Abstract
Light is one of the most important environmental signal for plants. Involvement of hormones, such as gibberellic acid, in light regulated development has been known for many years, though the molecular mechanisms remain still largely unknown. To shed light on possible interactions between phyto-hormones and photoperceptive photoreceptors of tomato, in a recent work we investigated the molecular effects of exogenous gibberellin to cryptochrome and phytochrome transcripts in wild type tomato as well as in a mutant genotype with a non-functional cryptochrome 1a and in a transgenic line overexpressing cryptochrome 2. Results highlight that following addition of gibberellin, cryptochrome and phytochrome transcription patterns are strongly modified, especially in cryptochrome 1a deficient plants. Our results suggest that cryptochrome mediated light responses can be modulated by gibberellin accumulation level, in tomato plants.
Keywords: cryptochromes, gibberellin, light, phytochromes, tomato
Many growth and development processes of plants, are regulated by both internal signals, such as hormones, and environmental cues. Light, one of the most important environmental signal for plants, is perceived by at least four different types of photoreceptors: the red (R)/far-red (FR) light sensing phytochromes and the UV-A blue light sensing cryptochromes, phototropins, and zeitlupes.1 In tomato (Solanum lycopersicum phytochromes are encoded by five genes (PHYA, PHYB1, PHYB2, PHYE and PHYF),2 whereas four cryptochrome genes have been discovered and analyzed so far: two CRY1-like (CRY1a and CRY1b), one CRY2 and one CRY-DASH gene.3,4
Involvement of plant hormones, such as gibberellic acid (GA), in light regulated development, which include seed germination and seedling photomorphogenesis, has been known for many years.5 GAs can modulate several molecular processes during the plant life cycle including germination, vegetative growth and flowering through transcriptional regulation of target genes.6 This transcriptional regulation also relies upon the activity of the nuclear GA-regulated DELLA proteins.7
Photoreceptor-hormone interactions have been reported to regulate a number of light responses; phytochromes and GAs are indeed involved (together with auxins and ethylene) in regulating shade-avoidance responses, that maximize light capture by positioning the leaves out of the shade.8 Several other examples could be reported; however there is little or no information about effects of phyto-hormones over photoreceptor proteins and their gene transcripts.
To gain information on possible interaction between phyto-hormones and photoperceptive photoreceptors of tomato, in a recent work9 we investigated the molecular effects of exogenous GA3 to CRYs and PHYs transcripts in wt tomato as well as in a mutant genotype with a non-functional CRY1a (cry1a-)10 and in a transgenic line overexpressing the cryptochrome 2 (CRY2OX).11 Tomato plants were grown hydroponically for 28 days under a light cycle of 16 h light/8 h darkness (LD) with a full nutrient solution;12 GA3 was added to nutrient solution of testplants on 29th day of growth, whereas control-plants were let in the standard nutrient solution.
The aerial parts of treated and control (without hormone) plants for each genotype (wt, cry1a- and CRY2OX) were harvested at distinct time points: ZT0 (ZT- Zeitgeber time=number of hours after the onset of illumination), ZT6, ZT12, ZT16 e ZT20. Total RNA extracted was reverse-transcribed and first strand cDNA achieved was used as template for cryptochrome and phytochrome gene expression assays by quantitative RT-PCR.9 Results highlight that, following addition of GA3, cryptochrome and phytochrome transcription patterns are strongly modified, especially in cry1a- plants (Fig. 1). In the latter genotype, GA3 produces strong downregulation of both cryptochrome and phytochrome transcripts at almost all the tested time points. Thus, the lack of a functional CRY1a protein produces a generic and strong signal of downregulation of the photoperceptive apparatus of tomato in GA3 treated plants with regard to the untreated ones, suggesting a pivotal role for CRY1a in mediating light and gibberellin stimuli.
Figure 1.
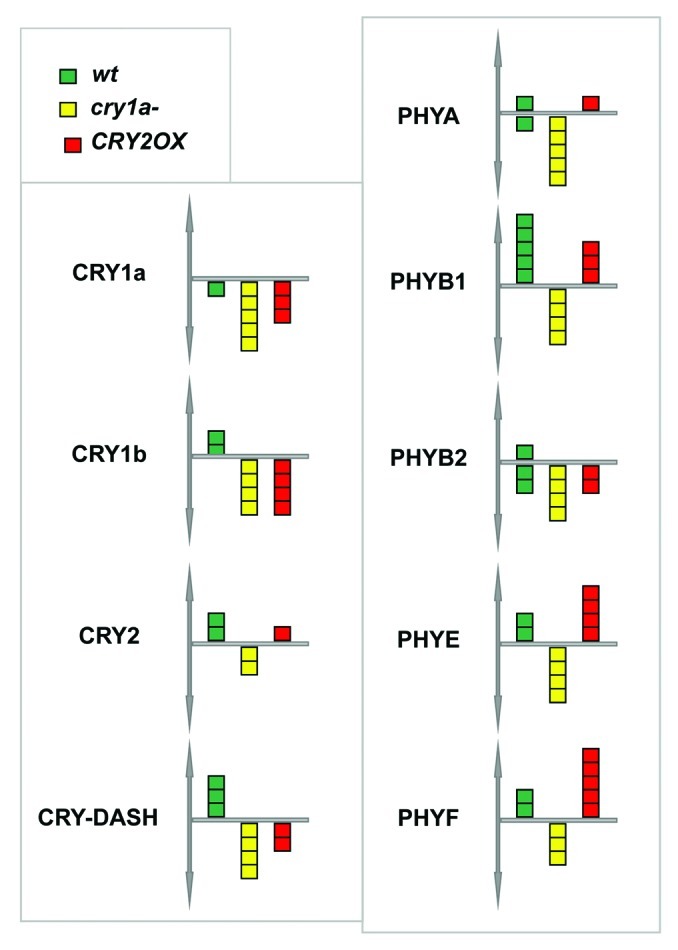
Representation of the behavior of each gene transcript encoiding cryptochromes and phytochromes after GA3 treatment in different genotypes. A square above x-axis represents a time point in which is present upregulation (P<0,05) of the considered gene, a square below x-axis symbolizes downregulation (P<0,05); different colors are used for each genotype.
Analyzing the behavior of cryptochrome transcripts following GA3 treatment in wt plants, we report they are less affected by rapid change of hormone concentration in the culture medium (Fig. 1).
The transcription pattern of the phytochrome gene family, following treatment with GA3, evidenced an opposite response in cry1a- plants with respect to wt and CRY2OX tomatoes. Indeed, when a functional form of CRY1a protein is absent, all five phytochromes are constantly downregulated; while, when CRY1a works normally (in wt and CRY2OX plants) the same genes appear to be mostly upregulated (Fig. 1). We demonstrated that exogenous GA3, in tomato, is able to modify the diurnal expression pattern of several photoreceptor genes, especially when a working form of cryptochrome 1a is absent. These results suggest the existence, in tomato, of a molecular network among cryptochrome 1a, GA3 and the other photoreceptor genes.
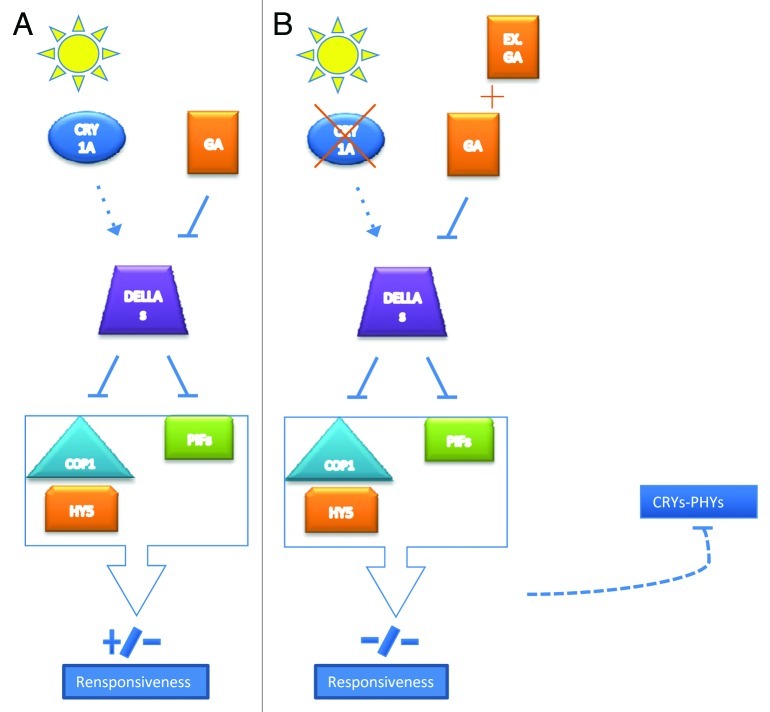
Recent studies13,14 have indicated that, in Arabidopsis, 1-2 type cryptochromes are able to control not only the GA content but also the GA sensing and/or signaling during fundamental biological plant processes like phototropism. Moreover, Achard and colleagues15 showed that phytochromes cause a decrease of GA level in the hypocotyl with concomitant accumulation of DELLA proteins. They also suggested additional photoreceptors could be involved in the regulation of photomorphogenesis via effects on DELLA function. Our results showed a significant effect of CRY1a on the transcription level of several photoreceptor genes of tomato plants after the addition of exogenous GA3. Given the copious evidences that assign DELLA proteins a key switching role between light and GA signals, we suggest a sort of CRY1 induced stabilizing effect on DELLA, antagonistic to the well known GA induced degradation, could take place. Following this hypothesis, in wt tomato during the day, accumulation of DELLA would depend on the divergent action of GA and CRY1a (and possibly other photoreceptors) and this would determine a fine modulation of responsiveness to light signals (Fig. 2A). On the other hand, the absence of a working CRY1a protein and the addition of exogenous GA3 would unbalance the system toward degradation of DELLA; this would cause the activation of COP and PIF signals and by consequence an overall reduction of responsiveness to light signals (Fig. 2B). We suppose the observed downregulation of CRY and PHY photoreceptor transcripts in cry1a- tomatoes, after GA3 treatment, could be the result of a negative feedback effect of the light signal transduction pathway in response to the reduced sensitivity to light signals (Fig. 2B). We are aware that further studies will be needed to confirm this hypothesis and to elucidate the complex network among cryptochromes and phytochromes, gibberellin and its signal transduction pathway.
Figure 2.
Proposed model of interactions between CRY1a, GA and DELLAs in tomato. Arrows represent activation; lines with flat ends represent inhibition. New interactions hypothesized in this study are shown as dashed lines.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20657
References
- 1.Möglich A, Yang XJ, Ayers RA, Moffat K. Structure and function of plant photoreceptors. Annu Rev Plant Biol. 2010;61:21–47. doi: 10.1146/annurev-arplant-042809-112259. [DOI] [PubMed] [Google Scholar]
- 2.Hauser BA, Cordonnier-Pratt MM, Daniel-Vedele F, Pratt LH. The phytochrome gene family in tomato includes a novel subfamily. Plant Mol Biol. 1995;29:1143–55. doi: 10.1007/BF00020458. [DOI] [PubMed] [Google Scholar]
- 3.Facella P, Lopez L, Chiappetta A, Bitonti MB, Giuliano G, Perrotta G. CRY-DASH gene expression is under the control of the circadian clock machinery in tomato. FEBS Lett. 2006;580:4618–24. doi: 10.1016/j.febslet.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Perrotta G, Ninu L, Flamma F, Weller JL, Kendrick RE, Nebuloso E, et al. Tomato contains homologues of Arabidopsis cryptochromes 1 and 2. Plant Mol Biol. 2000;42:765–73. doi: 10.1023/A:1006371130043. [DOI] [PubMed] [Google Scholar]
- 5.Neff MM, Street IH, Turk EM, Ward JM. Interaction of light and hormone signalling to mediate photomorphogenesis. In: Photomorphogenesis in Plants and Bacteria 2006; 439-73. [Google Scholar]
- 6.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–51. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 7.Harberd NP, Belfield E, Yasumura Y. The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell. 2009;21:1328–39. doi: 10.1105/tpc.109.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D. Reaching out of the shade. Curr Opin Plant Biol. 2005;8:462–8. doi: 10.1016/j.pbi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Facella P, Daddiego L, Giuliano G, Perrotta G. Gibberellin and auxin influence the diurnal transcription pattern of photoreceptor genes via CRY1a in tomato. PLoS One. 2012;7:e30121. doi: 10.1371/journal.pone.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weller JL, Perrotta G, Schreuder MEL, van Tuinen A, Koornneef M, Giuliano G, et al. Genetic dissection of blue-light sensing in tomato using mutants deficient in cryptochrome 1 and phytochromes A, B1 and B2. Plant J. 2001;25:427–40. doi: 10.1046/j.1365-313x.2001.00978.x. [DOI] [PubMed] [Google Scholar]
- 11.Giliberto L, Perrotta G, Pallara P, Weller JL, Fraser PD, Bramley PM, et al. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005;137:199–208. doi: 10.1104/pp.104.051987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckhout TJ, Bell PF, Luster DG, Chaney RL. Iron-stress induced redox activity in tomato (Lycopersicum esculentum Mill.) is localized on the plasma membrane. Plant Physiol. 1989;90:151–6. doi: 10.1104/pp.90.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Yu X, Foo E, Symons GM, Lopez J, Bendehakkalu KT, et al. A study of gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol. 2007;145:106–18. doi: 10.1104/pp.107.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuchida-Mayama T, Sakai T, Hanada A, Uehara Y, Asami T, Yamaguchi S. Role of the phytochrome and cryptochrome signaling pathways in hypocotyl phototropism. Plant J. 2010;62:653–62. doi: 10.1111/j.1365-313X.2010.04180.x. [DOI] [PubMed] [Google Scholar]
- 15.Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, et al. DELLAs contribute to plant photomorphogenesis. Plant Physiol. 2007;143:1163–72. doi: 10.1104/pp.106.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]