Abstract
Thermospermine, a structural isomer of spermine, is synthesized by a thermospermine synthase designated ACAULIS5 (ACL5). Thermospermine-deficient acl5 mutant of Arabidopsis thaliana shows severe dwarfism and excessive xylem differentiation. By screening for compounds that affect xylem differentiation in the acl5 mutant, we identified auxin analogs that remarkably enhanced xylem vessel differentiation in the acl5 mutant but not in the wild type. The xylem-inducing effect of auxin analogs was clearly suppressed by thermospermine, indicating that auxin-inducible xylem differentiation is normally limited by thermospermine. Here, we further characterized xylem-inducing effect of auxin analogs in various organs. Auxin analogs promoted protoxylem differentiation in roots and cotyledons in the acl5 mutant. Our results indicate that the opposite action between thermospermine and auxin in xylem differentiation is common in different organs and also suggest that thermospermine might be required for the suppression of protoxylem differentiation.
Keywords: thermospermine; auxin; 2,4-D; xylem differentiation; ACAULIS5; chemical biology
Xylem is a major conducting tissue for water, minerals and signaling molecules in vascular plants. Previous studies revealed that multiple signaling molecules regulate the differentiation of xylem vessel elements.1 Among these signals, auxin plays a pivotal in xylem development and auxin polar transport might be required to determine the pattern of xylem differentiation. In the auxin canalization hypothesis, polar auxin transport generates local auxin flow, which in turn specifies procambial cell fate.2-4 Auxin may interact with other signals for spatial and temporal regulation of xylem vessel differentiation, while the interaction mechanisms are not well understood.5
Thermospermine is a structural isomer of a major polyamine, spermine, and has recently been identified as another plant growth regulator that represses xylem differentiation and promotes stem elongation in Arabidopsis thaliana.6,7 The acaulis5 (acl5) mutant of A. thaliana exhibits excessive differentiation of xylem tissues and severe dwarfism8-10 and shows deficient biosynthesis of thermospermine.6 Exogenously supplied thermospermine remarkably suppresses xylem vessel differentiation in A. thaliana and Zinnia elegans.7 Although the mode of action of thermospermine remains unclear, genetic analyses of suppressor of acl5 (sac) mutants have suggested that thermospermine suppresses the inhibitory effect of an upstream open reading frame (uORF) located in the 5′ leader of the SAC51 mRNA on its translation.11,12 Consequently, thermospermine enhances translation of SAC51, which encodes a basic helix-loop-helix (bHLH) transcription factor, and SAC51 in turn may restrict xylem differentiation and promote stem elongation.
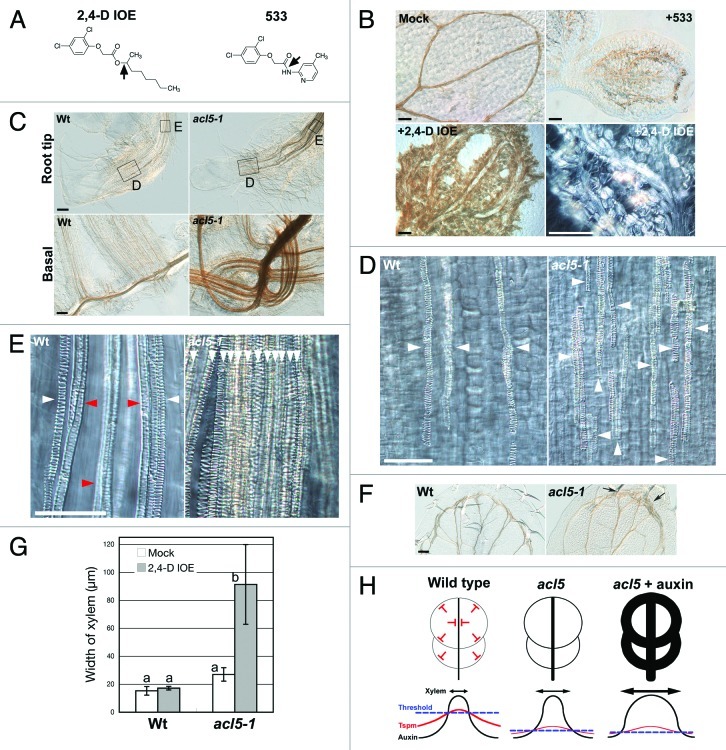
To investigate how thermospermine or SAC51-mediated thermospermine signaling regulates xylem differentiation, we screened for chemicals that affect xylem development in the acl5 mutant and identified the isooctyl ester of 2,4-dichlorophenoxyacetic acid (2,4-D IOE) as a potent inducer of xylem vessel differentiation (Fig. 1A and B).13 Our experiments using other auxin analogs indicated that 2,4-D, 2,4-D analogs, and IAA analogs also induced excessive xylem differentiation in acl5-1 but not in the wild type. The effectiveness of auxin analogs may be dependent on their cellular accumulation, tissue permeability and metabolic stability. For example, metabolism-resistant 4-Cl−IAA remarkably induced xylem differentiation in acl5 while IAA did not,13 suggesting that the high level of auxin is required to overcome the threshold for xylem induction.
Figure 1. Auxin analogs induce excessive xylem differentiation in the acl5 mutant. (A) Structure of 2,4-D IOE and 533. Arrows indicate the putative cleavage sites by esterases. (B) 533 and 2,4-D IOE induce excessive xylem differentiation in acl5-1. The acl5-1 mutant was grown for 7 d in the MS liquid medium in the absence (Mock) or presence of 10 µM 533 or 2,4-D IOE, and analyzed for xylem vessel differentiation in the cotyledons. (C-F) 2,4-D IOE promotes xylem differentiation in the acl5–1 mutant but not in the wild type. Wild type (Wt) and the acl5-1 mutant were grown for 10 d on the MS agar medium in the absence (Mock) or presence of 0.3 µM 2,4-D IOE. Xylem vessels in the root tips (upper panels in C, D, E), the basal parts of roots (lower panels in C) or leaf tips (F) were observed under a microscope. White and red arrowheads in D and E point to the protoxylem vessels and metaxylem vessels, respectively. Arrow in F indicates excessive xylem differentiation in acl5-1. Bars = 100 µm (B, C, F) or 50 µm (D, E). (G) Effect of 2,4-D IOE on the width of xylem in the cotyledon midvein. Wild type (Wt) and the acl5-1 mutant were grown for 7 d on the MS agar medium in the absence (Mock) or presence of 0.3 µM 2,4-D IOE. Values indicate means ± SEs (n = 9). Values designated by the same letter are not significantly different at the p = 0.05 level in the Student’s t-test. (H) A model of interaction between thermospermine and auxin in xylem development. The upper panels show that thermospermine (“T”) limits xylem differentiation (black line). In the lower panels, the black, red and blue lines represent auxin gradient, thermospremine (Tspm) gradient and the threshold for xylem differentiation, respectively. Arrows indicate xylem differentiation.
Savaldi-Goldstein et al.14 (2008) have also found that 2,4-D analogs with an amide linkage promote hypocotyl elongation in A. thaliana. These compounds act as “proauxins,” which diffuse efficiently to the tissue, where they undergo cleavage and release functional auxins.14,15 Our results suggested that 2,4-D IOE might permeate efficiently into the tissue and release active 2,4-D, which promotes xylem vessel differentiation through SCFTIR1/AFB auxin signaling pathway.13 To confirm this idea, we analyzed the effect of a bipartite pro-auxin named 53314 on xylem vessel differentiation in the acl5 mutant (Fig. 1A and B). 533 induced excessive xylem differentiation in acl5-1, indicating that pro-auxin is a potent inducer of xylem vessel differentiation probably due to its high tissue permeability.
Next, we addressed whether auxin analogs promote protoxylem differentiation or metaxylem differentiation in the acl5 mutant. Close-up observation of the cotyledons of acl5-1 indicated that 2,4-D IOE strongly promoted protoxylem differentiation (Fig. 1B, lower panels). This is consistent with the previous report that protoxylem-like vessel elements were predominant in the hypocotyls of the acl5 mutant.16 The xylem-inducing effect of 2,4-D IOE was also found in the roots and leaves as well as in the cotyledons (Fig. 1C-F). 2,4-D IOE remarkably induced excessive xylem differentiation in roots of acl5-1 but not in those of the wild type (Fig. 1C). The roots of acl5-1 grown with 2,4-D IOE had the increased number of protoxylem cell files and less metaxylem vessels (Fig. 1D and E). 2,4-D IOE also promoted xylem differentiation in the leaf tip margin of acl5-1 (Fig. 1F). In addition, effect of 2,4-D IOE on xylem differentiation was quantitatively analyzed by measuring the width of xylem in the cotyledon midvein (Fig. 1G). 2,4-D IOE induced about 3-fold increase in the width of xylem in acl5–1 but not in the wild type. These results indicate that thermospermine mainly suppresses protoxylem differentiation that can be stimulated by auxin (Fig. 1H). In addition, the opposite action between auxin and thermospermine may commonly regulate xylem differentiation in various organs.
In summary, our results demonstrate that auxin analogs show an inducing effect on protoxylem vessel differentiation in the absence of thermospermine. Thermospermine might be an opposing factor to auxin in the regulation of the timing and spatial pattern of protoxylem differentiation (Fig. 1H). Because ACL5 is expressed in provascular cells and xylem vessels and is upregulated by auxin,9,10,16 thermospermine may be synthesized in developing vascular cells in response to auxin and form a gradient around the auxin maxima to limit auxin-inducible xylem differentiation (Fig. 1H). Taking into account that transcription factors required for xylem differentiation are remarkably upregulated by 2,4-D in the acl5 mutant,13 thermospermine is critically required for suppressing the inductive effect of auxin on gene expression involved in xylem differentiation. Further analysis of the effect of auxin analogs and thermospermine using mutants related to auxin and thermospermine signaling will provide new insight into the molecular mechanism of xylem differentiation.
Acknowledgments
We thank the Arabidopsis Biological Resource Center for providing seeds and Prof. Masaru Niitsu (Josai University, Japan) for providing thermospermine. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan [Grants in Aid for Scientific Research (22770043 and 23119513 to H.M., 22370021 and 23012032 to T.T.)]; RCIS-Young Faculty Joint Research Award; Grant in Aid from the Ryobi Teien Memory Foundation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20784
References
- 1.Fukuda H, Hirakawa Y, Sawa S. Peptide signaling in vascular development. Curr Opin Plant Biol. 2007;10:477–82. doi: 10.1016/j.pbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Sachs T. Cell polarity and tissue patterning in plants. Development. 1991;(Suppl I):83–93. [Google Scholar]
- 3.Aloni R, Schwalm K, Langhans M, Ullrich CI. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta. 2003;216:841–53. doi: 10.1007/s00425-002-0937-8. [DOI] [PubMed] [Google Scholar]
- 4.Mattsson J, Ckurshumova W, Berleth T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003;131:1327–39. doi: 10.1104/pp.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehesranta SJ, Lichtenberger R, Helariutta Y. Cell-to-cell communication in vascular morphogenesis. Curr Opin Plant Biol. 2010;13:59–65. doi: 10.1016/j.pbi.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Kakehi J, Kuwashiro Y, Niitsu M, Takahashi T. Thermospermine is required for stem elongation in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:1342–9. doi: 10.1093/pcp/pcn109. [DOI] [PubMed] [Google Scholar]
- 7.Kakehi J, Kuwashiro Y, Motose H, Igarashi K, Takahashi T. Norspermine substitutes for thermospermine in the control of stem elongation in Arabidopsis thaliana. FEBS Lett. 2010;584:3042–6. doi: 10.1016/j.febslet.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Hanzawa Y, Takahashi T, Komeda Y. ACL5: an Arabidopsis gene required for internodal elongation after flowering. Plant J. 1997;12:863–74. doi: 10.1046/j.1365-313X.1997.12040863.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, et al. ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J. 2000;19:4248–56. doi: 10.1093/emboj/19.16.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clay NK, Nelson T. Arabidopsis thickvein mutation affects vein thickness and organ vascularization, and resides in a provascular cell-specific spermine synthase involved in vein definition and in polar auxin transport. Plant Physiol. 2005;138:767–77. doi: 10.1104/pp.104.055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai A, Hanzawa Y, Komura M, Yamamoto KT, Komeda Y, Takahashi T. The dwarf phenotype of the Arabidopsis acl5 mutant is suppressed by a mutation in an upstream ORF of a bHLH gene. Development. 2006;133:3575–85. doi: 10.1242/dev.02535. [DOI] [PubMed] [Google Scholar]
- 12.Imai A, Komura M, Kawano E, Kuwashiro Y, Takahashi T. A semi-dominant mutation in the ribosomal protein L10 gene suppresses the dwarf phenotype of the acl5 mutant in Arabidopsis thaliana. Plant J. 2008;56:881–90. doi: 10.1111/j.1365-313X.2008.03647.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto K, Noutoshi Y, Hayashi K, Shirasu K, Takahashi T, Motose H. A chemical biology approach reveals an opposite action between thermospermine and auxin in xylem development in Arabidopsis thaliana. Plant Cell Physiol. 2012;53:635–45. doi: 10.1093/pcp/pcs017. [DOI] [PubMed] [Google Scholar]
- 14.Savaldi-Goldstein S, Baiga TJ, Pojer F, Dabi T, Butterfield C, Parry G, et al. New auxin analogs with growth-promoting effects in intact plants reveal a chemical strategy to improve hormone delivery. Proc Natl Acad Sci USA. 2008;105:15190–5. doi: 10.1073/pnas.0806324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershater MC, Cummins I, Edwards R. Role of a carboxylesterase in herbicide bioactivation in Arabidopsis thaliana. J Biol Chem. 2007;282:21460–6. doi: 10.1074/jbc.M701985200. [DOI] [PubMed] [Google Scholar]
- 16.Muñiz L, Minguet EG, Singh SK, Pesquet E, Vera-Sirera F, Moreau-Courtois CL, et al. ACAULIS5 controls Arabidopsis xylem specification through the prevention of premature cell death. Development. 2008;135:2573–82. doi: 10.1242/dev.019349. [DOI] [PubMed] [Google Scholar]