Plant Physiol. 2012;158:1193–207. doi: 10.1104/pp.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie F, Liu Q, Wen C-K. Receptor signal output mediated by the ETR1 N terminus is primarily subfamily I receptor dependent. Plant Physiol. 2006;142:492–508. doi: 10.1104/pp.106.082628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamble RL, Qu X, Schaller GE. Mutational analysis of the ethylene receptor ETR1. Role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol. 2002;128:1428–38. doi: 10.1104/pp.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Helmbrecht EE, Stalans MB, Schmitt C, Patel N, Wen C-K, et al. Ethylene receptor ETHYLENE RECEPTOR1 domain requirements for ethylene responses in Arabidopsis seedlings. Plant Physiol. 2011;156:417–29. doi: 10.1104/pp.110.170621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu L, Xie F, Yu J, Wen C-K. Arabidopsis RTE1 is Essential to Ethylene Receptor ETR1 N-terminal Signaling Independent of CTR1. Plant Physiol. 2012;••• doi: 10.1104/pp.112.193979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Z, Wen C-K, Binder BM, Chen Y-F, Chang J, Chiang Y-H, et al. Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J Biol Chem. 2008;283:23801–10. doi: 10.1074/jbc.M800641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grefen C, Städele K, Růzicka K, Obrdlik P, Harter K, Horák J. Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol Plant. 2008;1:308–20. doi: 10.1093/mp/ssm015. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y-F, Gao Z, Kerris RJ, 3rd, Wang W, Binder BM, Schaller GE. Ethylene receptors function as components of high-molecular-mass protein complexes in Arabidopsis. PLoS One. 2010;5:e8640. doi: 10.1371/journal.pone.0008640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Malley RC, Rodriguez FI, Esch JJ, Binder BM, O’Donnell P, Klee HJ, et al. Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J. 2005;41:651–9. doi: 10.1111/j.1365-313X.2004.02331.x. [DOI] [PubMed] [Google Scholar]
- 22.Gao Z, Schaller GE. The role of receptor interactions in regulating ethylene signal transduction. Plant Signal Behav. 2009;4:1152–3. doi: 10.4161/psb.4.12.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci U S A. 2000;97:5663–8. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnick JS, Wen C-K, Shockey JA, Chang C. REVERSION-TO-ETHYLENE SENSITIVITY1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:7917–22. doi: 10.1073/pnas.0602239103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Liu Q, Xie F, Wen C-K. RTE1 is a Golgi-associated and ETR1-dependent negative regulator of ethylene responses. Plant Physiol. 2007;145:75–86. doi: 10.1104/pp.107.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y-F, Shakeel SN, Bowers J, Zhao X-C, Etheridge N, Schaller GE. Ligand-induced degradation of the ethylene receptor ETR2 through a proteasome-dependent pathway in Arabidopsis. J Biol Chem. 2007;282:24752–8. doi: 10.1074/jbc.M704419200. [DOI] [PubMed] [Google Scholar]