Abstract
Reactive oxygen species (ROS) act as signaling molecules for regulating plant responses to abiotic and biotic stress and there exist source- and kind-specific pathways for ROS signaling. Recently, we created a novel system for producing H2O2 in Arabidopsis chloroplasts by chemical-dependent thylakoid membrane-bound ascorbate peroxidase (tAPX) silencing using an estrogen-inducible RNAi method. Microarray analysis revealed that the expression of a large set of genes was altered in response to tAPX silencing, some of which are known to be involved in pathogen response/resistance. Furthermore, we found that tAPX silencing enhances the levels of salicylic acid (SA) and the response to SA, a central regulator for biotic stress response. In this addendum, we describe the relationship between chloroplastic H2O2 and SA in stress response, and discuss the function of the kind- and source-specific ROS signaling in SA-mediated stress response.
Keywords: ascorbate peroxidase, chloroplast, isochorismate synthase, oxidative signaling, Reactive oxygen species, salicylic acid, stress response
Reactive oxygen species (ROS) act as signaling molecules involved in responses to abiotic and biotic stress in plants.1-4 It has gradually been accepted that source- and kind-specific pathways exist for ROS signaling.5 To understand the role of ROS in plant responses to stress, the molecular mechanism and signaling crosstalk of each pathway must be analyzed.
Chloroplasts are one of the most significant sources of ROS in pant cells. Thylakoid membrane-bound ascorbate peroxidase (tAPX) is a major H2O2-scavenging enzyme in chloroplasts.6-8 To clarify the signaling function of chloroplastic H2O2, we recently created a novel system for producing H2O2 in Arabidopsis chloroplasts by estrogen-inducible silencing of thylakoid membrane-bound ascorbate peroxidase (tAPX), a major H2O2-scavenging enzyme in chloroplasts.9 Microarray analysis revealed that tAPX silencing affects the expression of 774 genes. Functional classification of the chloroplastic H2O2-responsive genes and physiological analyses using the tAPX-silencing system indicated that chloroplastic H2O2 negatively regulates the response to chilling, and has antagonistic and synergistic roles in the response to high light. Furthermore, we found that tAPX silencing enhances the levels of salicylic acid (SA) and the response to SA, a central regulator for biotic stress response,10,11 indicating crosstalk between chloroplastic H2O2 and SA in stress response.9 In this addendum, we provide further data supporting the crosstalk, and discuss the function of source-specific H2O2 signaling pathways for regulating the SA response.
To study the effect of chloroplastic H2O2 on the SA response we checked the sensitivity of tAPX-silenced plants to SA treatment. As described in Maruta et al.,9 at 2 d after estrogen (100 µM) treatment, the expression of tAPX was drastically suppressed at the protein level in the IS-tAPX plants. IS-GUS plants were used as a control. Seventeen-day-old IS-GUS and IS-tAPX plants grown under continuous light at 100 µmol photons/m2/s were treated with estrogen. At 2 d after the treatment, plants were further treated with a high concentration (5 mM) of SA for 4 d. As shown in Figure 1, the leaves of IS-GUS plants and estrogen-untreated IS-tAPX plants were visibly damaged by SA treatment to the same degree. Conversely, the leaves of estrogen-treated IS-tAPX plants were insensitive to the treatment, suggesting that chloroplastic H2O2 causes SA insensitivity. This result was unexpected, because our previous findings revealed that tAPX silencing enhances the levels of SA and the SA response. However, the SA-insensitive phenotype of the tAPX-silenced plants strongly supports the possibility that chloroplastic H2O2 is involved in the regulation of the SA response. It is possible that chloroplastic H2O2 induces the expression of gene(s) involved in the reduction of SA toxicity, though no such gene has yet been identified.
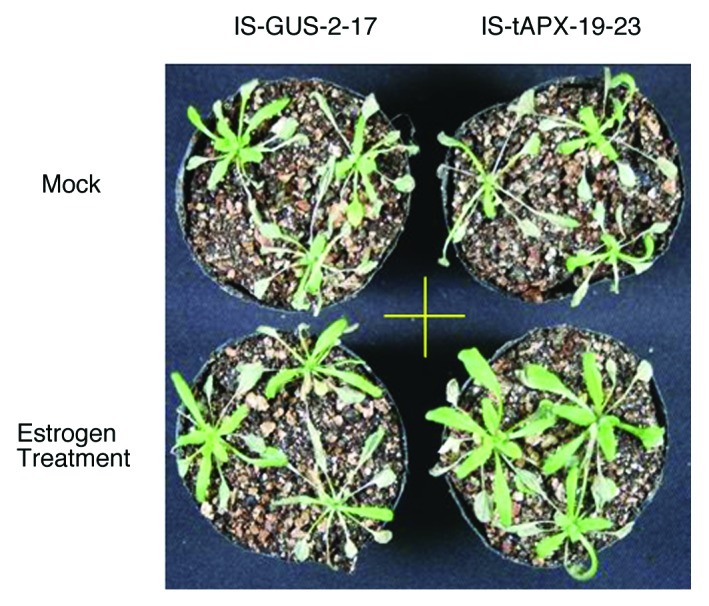
Figure 1. tAPX-silenced plants show SA insensitivity. Plants were grown under continuous light at 100 µmol/m2/s. Seventeen-day-old IS-GUS-2–17 and IS-tAPX-19–23 plants were sprayed with 100 µM estrogen. At 2 d after the treatment, plants were further sprayed with 5 mM SA. The plants 4 d after SA treatment were photographed. The same results were obtained in three independent experiments. A representative photograph is shown.
Arabidopsis has two isochorismate synthase (ICS) genes, ICS1 and ICS2, known to be involved in SA biosynthesis.12 Garcion et al.12 reported that both ICS enzymes are located in chloroplasts and ICS1 has a dominant role in the biosynthesis of SA. Furthermore, ICS1, but not ICS2, was highly responsive to a pathogen infection which enhanced levels of SA.13 Thus, the physiological function of ICS2 is largely unknown. Our previous microarray and quantitative RT-PCR (q-PCR) analyses have revealed that the expression of ICS2 but not ICS1 increased in response to tAPX silencing, resulting in enhanced levels of SA.9 In fact, there was no effect of tAPX silencing on the transcript levels of ICS1 (Fig. 2). These findings indicated that chloroplastic H2O2 enhances the levels of SA through ICS2 expression. Mutants lacking catalase 2, encoding a major H2O2-scavenging enzyme (CAT2) in peroxisomes, have been used to investigate the function of peroxisome-derived H2O2.14 It was found that the CAT2-defective mutants show cell death phenotypes under long-day conditions, and markedly accumulate ICS1 transcripts and SA.15 Interestingly, the lack of ICS1 inhibited the accumulation of SA in the mutants and rescued the phenotypes.15 These findings suggest that peroxisomal and chloroplastic H2O2 enhance SA biosynthesis through ICS1 and ICS2 expression, respectively.
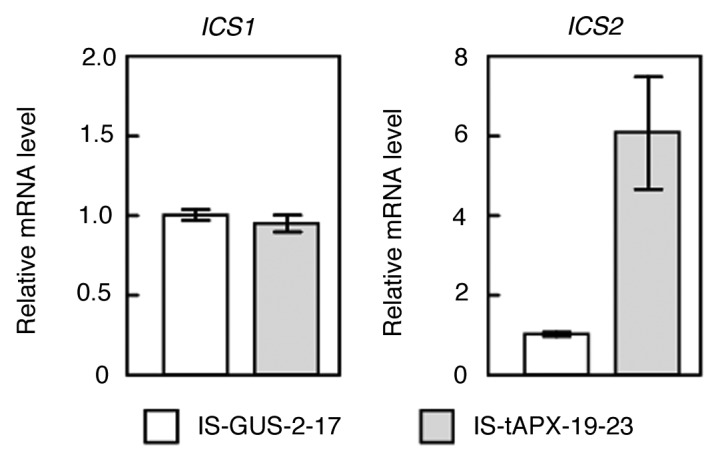
Figure 2. tAPX silencing induces the transcription of ICS2 but not ICS1. Seventeen-day-old IS-GUS-2–17 and IS-tAPX-19–23 plants were sprayed with 100 µM estrogen. At 2 d after the treatment, the transcript levels of ICS1 and ICS2 were measured by q-PCR. Error bars indicate SD (n = 3). Significant differences: *, p < 0.05 vs. the value for IS-GUS-2–17 plants.
Taken together, our previous findings and the present results clearly show the role for chloroplastic H2O2 in the response to SA. SA acts as an antagonist of abscisic acid (ABA) signaling,16 which is required plant responses to drought,17 chilling,18 and high light.19 Therefore, it is possible that the negative effect of chloroplastic H2O2 on the chilling and high light responses is at least partially due to inhibition of ABA signaling by SA accumulation. Interestingly, comparison of the data from IS-tAPX plants and CAT2-defective mutants suggests a functional difference between peroxisomal and chloroplastic H2O2 in regulating the SA response. Analysis of double mutants of IS-tAPX and SA biosynthesis/signaling would reveal the role for the ICS2 pathway in the chloroplastic H2O2-mediated stress response, and the physiological significance of source-specific H2O2 signaling pathways.
Acknowledgments
This work was supported by Grants-in-aid for Scientific Research (A) (S.S.: 22248042) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and in-part by the Strategic Project to Support the Formation of Research Bases at Private Universities: Matching Fund Subsidy from MEXT, 2011–2015 (S1101035).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20906
References
- 1.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–8. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 3.Foyer CH, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100. doi: 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–9. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, et al. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006;141:436–45. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asada K. THE WATER-WATER CYCLE IN CHLOROPLASTS: Scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–39. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 7.Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S. Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 2002;32:915–25. doi: 10.1046/j.1365-313X.2002.01476.x. [DOI] [PubMed] [Google Scholar]
- 8.Maruta T, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, et al. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010;51:190–200. doi: 10.1093/pcp/pcp177. [DOI] [PubMed] [Google Scholar]
- 9.Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, et al. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem. 2012;287:11717–29. doi: 10.1074/jbc.M111.292847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loake G, Grant M. Salicylic acid in plant defence--the players and protagonists. Curr Opin Plant Biol. 2007;10:466–72. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Vlot AC, Dempsey DA, Klessig DF. Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 12.Garcion C, Lohmann A, Lamodière E, Catinot J, Buchala A, Doermann P, et al. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 2008;147:1279–87. doi: 10.1104/pp.108.119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–5. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 14.Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, et al. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007;52:640–57. doi: 10.1111/j.1365-313X.2007.03263.x. [DOI] [PubMed] [Google Scholar]
- 15.Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, Langlois-Meurinne M, et al. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol. 2010;153:1692–705. doi: 10.1104/pp.110.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, et al. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell. 2008;20:1678–92. doi: 10.1105/tpc.107.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, et al. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell. 2005;17:3470–88. doi: 10.1105/tpc.105.035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang SD, Seo PJ, Yoon HK, Park CM. The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell. 2011;23:2155–68. doi: 10.1105/tpc.111.084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, Truman W, Smirnoff N, et al. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell. 2009;21:2143–62. doi: 10.1105/tpc.108.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]


