Abstract
To date, Arabidopsis purple acid phosphatase 2 (AtPAP2) is the only known plant protein that is dual-targeted to chloroplasts and mitochondria by a C-terminal targeting signal. Using in vitro organelle import and green fluorescence protein (GFP) localization assays, we showed that AtPAP2 is located on, but not imported across the outer membrane (OM) of chloroplasts and mitochondria and exposed its N-terminal enzymatic domain to the cytosol. It was also found that a short stretch of 30 amino acids (a.a.) at the C-terminal region (a.a. 615-644) that contains a stretch of 18 hydrophobic residues, a WYAK motif and 8 hydrophilic residues is sufficient for dual-targeting. Mutation of WYAK to WYAE had no effect on dual-targeting ability suggesting that the charge within this flanking region alone is not an important determinant for dual-targeting.
Keywords: dual-targeting, mitochondria, outer membrane, plastid, purple acid phosphatase
Tail-anchored (TA) proteins possess an N-terminal functional domain and a single transmembrane domain (TMD) at the C-terminus followed by a hydrophilic tail.1 Newly synthesized TA proteins are released from free ribosomes with the C-terminal hydrophobic region inserted into various membranes, such as the endoplasmic reticulum, chloroplast outer envelope, mitochondrial outer membrane, and the peroxisomal membrane.2 The functional domain of TA proteins orients to the cytosol and the TMD of TA proteins is inserted into membranes post-translationally. Sorting of proteins by the C-terminal tail (CT) to their specific intracellular destinations is essential for their functions.3 For instance, overexpression of a C-terminal TMD-truncated AtPAP2 in Arabidopsis abolishes its faster plant growth phenotype.4
Over 500 proteins in Arabidopsis have been predicted to have TA structures, of which, 130 have had their subcellular localization experimentally confirmed based on either GFP targeting or mass spectrometry.2 Most TA proteins were assigned to the ER and secretory membranes, 27 proteins to mitochondria and 31 proteins to plastids.2,5 These include several isoforms of Tom20 and Tom22 (also known as Tom9) of the mitochondrial outer membrane translocon6,7 and the GTPase receptors of the outer membrane translocon of plastids, including AtToc33, AtToc34.5,8
Arabidopsis purple acid phosphatase 2 (AtPAP2) is the only plant TA protein shown to be dual-targeted to chloroplasts and mitochondria.4 It was predicted to carry a putative N-terminal signal peptide, a phosphatase domain and a transmembrane domain (TMD) followed by a short hydrophilic C-terminal tail (CT) (a.a. 614–636) by the TMHMM analysis.4 AtPAP2 was detected in the membrane fraction using immunoblotting.4 An in vivo targeting assay using chimeric GFP vectors showed that the C-terminal TMD motif of AtPAP2, but not the predicted N-terminal signal peptide, can direct GFP to both plastids and mitochondria in Arabidopsis PSB-D protoplasts.4 In transgenic Arabidopsis, deletion of the N-terminal signal peptide did not affect the AtPAP2 overpression phenotype such as earlier flowering and enhanced seed yield, whereas deletion of the C-terminal TMD domain abolished the fast-growing phenotype.4 The goal of this study was to verify the precise localization of AtPAP2 and to examine its dual-targeting capabilities by in vitro import and GFP localization assays.
AtPAP2 is sensitive to externally added proteases
To verify if AtPAP2 is imported into mitochondria and plastids, in vitro import assays using isolated organelles were performed.11 The [35S] Met-labeled precursor of AtPAP2 was synthesized using rabbit reticulocyte TNT in vitro transcription/translation lysate (Promega, Melbourne, Australia). The precursor was incubated with isolated Arabidopsis mitochondria under conditions that support import, followed by proteinase K treatment or rupture of the outer membrane following import and subsequent proteinase K treatment (Fig. 1).12 Mitochondria was re-isolated and proteins resolved on sodium dodecyl sulfate-PAGE (SDS-PAGE) and gels were stained, dried, exposed to a BAS TR2040S plate for 24 h and visualized using a BAS 2500 (Fuji, Tokyo). Results showed that the 74 kDa precursor protein of AtPAP2 was located on the outer membrane of the mitochondria: AtPAP2 was sensitive to protease digestion before and after rupture of the outer membrane of mitochondria (Fig. 1A, lanes 3, 5, 7, 9) in a valinomycin-independent manner (Fig. 1A, lanes 4–5). Valinomycin depolarizes the membrane potential across the inner membrane of mitochondria. Precursor proteins such as alternative oxidase (AOX) and component 23 of the inner mitochondrial membrane translocase (TIM23), located in the mitochondrial inner membrane are insensitive to added protease when the outer membrane was ruptured (Fig. 1A). The addition of valinomycin blocks the import of proteins into the inner mitochondrial membrane and rupture of the mitochondria OM results in the complete digestion of the precursor proteins (Fig. 1A).
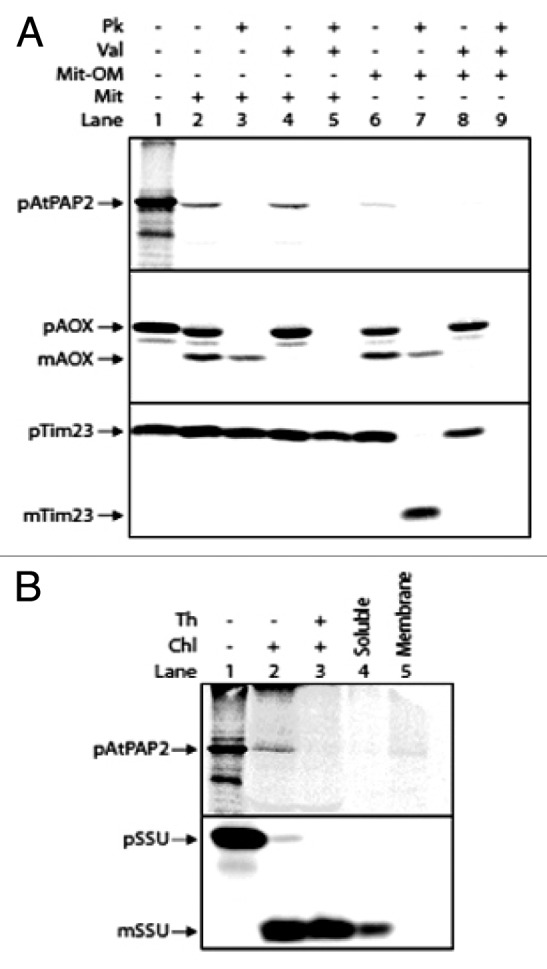
Figure 1. AtPAP2 protein import experiments. (A) In vitro import of radiolabeled AtPAP2 into isolated mitochondria. Lane 1, precursor protein alone. Lane 2, precursor protein incubated with Arabidopsis mitochondria. Lane 3, as in lane 2 except that proteinase K was added after mitochondrial incubation to degrade unimported proteins; Lanes 4–5 as in lanes 2–3, except that valinomycin was added prior to import to dissipate the membrane potential. Lanes 6–9, as lanes 2–5, except that the mitochondrial outer membrane was ruptured following import and prior to proteinase K digestion. Import control of Tim23 and AOX verified that the import of Tim23 and AOX precursors were inhibited in the presence of valinomycin due to the lack of a membrane potential (lanes 1–5) and the inner membrane was intact, as indicated by the proteases-insensitive Tim23 and AOX facing to the ruptured mitochondria outer membrane (lanes 6–9) (B) Import of AtPAP2 into isolated chloroplasts. Lane 1, 35S-labeled AtPAP2 precursor protein; lane 2, AtPAP2 precursor protein incubated with Arabidopsis chloroplasts; lane 3, as lane 2, except that thermolysin was added after import to degrade un-imported proteins. Lanes 4 and 5, soluble and membrane fractions following alkaline extractions of chloroplasts. Abbreviations: Chl, chloroplast ; Mit, mitochondria; PK, proteinase K; Th, thermolysin; Val, valinomycin; p, precursor protein band; m, mature protein band. (+), presence; (-), absence.
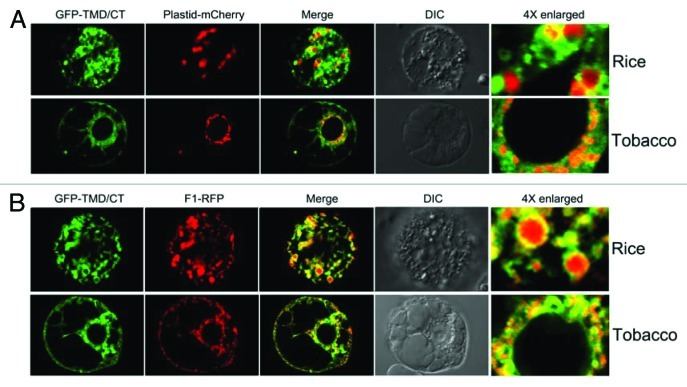
In isolated chloroplasts, membrane-integrated AtPAP2 was digested by treatment with thermolysin, while the precursor of the small subunit of Rubisco (SSU) was imported into chloroplasts and processed into the mature thermolysin-insensitive form (Fig. 1B). These results clearly indicate that AtPAP2 is located on the outer membranes of mitochondria and chloroplasts and its N-terminal functional phosphatase domain is orientated toward the cytosol consistent with being typical TA proteins. If the large N-terminal portion was located inside the outer membrane of mitochondria and plastids, a large protease protected fragment would be evident upon import following protease treatment of intact organelles. Furthermore, transient expression in rice and tobacco BY2 protoplasts showed that all the GFP constructs fused with the TMD/CT region of AtPAP2 were co-localized with mitochondrial (F1-RFP) and plastidic (plastid-mCherry) markers and generated “ring-like” signals surrounding these organelles (Fig. 2). These indicated that AtPAP2 proteins were localized to the outer membranes of these two organelles but not imported across the outer membrane.
Figure 2. Targeting of the GFP fused with C-terminal tail of AtPAP2 in rice and tobacco BY2 protoplasts. The GFP fusion construct (P2C1) were co-transfected with the plastidial (plastid-mCherry) marker (A) and mitochondrial (F1-RFP) marker (B). Scale bar, 50μm.
AtPAP2 dual-localization is dependent on a C-terminal targeting signal
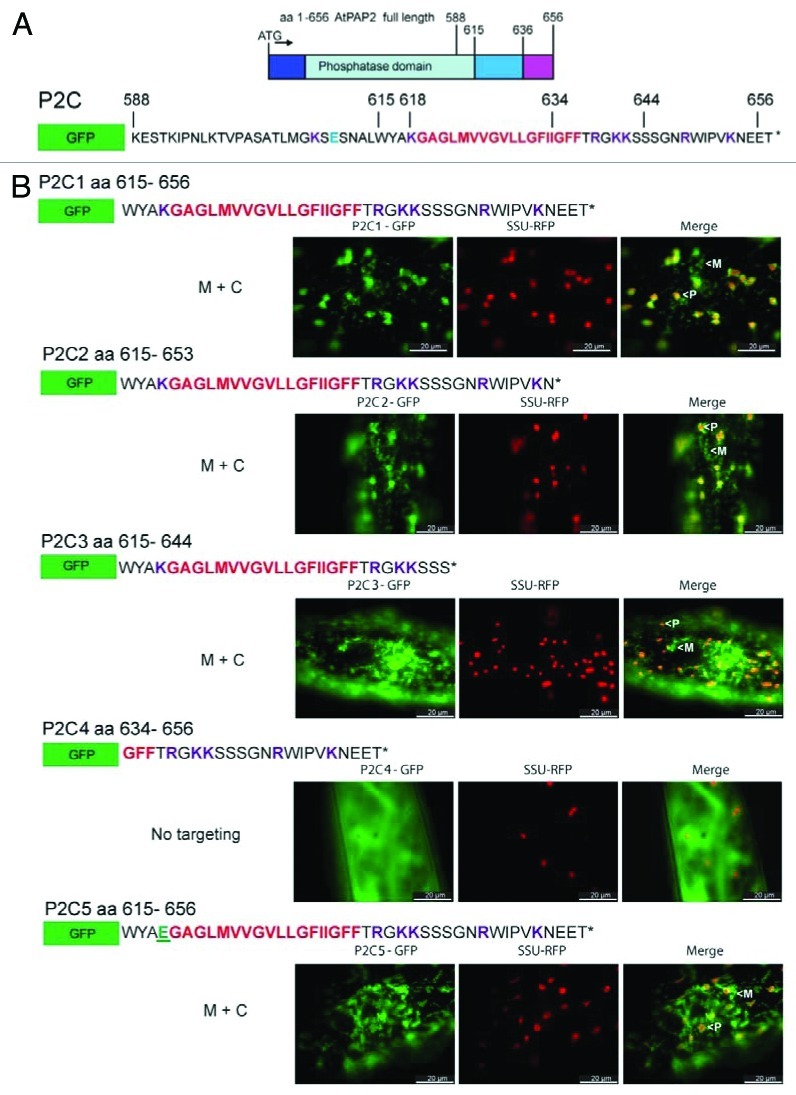
To characterize the targeting sequence at the C-terminus of AtPAP2, a comprehensive series of GFP C-terminal chimeric constructs were generated. The GFP fusion constructs were transiently introduced into onion epidermal cells and GFP localization was examined by fluorescence microscopy (Fig. 3). When the full length TMD/CT of AtPAP2 (615–656, P2C1) and TMD/CT lacking the conserved EE doublets in the C-tail (615–653, P2C2) were appended to the C-terminus of GFP, respectively, the fusion proteins were targeted to both plastids and mitochondria, as evidenced by the co-localization with plastid marker (SSU-RFP) and mitochondrial marker (mito-mCherry) (data not shown). The dual targeting characteristics could be maintained even by a shorter sequence (615–644, P2C3). However, the AtPAP2 C-tail alone (residues 634–656, P2C4), without the hydrophobic TMD motif, was unable to target GFP to plastids and mitochondria, and instead this fusion protein diffused in the cytosol. This indicated that the TMD is essential for dual-targeting to mitochondria and plastids.
Figure 3. Targeting of GFP fused with various C-terminal extensions of AtPAP2. Hydrophobic motifs are in red; positively and negatively charged residues are in purple and blue, respectively; * denotes stop codon. (A) Full length AtPAP2 has been previously shown to be dual-targeted to the outer-membranes of plastids and mitochondria, by its C-terminal sequence.4 (B) A series of GFP constructs containing AtPAP2 with a number of modified C-terminal extensions were biolistically transformed into onion epidermal cells, alongside a plastidial RFP marker. Plastids and mitochondria have been identified in the merged micrograph by a (P) or (M), respectively. The K to E residue substitution in P2C5 has been shown in green.
While 500 proteins in Arabidopsis are predicted to have a TA structure, how they are sorted to the various membranes and maintain their localization specificity remains unknown. Using proteomic methods, 42 and 25 Arabidopsis proteins were identified on the outer membranes of mitochondria13 and chloroplasts,14 respectively. Of these, 13 mitochondrial and 5 chloroplast proteins are putative TA proteins (data not shown), of which AtToc33,5 AtToc345 and TOM2015 have been well characterized previously (Table 1). Different sorting pathways for plastid OM proteins (Toc34, Toc159, Toc75) have been proposed.16 Soluble sorting factors, such as the ankryin repeat containing protein AKR2A, which interacts with AtToc33, AtToc34 and OEP9 at their C-termini, assists their transport to the plastid membrane.5
Table 1. C-terminal hydrophobic motifs of AtPAP2 and preprotein receptors of TOC and TOM.
| Name | AGI | Sequence (N-terminus to C-terminus) | Hydrophobicity (TMD) K&D | Positive charges | Negative charges | ||
|---|---|---|---|---|---|---|---|
| |
|
|
H |
μH |
|
|
|
| Plastids |
|
|
|
|
|
|
|
| AtPAP2 |
At1g13900 |
LMGKSESNALWYAKGAGLMVVGVLLGFIIGFFTRGKKSSSGNRWIPVKP* |
2.39 |
0.66 |
|
7 |
1 |
| AtToc33 |
At1g02280 |
KKMVDGSYSDDKGKKLIPLIIGAQYLIVKMIQGAIRNDIKTSGKPL* |
1.51 |
1.32 |
|
8 |
4 |
| AtToc34 |
At5g05000 |
KLVEGPNPNERGKKLIPLMFAFQYLLVMKPLVRAIKSDVSRESKPAWELR* |
1.73 |
0.17 |
|
8 |
4 |
| Mitochondria |
|
|
|
|
|
|
|
| AtTom20–1 |
At3g27070 |
KNKKSSDEKYIVMGWVILAIGVVACISFRKLR* |
2.37 |
0.56 |
|
7 |
2 |
| AtTom20–2 |
At1g27390 |
KKRNTEFTYDVCGWIILACGIVAWVGMAKSLGPPPPAR* |
2.17 |
0.56 |
|
5 |
2 |
| AtTom20–3 |
At3g27080 |
NKKSSDAKYDAMGWVILAIGVVAWISFAKANVPVSPPR* |
2.13 |
0.43 |
|
5 |
2 |
| AtTom20–4 |
At5g40930 |
QKKTSEFKYDVFGWVILASYVVAWISFANSQTPVSRQ* |
1.97 |
0.49 |
|
4 |
2 |
| AtTom22-I |
At1g04070 |
KKLLKSTGKAAWIAGTTFLILAVPLILELEQDHRLGEIDFEQASLLGTPPVGAML* |
2.14 |
0.07 |
|
5 |
6 |
| AtTom22-V |
At5g43970 |
SKKLLRSTGKAAWIAGTTFLILVVPLIIEMDREAQINEIELQQASLLGAPPSPMQRGL* |
2.31 |
0.18 |
|
6 |
5 |
The mean hydrophobicities were calculated by HydroMCalc (http://www.bbcm.univ.trieste.it/~tossi/HydroCalc/HydroMCalc.html#Hiscale) using Kyte and Doolittle (K&D) hydrophobicity scales. H and μH are the total sum of all residue hydrophobicity indices and the vectorial sum of all the hydrophobicity indices divided by the number of residues, respectively. Hydrophobic motifs are underlined; positively and negatively charged residues are in blue and red color; * denotes stop codon.
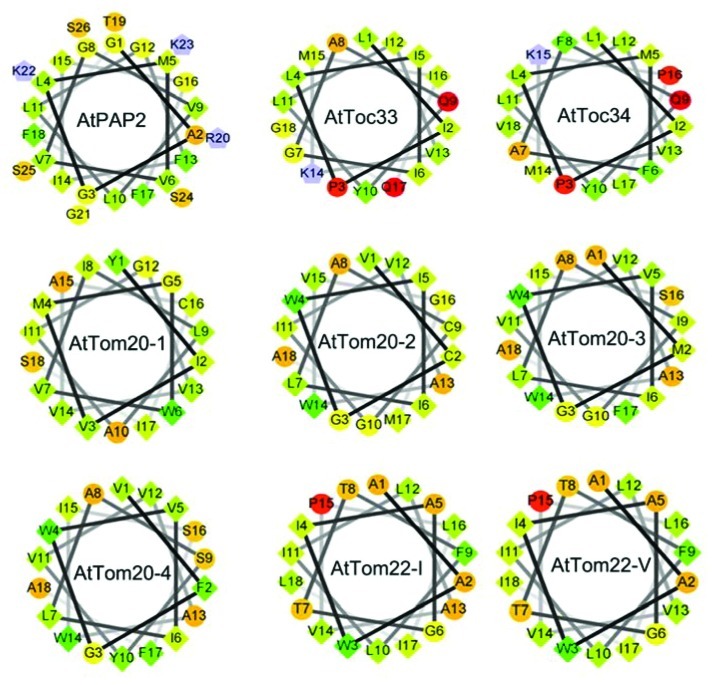
To date AtPAP2 is the only TA protein dual-targeted to the outer membranes of both chloroplasts and mitochondria. The hydrophobicity of the TMD17 and the positively charged amino acids Lys (K) and Arg (R) flanking the TMD sequence were proposed to be determinants for the sorting of TA proteins.18 Our results showed that the alteration of a positively charged residue (K) to a negatively charged residue (E) adjacent to the first a.a. of TMD sequence did not affect the dual targeting ability. This suggests the TMD motif and the hydrophilic residues at the C-terminus of the TMD motif are the major determinants of the dual-targeting. Helical wheel projections of the TMDs of TA proteins targeted to OM of chloroplasts and mitochondria is showed in Figure 4. Generally, the TMDs of TA proteins (AtToc 33/34) targeted to plastid OM contain K, Q and P, which are not favored by hydrophobic transmembrane helix. In contrast, the TMDs of TA proteins targeted to mitochondria OM (AtTom20, AtTom22) are very hydrophobic. For AtPAP2, the 18 aa of the TMD are extremely hydrophobic and unexpectedly, contain 5 glycine residues. Due to its high conformational flexibility, it is entropically unfavorable to include glycine residues in the relatively constrained α-helical structure. The inclusion of glycine residues may increase the flexibility of the TMD of AtPAP2, and whether this facilitates binding to sorting proteins specific to both chloroplasts and mitochondria OM is a subject for further studies.
Figure 4. Helical wheel projections of the predicted C-terminal helices of AtPAP2 and TOC and TOM complex members. The C-terminal hydrophobic sequences were analyzed by the program (http://rzlab.ucr.edu/scripts/wheel/wheel.cgi). Numbers indicate the order of the sequence of amino acids. Hydrophobicity is highlighted based on Kyte-Doolittle scale19 (Diamond green very hydrophobic and yellow lowest hydrophobic; circle red very hydrophilic). Residues that are potentially positively charged are presented as pentagons.
Acknowledgments
This project was supported by the Initiatives for the Clean Energy and Environment (ICEE) of the University of Hong Kong, the General Research Fund (HKU772710M) of the HKSAR, China and an Australian Research Council Discovery Grant (DP DP0986245) to J.W.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20769
References
- 1.Kalbfleisch T, Cambon A, Wattenberg BW. A bioinformatics approach to identifying tail-anchored proteins in the human genome. Traffic. 2007;8:1687–94. doi: 10.1111/j.1600-0854.2007.00661.x. [DOI] [PubMed] [Google Scholar]
- 2.Kriechbaumer V, Shaw R, Mukherjee J, Bowsher CG, Harrison AM, Abell BM. Subcellular distribution of tail-anchored proteins in Arabidopsis. Traffic. 2009;10:1753–64. doi: 10.1111/j.1600-0854.2009.00991.x. [DOI] [PubMed] [Google Scholar]
- 3.Schleiff E, Becker T. Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat Rev Mol Cell Biol. 2011;12:48–59. doi: 10.1038/nrm3027. [DOI] [PubMed] [Google Scholar]
- 4.Sun F, Suen PK, Zhang Y, Liang C, Carrie C, Whelan J, et al. A dual-targeted purple acid phosphatase in Arabidopsis thaliana moderates carbon metabolism and its overexpression leads to faster plant growth and higher seed yield. New Phytol. 2012;194:206–19. doi: 10.1111/j.1469-8137.2011.04026.x. [DOI] [PubMed] [Google Scholar]
- 5.Dhanoa PK, Richardson LGL, Smith MD, Gidda SK, Henderson MPA, Andrews DW, et al. Distinct pathways mediate the sorting of tail-anchored proteins to the plastid outer envelope. PLoS One. 2010;5:e10098. doi: 10.1371/journal.pone.0010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maćasev D, Whelan J, Newbigin E, Silva-Filho MC, Mulhern TD, Lithgow T. Tom22′, an 8-kDa trans-site receptor in plants and protozoans, is a conserved feature of the TOM complex that appeared early in the evolution of eukaryotes. Mol Biol Evol. 2004;21:1557–64. doi: 10.1093/molbev/msh166. [DOI] [PubMed] [Google Scholar]
- 7.Werhahn W, Niemeyer A, Jänsch L, Kruft V, Schmitz UK, Braun HP. Purification and characterization of the preprotein translocase of the outer mitochondrial membrane from Arabidopsis. Identification of multiple forms of TOM20. Plant Physiol. 2001;125:943–54. doi: 10.1104/pp.125.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutensohn M, Schulz B, Nicolay P, Flügge UI. Functional analysis of the two Arabidopsis homologues of Toc34, a component of the chloroplast protein import apparatus. Plant J. 2000;23:771–83. doi: 10.1046/j.1365-313x.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 9.Ivanova Y, Smith MD, Chen KH, Schnell DJ. Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol Biol Cell. 2004;15:3379–92. doi: 10.1091/mbc.E03-12-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue H, Rounds C, Schnell DJ. The molecular basis for distinct pathways for protein import into Arabidopsis chloroplasts. Plant Cell. 2010;22:1947–60. doi: 10.1105/tpc.110.074328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whelan J, Tanudji MR, Smith MK, Day DA. Evidence for a link between translocation and processing during protein import into soybean mitochondria. Biochim Biophys Acta. 1996;1312:48–54. doi: 10.1016/0167-4889(96)00014-6. [DOI] [PubMed] [Google Scholar]
- 12.Lister R, Mowday B, Whelan J, Millar AH. Zinc-dependent intermembrane space proteins stimulate import of carrier proteins into plant mitochondria. Plant J. 2002;30:555–66. doi: 10.1046/j.1365-313X.2002.01316.x. [DOI] [PubMed] [Google Scholar]
- 13.Duncan O, Taylor NL, Carrie C, Eubel H, Kubiszewski-Jakubiak S, Zhang BT, et al. Multiple lines of evidence localize signaling, morphology, and lipid biosynthesis machinery to the mitochondrial outer membrane of Arabidopsis. Plant Physiol. 2011;157:1093–113. doi: 10.1104/pp.111.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferro M, Brugière S, Salvi D, Seigneurin-Berny D, Court M, Moyet L, et al. AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol Cell Proteomics. 2010;9:1063–84. doi: 10.1074/mcp.M900325-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lister R, Carrie C, Duncan O, Ho LHM, Howell KA, Murcha MW, et al. Functional definition of outer membrane proteins involved in preprotein import into mitochondria. Plant Cell. 2007;19:3739–59. doi: 10.1105/tpc.107.050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann NR, Theg SM. Chloroplast outer membrane protein targeting and insertion. Trends Plant Sci. 2005;10:450–7. doi: 10.1016/j.tplants.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Maggio C, Barbante A, Ferro F, Frigerio L, Pedrazzini E. Intracellular sorting of the tail-anchored protein cytochrome b5 in plants: a comparative study using different isoforms from rabbit and Arabidopsis. J Exp Bot. 2007;58:1365–79. doi: 10.1093/jxb/erl303. [DOI] [PubMed] [Google Scholar]
- 18.Borgese N, Colombo S, Pedrazzini E. The tale of tail-anchored proteins: coming from the cytosol and looking for a membrane. J Cell Biol. 2003;161:1013–9. doi: 10.1083/jcb.200303069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–32. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]