Abstract
Since its initial discovery as a high affinity Ca2+-binding protein in the sarcoplasmic reticulum and endoplasmic reticulum (ER), calreticulin (CRT) has been documented to be a multifunctional protein in both animal and plant cells. This protein is well recognized as a Ca2+-binding molecular chaperone that facilitates the folding of newly synthesized glycoproteins and regulates the Ca2+ homeostasis in the ER lumen. However, functional relevance associated with its localization in other cellular compartments has also been reported. Recent studies suggest that both isoforms of plant CRTs (AtCRT1/2 and AtCRT3) are involved in regulating plant defense against biotrophic pathogens. Here we discuss the cellular functions of CRT and its connection to the emerging functions of AtCRTs in plant immunity.
Keywords: Pseudomonas syringae, ER retention signal, calcium binding, calreticulin, molecular chaperone, plant immunity, salicylic acid
Calcium (Ca2+) is a ubiquitous second messenger that plays a vital role in numerous signaling pathways in both plant and animal cells.1,2 Whereas a large proportion of the Ca2+-binding proteins function as decoders for stimulus-specific messages encrypted in intracellular Ca2+ oscillations/changes,3 some other Ca2+-binding proteins including calreticulin (CRT) and calsequestrin have distinct cellular functions. The first CRT was identified as a high affinity Ca2+-binding protein from the rabbit skeletal muscle sarcoplasmic reticulum (SR) in 1974.4 Further studies revealed that CRT not only plays a key role in the intracellular Ca2+ storage and endoplasmic reticulum (ER) Ca2+ homeostasis,5-7 but also functions as a crucial molecular chaperone.8-12
CRT Isoforms in Plants and Animals
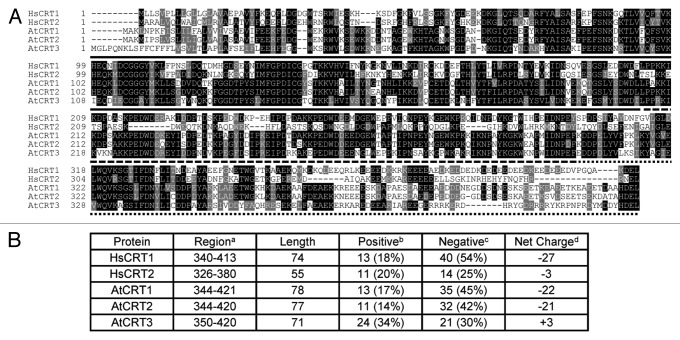
A typical CRT protein is composed of three distinct domains: the N-terminal region (N-domain), the central proline-rich domain (P-domain) and the carboxyl-terminal region (C-domain), followed by an ER retention signal H/KDEL (Fig. 1A).13 Phylogenetic studies revealed two distinct isoforms of CRT in higher plants.14 Unlike the highly acidic C-domains in the AtCRT1/2 isoform and their mammalian counterpart HsCRT1 (Fig. 1B), the electrically neutral C-domain of the AtCRT3 isoform loses its high capacity Ca2+-binding ability.15 For many years, mammalian CRT was considered to be one gene, one protein with diverse functions.16 However, recent studies have revealed that, like higher plants, humans also possess a second CRT isoform HsCRT2 which also contains an electrically neutral C-domain (Fig. 1B) and fails to bind Ca2+ with high capacity.17,18
Figure 1. Sequence comparison between human and Arabidopsis CRTs. (A) Sequence comparison was performed by the CLUSTALW program (www.genome.jp/tools/clustalw/) and BOXSHADE program (www.ch.embnet.org/software/BOX_form.html). Accession numbers are as follows: HsCRT1 (NP_004334.1), HsCRT2 (NP_659483.2), AtCRT1 (NP_176030.1), AtCRT2 (NP_172392.1), AtCRT3 (NP_563816.1). The N-, P- and C-domains are indicated by solid, dashed and dotted lines, respectively. (B) C-terminal amino acid sequence comparison. a The region covering the C-terminal amino acid sequence of CRTs (the H/KDEL ER retention signal is excluded). b Number of amino acids with positively charged side chains (K, R, H). c Number of amino acids with negatively charged side chains (D, E). d Net charge is calculated as Positive - Negative.
Functional Redundancy and Multifunctionality of AtCRTs in Plant Immunity
Although the physiological function of HsCRT2 remains largely unknown, HsCRT1 has been reported to be involved in a variety of biological processes and pathways (for a review see ref. 19). Similarly, plant CRTs are also revealed to be multifunctional proteins that play significant roles in plant growth, development and stress responses (for a review see ref. 20). Particularly, recent studies suggest that both CRT isoforms are important regulators of plant immunity.11,12,15 As shown in Figure 2, different atcrt mutants display distinct disease symptoms when infected with Pseudomonas syringae pv tomato DC3000 (Pst DC3000). While atcrt1 and atcrt2 mutant plants are more resistant to the Pst DC3000 infection than wild-type plants, atcrt3 mutants are more sensitive (Fig. 2). Furthermore, atcrt1 atcrt2 double mutant plants show elevated defense responses to Pst DC3000 when compared with either of the single mutants (Fig. 2), suggesting a functional redundancy of AtCRT1 and AtCRT2 in regulating plant defense against biotrophic pathogens. AtCRT3 functions as a potential component of ER quality control (ER-QC) and is indispensible for the abundance of functional leucine-rich repeat receptor kinase EFR.11,12 EFR is a pattern-recognition receptor that can specifically recognize bacterial elongation factor (EF)-Tu and its surrogate peptide elf18. This recognition further initiates pathogen-associated molecular pattern-triggered immunity (PTI).11,12 In contrast, EFR function was only partially compromised in the atcrt1 atcrt2 double mutant, suggesting that although AtCRT1/2 isoform might affect EFR-mediated PTI, their contribution is relatively minor.11 On the other hand, our recent results showed that the C-terminal Ca2+-binding capacity of AtCRT2 is responsible for its regulation of endogenous SA accumulation; the chaperon function associated with the N-terminal portion of AtCRT2 serves to suppress the defense response resulting from the elevated SA levels. Whether the Ca2+-binding property of AtCRT2 affects its chaperon function remains to be answered. However, these results revealed that multiple functions could be performed and integrated by a single CRT protein.15
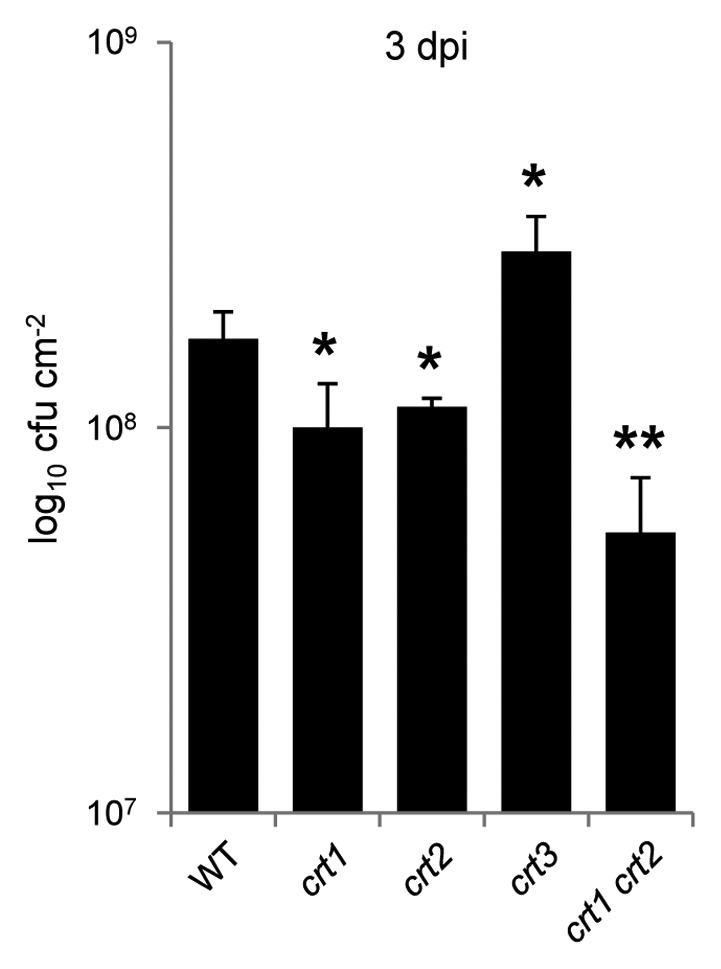
Figure 2.Arabidopsis crt mutants show differential responses to the Pst DC3000 infection. Five-week-old wild-type plants (WT) and T-DNA insertional mutants of crt were infiltrated with 5 × 105 cfu/mL of Pst DC3000. crt1, Salk_142821; crt2, Salk_005910; crt3, Salk_051336; crt1 crt2, Salk_142821 × Salk_005910. Bacterial populations were calculated at day 3 after pathogen infection (3 dpi).15 Data are presented as mean ± s.d. (n = 3). *p < 0.05 and **p < 0.01 between WT and crt mutant plants.
The Role of the H/KDEL ER Retention Signal
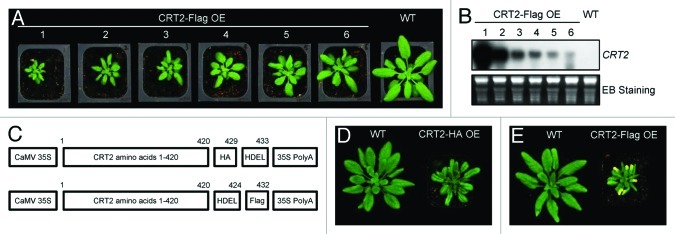
Almost all known CRTs contain a C-terminal tetrapeptide H/KDEL as the ER retention/retrieval signal.20 However, it has been suggested that CRTs are most likely retained in the ER by mechanisms that rely primarily on signals other than H/KDEL motifs.21 Consistent with this hypothesis, HsCRT2 is localized in the ER lumen18 with the absence of a C-terminal H/KDEL motif (Fig. 1A). However, a recent study demonstrated that ERD2b, a Golgi-localized HDEL receptor, is required for AtCRT3 protein accumulation,11 which indicates that the HDEL signal is critical for the ER retention of the AtCRT3 isoform. When the HDEL motif in AtCRT2 is masked by a C-terminal Flag tag (DYKDDDDK, Fig. 3C), overexpression (OE) of AtCRT2-Flag protein results in a dwarf phenotype (Fig. 3A, B) similar to that observed in transgenic plants overexpressing AtCRT2-HA-HDEL protein.15 However, six-week-old AtCRT2-Flag OE plants display chlorosis and autonomous lesions when plants are grown at 20°C (Fig. 3E), a phenotype closely related to abnormal oxidative burst, whereas AtCRT2-HA OE plants of the same age fail to show the same phenotype (Fig. 3D). These observations support that the H/KDEL ER retention signal, and very likely its ER localization, has a role in the normal function of AtCRT2.
Figure 3. Phenotype of transgenic plants overexpressing AtCRT2-Flag. (A) One-month-old wild-type (WT) and transgenic plants overexpressing AtCRT2-Flag (CRT2-Flag OE). (B) northern blot showing the expression levels of AtCRT2 in CRT2-Flag OE and WT. The ethidium bromide (EB)-stained rRNA is shown for loading control. (C) Schematic illustration of the AtCRT2 overexpression constructs. The expression of AtCRT2 is driven by cauliflower mosaic virus (CaMV) 35S promoter in both constructs. An HA tag was inserted upstream of ER retention signal (HDEL) in the 35S:AtCRT2-HA construct, while a Flag tag was inserted after HDEL in the 35S:AtCRT2-Flag construct. The numbers of amino acids are marked. (D) Six-week-old WT and transgenic plants overexpressing AtCRT2-HA (CRT2-HA OE). (E) Six-week-old WT and transgenic plants overexpressing AtCRT2-Flag (CRT2-Flag OE).
The Cellular Localization of CRTs
Despite the presence of the C-terminal ER retention signal and its first identification in the lumen of SR and ER,4,22,23 both animal and plant CRTs are also found outside the ER compartment (Table 1). For example, the cytosolic concentration of animal CRT increases under apoptotic stress conditions,24 and the C-terminal Ca2+-binding domain is crucial for its retrotranslocation from the ER to the cytoplasm.25 The nuclear-localized mouse CRT directly interacts with glucocorticoid receptor (GR) and facilitates its export from the nucleus to the cytoplasm.26 Furthermore, this process is Ca2+-dependent and removal of the C-domain resulted in loss of the EGTA (a Ca2+ chelator)-dependent inhibition of GR export.26 These reports suggest a possible role of the C-domain in controlling the subcellular localization of CRT. Our previous study suggested that the C-domain of AtCRT2 is required for its regulation of endogenous SA biosynthesis. It was shown that AtCRT2-HA OE plants accumulated much higher SA levels than wild-type plants, while transgenic plants overexpressing a chimeric protein with N- and P-domains of AtCRT2 and C-domain of AtCRT3 failed to.15 Hence, considering that plant CRTs are also found in the cytoplasm and nucleus,20 and that animal CRTs play diverse roles in different cellular compartments,24-26 determining the subcellular localization of AtCRT2 is the highest priority for studying its functional regulation during SA-mediated disease resistance.
Table 1. Subcellular localization of animal and plant CRTs.
| Animal |
Plant |
||
|---|---|---|---|
| Localization | References | Localization | References |
| Sarcoplasmic reticulum |
22,23 |
Endoplasmic reticulum (Plasmodesmata) |
11,27,28 (29,30) |
| Endoplasmic reticulum |
23 |
||
| Golgi apparatus |
31 |
Golgi apparatus |
28 |
| Cytosol |
25 |
Cytosol |
20 |
| Nucleus |
32 |
Nucleus |
20 |
| Cell Surface | 33 | Cell Surface | 28 |
Acknowledgments
This research was supported by National Science Foundation grant no. 1021344 and the National Research Initiative Competitive Grant no. 2008–35100–04566 from the US. Department of Agriculture, National Institute of Food and Agriculture. We thank Lorie Mochel, Washington State University, for her help with preparing the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20721
References
- 1.Poovaiah BW, Reddy AS. Calcium and signal transduction in plants. CRC Crit Rev Plant Sci. 1993;12:185–211. doi: 10.1080/07352689309701901. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14(Suppl):S401–17. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostwald TJ, MacLennan DH. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J Biol Chem. 1974;249:974–9. [PubMed] [Google Scholar]
- 5.Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH. Overexpression of calreticulin increases intracellular Ca2+ storage and decreases store-operated Ca2+ influx. J Biol Chem. 1996;271:9332–9. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- 6.Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, et al. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–68. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson S, Wyatt SE, Love J, Thompson WF, Robertson D, Boss WF. The Ca(2+) status of the endoplasmic reticulum is altered by induction of calreticulin expression in transgenic plants. Plant Physiol. 2001;126:1092–104. doi: 10.1104/pp.126.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman MYu A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–9. [PubMed] [Google Scholar]
- 9.Nauseef WM, McCormick SJ, Clark RA. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J Biol Chem. 1995;270:4741–7. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- 10.Jin H, Hong Z, Su W, Li J. A plant-specific calreticulin is a key retention factor for a defective brassinosteroid receptor in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2009;106:13612–7. doi: 10.1073/pnas.0906144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, et al. Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci U S A. 2009;106:15973–8. doi: 10.1073/pnas.0905532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Häweker H, et al. Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J. 2009;28:3439–49. doi: 10.1038/emboj.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith MJ, Koch GL. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding ER/SR protein. EMBO J. 1989;8:3581–6. doi: 10.1002/j.1460-2075.1989.tb08530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persson S, Rosenquist M, Svensson K, Galvão R, Boss WF, Sommarin M. Phylogenetic analyses and expression studies reveal two distinct groups of calreticulin isoforms in higher plants. Plant Physiol. 2003;133:1385–96. doi: 10.1104/pp.103.024943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y, Xi J, Du L, Roje S, Poovaiah BW. A dual regulatory role of Arabidopsis calreticulin-2 in plant innate immunity. Plant J. 2012;69:489–500. doi: 10.1111/j.1365-313X.2011.04807.x. [DOI] [PubMed] [Google Scholar]
- 16.Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344:281–92. doi: 10.1042/0264-6021:3440281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson S, Rosenquist M, Sommarin M. Identification of a novel calreticulin isoform (Crt2) in human and mouse. Gene. 2002;297:151–8. doi: 10.1016/S0378-1119(02)00880-6. [DOI] [PubMed] [Google Scholar]
- 18.Nomura R, Orii M, Senda T. Calreticulin-2 is localized in the lumen of the endoplasmic reticulum but is not a Ca2+ -binding protein. Histochem Cell Biol. 2011;135:531–8. doi: 10.1007/s00418-011-0817-z. [DOI] [PubMed] [Google Scholar]
- 19.Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–66. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- 20.Jia XY, He LH, Jing RL, Li RZ. Calreticulin: conserved protein and diverse functions in plants. Physiol Plant. 2009;136:127–38. doi: 10.1111/j.1399-3054.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- 21.Pagny S, Cabanes-Macheteau M, Gillikin JW, Leborgne-Castel N, Lerouge P, Boston RS, et al. Protein recycling from the Golgi apparatus to the endoplasmic reticulum in plants and its minor contribution to calreticulin retention. Plant Cell. 2000;12:739–56. doi: 10.1105/tpc.12.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989;264:21522–8. [PubMed] [Google Scholar]
- 23.Milner RE, Baksh S, Shemanko C, Carpenter MR, Smillie L, Vance JE, et al. Calreticulin, and not calsequestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum. J Biol Chem. 1991;266:7155–65. [PubMed] [Google Scholar]
- 24.Tarr JM, Young PJ, Morse R, Shaw DJ, Haigh R, Petrov PG, et al. A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol. 2010;401:799–812. doi: 10.1016/j.jmb.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 25.Afshar N, Black BE, Paschal BM. Retrotranslocation of the chaperone calreticulin from the endoplasmic reticulum lumen to the cytosol. Mol Cell Biol. 2005;25:8844–53. doi: 10.1128/MCB.25.20.8844-8853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holaska JM, Black BE, Rastinejad F, Paschal BM. Ca2+-dependent nuclear export mediated by calreticulin. Mol Cell Biol. 2002;22:6286–97. doi: 10.1128/MCB.22.17.6286-6297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denecke J, Carlsson LE, Vidal S, Höglund AS, Ek B, van Zeijl MJ, et al. The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell. 1995;7:391–406. doi: 10.1105/tpc.7.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borisjuk N, Sitailo L, Adler K, Malysheva L, Tewes A, Borisjuk L, et al. Calreticulin expression in plant cells: developmental regulation, tissue specificity and intracellular distribution. Planta. 1998;206:504–14. doi: 10.1007/s004250050427. [DOI] [PubMed] [Google Scholar]
- 29.Baluska F, Samaj J, Napier R, Volkmann D. Maize calreticulin localizes preferentially to plasmodesmata in root apex. Plant J. 1999;19:481–8. doi: 10.1046/j.1365-313X.1999.00530.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen MH, Tian GW, Gafni Y, Citovsky V. Effects of calreticulin on viral cell-to-cell movement. Plant Physiol. 2005;138:1866–76. doi: 10.1104/pp.105.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns K, Michalak M. Interactions of calreticulin with proteins of the endoplasmic and sarcoplasmic reticulum membranes. FEBS Lett. 1993;318:181–5. doi: 10.1016/0014-5793(93)80017-O. [DOI] [PubMed] [Google Scholar]
- 32.Brünagel G, Shah U, Schoen RE, Getzenberg RH. Identification of calreticulin as a nuclear matrix protein associated with human colon cancer. J Cell Biochem. 2003;89:238–43. doi: 10.1002/jcb.10502. [DOI] [PubMed] [Google Scholar]
- 33.Gray AJ, Park PW, Broekelmann TJ, Laurent GJ, Reeves JT, Stenmark KR, et al. The mitogenic effects of the B beta chain of fibrinogen are mediated through cell surface calreticulin. J Biol Chem. 1995;270:26602–6. doi: 10.1074/jbc.270.44.26602. [DOI] [PubMed] [Google Scholar]