Abstract
Hormones exert pleiotropic effects on plant growth and development throughout the life cycle. Many of these effects are mediated at molecular level via altering gene expression. In this study, we investigated the exogenous effect of plant hormones, including auxin, cytokinin, abscisic acid, ethylene, salicylic acid and jasmonic acid, on the transcription of rice genes at whole genome level using microarray. Our analysis identified a total of 4171 genes involved in several biological processes, whose expression was altered significantly in the presence of different hormones. Further, 28% of these genes exhibited overlapping transcriptional responses in the presence of any two hormones, indicating crosstalk among plant hormones. In addition, we identified genes showing only a particular hormone-specific response, which can be used as hormone-specific markers. The results of this study will facilitate further studies in hormone biology in rice.
Keywords: gene expression, hormones, microarray, rice (Oryza sativa), transcriptional response
Introduction
Hormones govern almost every aspect of plant life cycle from germination to seed development. Several molecular genetics studies have demonstrated the crucial role of various hormones in plant growth and development processes. The analyses of mutants with altered hormone biosynthesis and signaling have revealed the role of auxin, brassinosteroid, cytokinin and gibberellin in cell expansion along the longitudinal axes and ethylene along the transverse axes. Abscisic acid acts antagonistically on plant growth and is majorly involved in abiotic stress responses. Jasmonic acid and salicylic acid are involved in plant defense responses against pathogens.
Our knowledge about the molecular mechanisms underlying hormone perception and signal transduction has increased greatly during the past decade.1 Several novel components involved in the hormone signal transduction pathways have been identified. The transcriptional responses of plant hormones have been analyzed at individual gene, gene family and whole genome levels in many plant species.2-11 Based on gene expression and genetic studies in loss-of-function mutants, several genes involved in common signaling pathways of two or more hormones have also been identified and extensive crosstalk and signal integration has been uncovered.12-15 Various genetic analyses suggested that hormones work via distinct pathways to elicit their responses.12 However, it has also been proposed that plant hormones regulate similar biological processes via different signaling pathways.6,16
Most of our knowledge in hormone biology is based on the work done in Arabidopsis. Only a few studies have been performed to show the effect of different hormones at whole genome level in crop plant rice.8,17-19 Further, the comparison of transcriptional responses of different plant hormones is also not available in rice. In this study, we performed microarray analysis in rice to study the effect of different hormones, including auxin, cytokinin, abscisic acid, ethylene, salicylic acid and jasmonic acid, on gene expression at whole genome level. We further examined the overlap in the transcriptional responses to different hormones and identified several hormone-specific marker genes.
Results and Discussion
Plant hormones modulate global gene expression
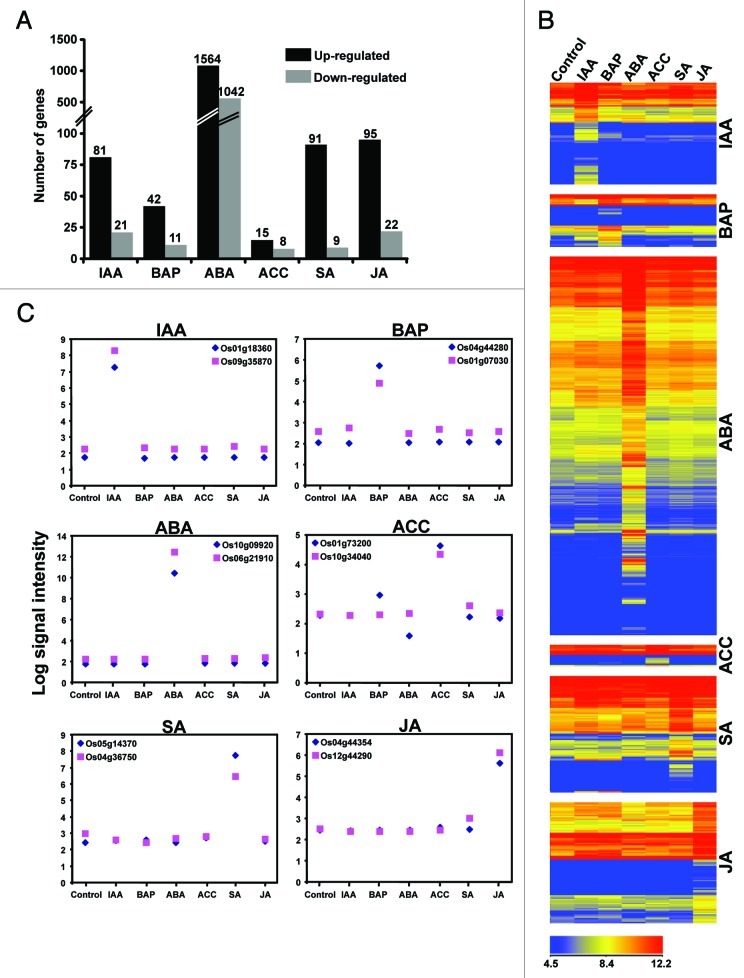
Plant hormones control various cellular processes by regulating the expression of several genes. To reveal the transcriptional responses of different hormones in rice, microarray analysis was performed with total RNA isolated from seedlings treated exogenously with various plant hormones involved in development and stress responses as per interest of our laboratory, including auxin (IAA), cytokinin (BAP), abscisic acid (ABA), ethylene derivative (ACC), salicylic acid (SA) and jasmonic acid (JA), using Affymetrix rice whole genome arrays. The microarray data are available at Gene Expression Omnibus database at NCBI under the series accession number GSE37557. The microarray data analysis identified a total of 4171 genes differentially expressed significantly (≥ 2-fold with at least 0.01 P-value) under at least one hormone condition tested. The list of all these genes with fold-change and P-value under different hormone conditions is given in Supplementary Table S1. Among these, 428, 279, 3635, 183, 798 and 615 genes were differentially expressed on IAA, BAP, ABA, ACC, SA and JA application, respectively (Fig. 1A). The results indicate that ABA altered the expression of largest number of genes, while ACC least number of genes under our experimental conditions. In each case, the number of upregulated genes was higher than the number of downregulated genes.
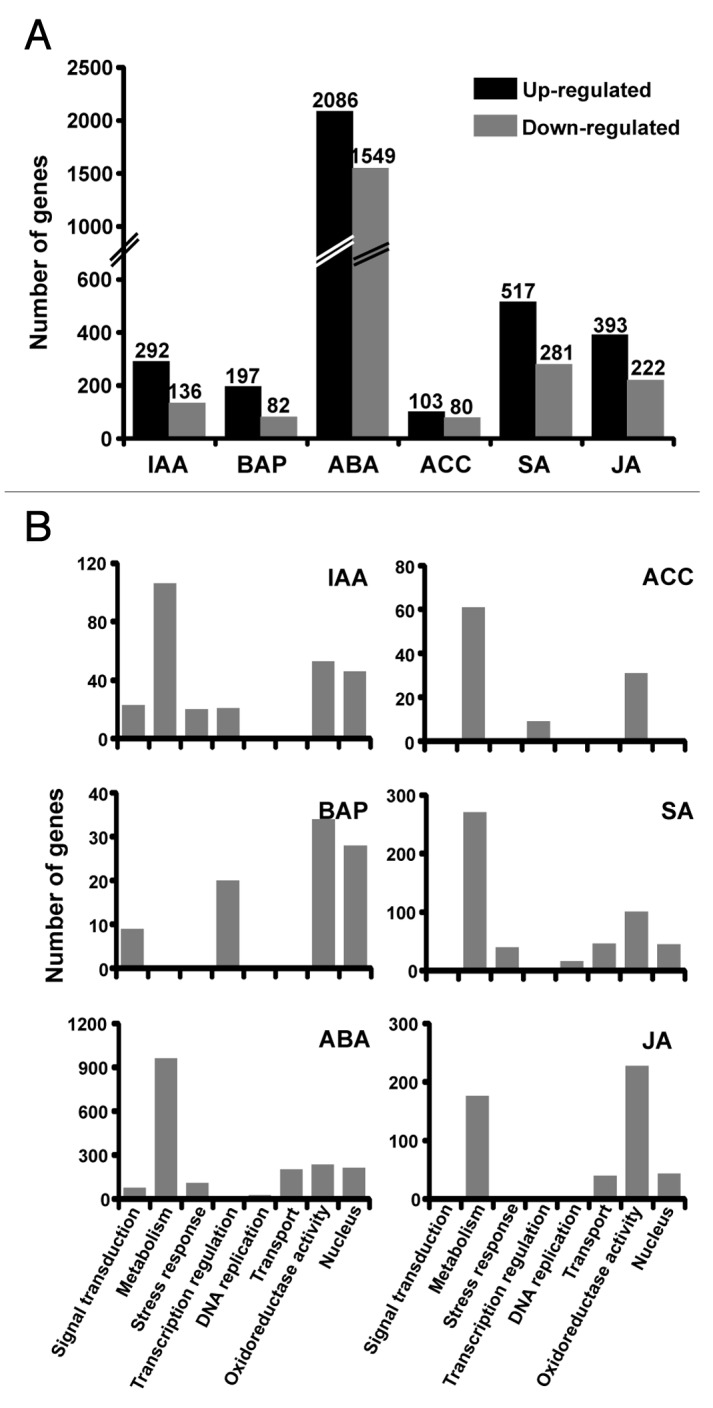
Figure 1. Differential expression of rice genes in the presence of different plant hormones. (A) Number of genes significantly (at least 2-fold with P-value ≤ 0.01) up- and downregulated in the presence of different plant hormones is shown. (B) The representative significantly enriched gene ontology (GO) categories in the genes showing differential expression are given. Only the data of significantly enriched (P-value < 0.001) GO categories are shown. IAA, auxin; BAP, cytokinin; ABA, abscisic acid; ACC, ethylene derivative; SA, salicylic acid; JA, jasmonic acid.
To gain insights into the putative functions of hormone-responsive genes, their annotation was explored from the Rice Genome Annotation Project database (http://rice.plantbiology.msu.edu/). Several genes well-known to be induced by different hormones were represented in our list of hormone-responsive genes. For example, Aux/IAA and GH3 gene family members, type-A response regulators and several stress-responsive genes were included in the IAA-, BAP- and ABA-induced gene lists, respectively. These results confirmed the reliability of our microarray experiments. The analysis revealed that genes involved in various cellular processes and pathways were regulated by different plant hormones. Several genes encoding for transcription factors belonging to different families were also represented in the hormone-responsive genes, indicating the regulatory role of plant hormones in transcription. Further, we analyzed various gene ontology (GO) categories enriched in the hormone-responsive genes (Fig. 1B). Some GO categories were enriched in all/most of the hormone-responsive genes and others were enriched under two or more hormone conditions. For example, the molecular function category, oxidoreductase activity, was highly enriched in all the hormone-responsive genes. Likewise, genes involved in metabolic processes and localized in nucleus were also enriched in most of the hormone-responsive gene lists. The genes related to stress response were found to be enriched on ABA, IAA and SA application as expected. Overall, these results suggest that different hormones largely regulate similar cellular processes in rice. Similar results have been obtained based on microarray analysis in Arabidopsis.6,16
Overlapping transcriptional responses of hormones
Many studies have revealed crosstalk among different plant hormones, which suggest that they might regulate the expression of similar set of genes. Therefore, to analyze the overlapping transcriptional responses of different hormones, we identified the genes responsive to one or more hormones. The largest fraction (72%) of genes showed response to specific hormone (Fig. 2A). A smaller fraction (28%) of genes was responsive to two or more hormones. Only four genes showed response to all the hormones tested in the study. These results indicate that plant hormone may work independently or coordinately to regulate plant growth and development. Further, we analyzed the overlap of genes regulated by any two hormones (Fig. 2B). As indicated by above analysis, a smaller fraction of genes showed overlapping transcriptional response of any two hormones. We observed that a larger fraction of the genes regulated by any two hormones exhibit similar (upregulated or downregulated in both) response as compared with the opposite (upregulated in one and downregulated in the other) response (Fig. 2C, Table S2). Most significantly, 60% (256) of the auxin-responsive genes showed response to stress hormone ABA and a significant fraction with SA (38%) and JA (29%) as well. Further, among the genes commonly regulated by IAA and SA, and IAA and ABA, 91% and 81% genes, respectively, exhibited similar responses. Although auxin has been implicated mainly in plant growth and developmental processes, some recent evidences suggest its crucial role in stress responses as well.20-23 In our previous studies, we also found several auxin-responsive genes regulated by abiotic and biotic stresses also.8,24 These results suggest that auxin might be acting coordinately with other hormones to regulate stress responses. We observed a significant overlap between the transcriptional responses of BAP and ABA, ABA and SA, ABA and JA, and JA and SA also. More than 65% of the gene commonly regulated by SA/JA with other hormones (except for BAP) showed similar response. However, the larger fraction (58%) of genes regulated by IAA and ACC showed opposite response as compared with the fraction of genes (42%) which exhibited similar response. Likewise, 64% of the genes regulated by both BAP and SA showed opposite regulation as compared with 36% genes showing similar regulation. These results indicate both similar and antagonistic effects of different hormones.
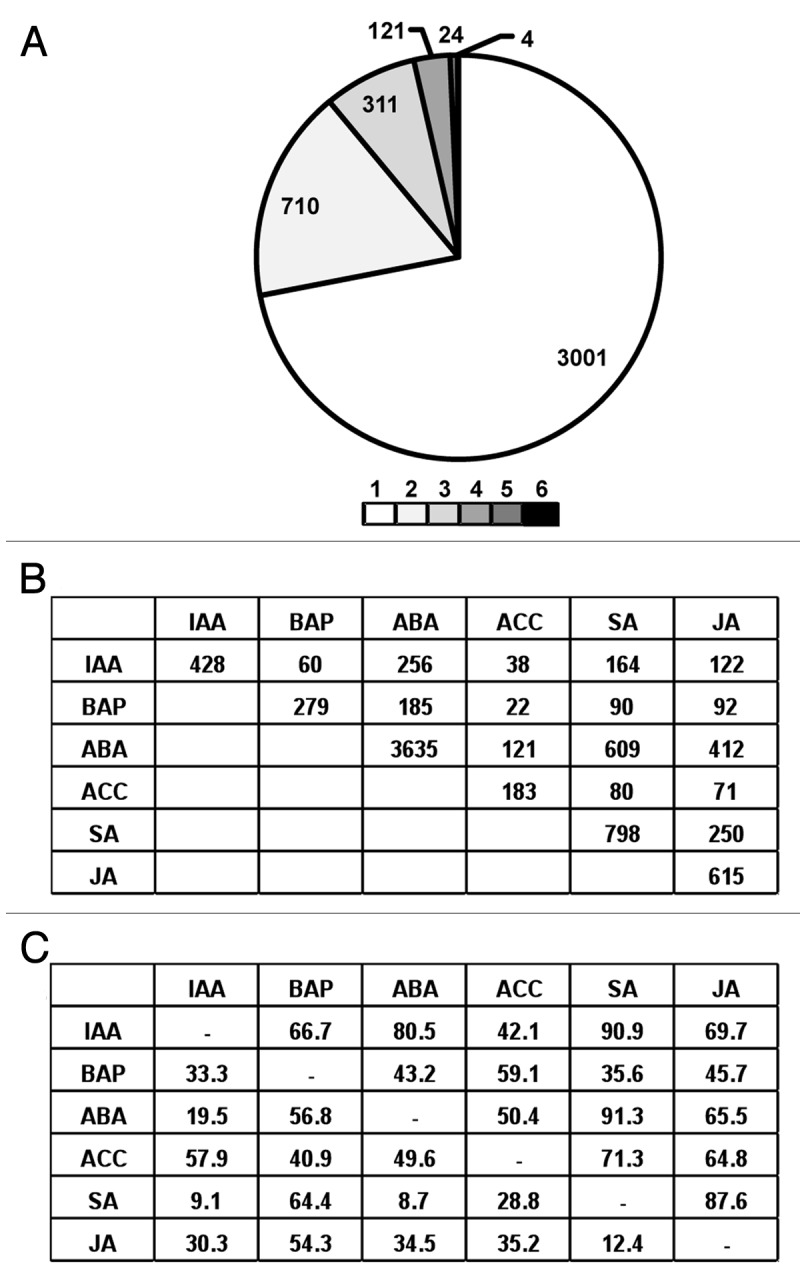
Figure 2. Overlapping transcriptional responses of different plant hormones. (A) Distribution of 4171 genes affected by one or more (up to six) hormones is shown. (B) The number of rice genes commonly regulated by any two hormones is indicated. (C) The percentages of rice genes regulated by any two hormones showing similar regulation (up- or downregulated by both hormones) and opposite regulation (upregulated by one hormone and downregulated by other hormones) are indicated on the upper (above the blank cells) and lower (below the blank cells) side of the table, respectively. Hormone abbreviations are same as given in Figure 1.
Many evidences are available to show the crosstalk among different hormones and have been reviewed comprehensively.12-15,25,26 The interactions between auxin and cytokinin,27 auxin and ABA,28,29 auxin and SA,23 auxin and JA,21 ethylene and ABA,30 ethylene and cytokinin,31 and JA and SA,32 are well investigated and have been proposed to work coordinately or antagonistically to regulate different plant responses. However, the exact molecular mechanisms underlying the similar and antagonistic responses of different hormones remain to be understood. The overlapping transcriptional responses of different hormones revealed in this study also suggest a comprehensive crosstalk among them building a complex network to mutually regulate signaling pathways. However, based on microarray data analysis in Arabidopsis, it has also been suggested that different plant hormones regulate similar biological processes through largely non-overlapping transcriptional responses.6,16 Overall, the crosstalk among different plant hormones appears to be too complex than anticipated and further investigations are required.
Identification of hormone-specific marker genes
As mentioned earlier, a larger fraction of genes exhibited response to specific hormone condition only. Our further analysis identified sets of hormone-specific genes (Fig. 3A). The analysis revealed 72% of the ABA-responsive genes showed the specific response to ABA only. However, for other hormones, only 13–24% genes exhibited specific response. The heatmaps showing hierarchical clustering of expression profiles of all the hormone-specific genes have been shown in Figure 3B. The hormone-specific expression of representative genes has been depicted graphically in Figure 3C. The validation of expression profiles of 10 selected hormone-specific genes was done by real-time PCR analysis. The comparative analysis showed a very good agreement of the expression patterns revealed by microarray and real-time PCR analyses (Fig. S1). Many of these hormone-specific marker genes include well-characterized hormone-responsive genes and orthologs of hormone-specific markers genes identified in Arabidopsis,16 such as IAA-induced Aux/IAA, GH3 and SAUR genes (for example, Os05 g42150, Os01 g18360 and Os09 g37430), BAP-induced response regulator (Os04 g44280), ABA-induced carotenoid dioxygenase (Os07 g05940) and many stress-responsive genes and ACC-induced ethylene receptor (Os04 g08740). In addition, several hormone-specific genes have no predicted function and have not been associated with a hormone pathway as of now. These genes identified in this study might represent the novel components of the hormone signaling pathways. However, the presence of many more genes showing crosstalk among different hormones and the genes showing hormone-specific response other than reported in this study cannot be ruled out, because the changes in gene expression might be highly specific to the developmental stage, tissue-type and/or cell-type being analyzed. These genes specifically regulated by only one hormone application can be used as hormone-specific marker genes in hormone related studies in rice.
Figure 3. Rice genes specifically regulated by single hormone. (A) Number of genes significantly up- and downregulated only in the presence of specific plant hormone is shown. (B) Heatmap showing the expression profiles of genes specifically regulated by single hormone. The color scale at the bottom represents average log signal intensity values. (C) Expression patterns of representative hormone-specific marker genes. Hormone abbreviations are same as given in Figure 1.
In conclusion, we have identified genes responsive to several plant hormones in rice followed by their overlapping transcriptional responses and hormone-specific marker genes. The analysis and results presented in the study will provide a resource to facilitate further studies in the area of hormone biology, particularly in rice. The integration of these data with further experiments will help understanding the effect of different hormones on plant growth and development.
Materials and Methods
Plant material and hormone treatments
Rice (Oryza sativa subspecies indica variety IR64) seeds were treated and grown as described previously.2 Different hormone treatments were given to the 7-d-old light-grown rice seedlings as described.33 The seedlings were transferred to the beakers containing 50 µM solution of indole-3-acetic acid (auxin) and benzyl aminopurine (cytokinin) and 100 µM solution of abscisic acid, 1-aminocyclopropane-1-carboxylic acid (ethylene derivative), salicylic acid and jasmonic acid for hormone treatments. The seedlings were kept in respective solutions for 3 h, at 28 ± 1°C before harvesting. The seedlings kept in water for 3 h, at 28 ± 1°C served as control. At least three independent biological replicates of each tissue sample were harvested.
Microarray hybridization and data analysis
Total RNA was isolated using TRI reagent followed by various quality controls.33 Microarray hybridizations were performed using Affymetrix GeneChip Rice Genome Arrays following the manufacturer’s protocol as described previously.34 Two independent biological replicates of each tissue sample were used for the microarray hybridization. After the microarray experiment, raw data for each sample was extracted as CEL files. The CEL files were imported into Genespring GX (version 11) software (Agilent Technologies) for further analysis. The Gene Chip Robust Multi Array (GCRMA) algorithm was used for normalization and probe summarization. The statistical analysis was performed using one-way ANOVA and Benjamini-Hoschberg multiple testing correction was applied to the data. Subsequently, differentially expressed genes showing a fold change of at least 2-fold with a P-value of at least 0.01 were identified for different hormone conditions as compared with the control. The tab delimited files for average log signal intensity and fold-change values were extracted for further analysis in Microsoft Excel. Gene ontology enrichment analysis was performed using Gene Ontology Enrichment Analysis Software Toolkit.35
Real-time PCR analysis
To validate the differential expression of selected genes, real-time PCR analysis was performed as described earlier33 using gene-specific primers. Three biological replicates of each tissue sample were used for real-time PCR analysis and PCR reactions for each biological replicate were performed in three technical replicates. The gene-specific primer sequences used in this study are given in Supplementary Table S3.
Supplementary Material
Acknowledgments
MJ acknowledges the financial support from the Department of Biotechnology, Government of India, New Delhi, under Innovative Young Biotechnologists Award scheme and core grant from NIPGR.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20910
References
- 1.Santner A, Estelle M. Recent advances and emerging trends in plant hormone signalling. Nature. 2009;459:1071–8. doi: 10.1038/nature08122. [DOI] [PubMed] [Google Scholar]
- 2.Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa) Funct Integr Genomics. 2006;6:47–59. doi: 10.1007/s10142-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 3.Jain M, Kaur N, Tyagi AK, Khurana JP. The auxin-responsive GH3 gene family in rice (Oryza sativa) Funct Integr Genomics. 2006;6:36–46. doi: 10.1007/s10142-005-0142-5. [DOI] [PubMed] [Google Scholar]
- 4.Jain M, Tyagi AK, Khurana JP. Molecular characterization and differential expression of cytokinin-responsive type-A response regulators in rice (Oryza sativa) BMC Plant Biol. 2006;6:1. doi: 10.1186/1471-2229-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S. Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2002;130:1319–34. doi: 10.1104/pp.011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goda H, Sasaki E, Akiyama K, Maruyama-Nakashita A, Nakabayashi K, Li W, et al. The AtGenExpress hormone and chemical treatment data set: experimental design, data evaluation, model data analysis and data access. Plant J. 2008;55:526–42. doi: 10.1111/j.1365-313X.2008.03510.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhong GY, Burns JK. Profiling ethylene-regulated gene expression in Arabidopsis thaliana by microarray analysis. Plant Mol Biol. 2003;53:117–31. doi: 10.1023/B:PLAN.0000009270.81977.ef. [DOI] [PubMed] [Google Scholar]
- 8.Jain M, Khurana JP. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J. 2009;276:3148–62. doi: 10.1111/j.1742-4658.2009.07033.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang JR, Hu H, Wang GH, Li J, Chen JY, Wu P. Expression of PIN genes in rice (Oryza sativa L.): tissue specificity and regulation by hormones. Mol Plant. 2009;2:823–31. doi: 10.1093/mp/ssp023. [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Jhanwar S, Tyagi AK, Jain M. Genome-wide survey and expression analysis suggest diverse roles of glutaredoxin gene family members during development and response to various stimuli in rice. DNA Res. 2010;17:353–67. doi: 10.1093/dnares/dsq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharoni AM, Nuruzzaman M, Satoh K, Moumeni A, Attia K, Venuprasad R, et al. Comparative transcriptome analysis of AP2/EREBP gene family under normal and hormone treatments, and under two drought stresses in NILs setup by Aday Selection and IR64. Mol Genet Genomics. 2012;287:1–19. doi: 10.1007/s00438-011-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzarrini S, McCourt P. Cross-talk in plant hormone signalling: what Arabidopsis mutants are telling us. Ann Bot. 2003;91:605–12. doi: 10.1093/aob/mcg064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Wei Y, Lu Y, Wang X. Mechanisms of brassinosteroids interacting with multiple hormones. Plant Signal Behav. 2009;4:1117–20. doi: 10.4161/psb.4.12.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaillais Y, Chory J. Unraveling the paradoxes of plant hormone signaling integration. Nat Struct Mol Biol. 2010;17:642–5. doi: 10.1038/nsmb0610-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Depuydt S, Hardtke CS. Hormone signalling crosstalk in plant growth regulation. Curr Biol. 2011;21:R365–73. doi: 10.1016/j.cub.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Nemhauser JL, Hong F, Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–75. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Yazaki J, Kishimoto N, Nagata Y, Ishikawa M, Fujii F, Hashimoto A, et al. Genomics approach to abscisic acid- and gibberellin-responsive genes in rice. DNA Res. 2003;10:249–61. doi: 10.1093/dnares/10.6.249. [DOI] [PubMed] [Google Scholar]
- 18.Jan A, Komatsu S. Functional characterization of gibberellin-regulated genes in rice using microarray system. Genomics Proteomics Bioinformatics. 2006;4:137–44. doi: 10.1016/S1672-0229(06)60026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirose N, Makita N, Kojima M, Kamada-Nobusada T, Sakakibara H. Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol. 2007;48:523–39. doi: 10.1093/pcp/pcm022. [DOI] [PubMed] [Google Scholar]
- 20.Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002;129:661–77. doi: 10.1104/pp.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell PJ, Schmelz EA, Moussatche P, Lund ST, Jones JB, Klee HJ. Susceptible to intolerance--a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J. 2003;33:245–57. doi: 10.1046/j.1365-313X.2003.01619.x. [DOI] [PubMed] [Google Scholar]
- 22.Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–9. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol. 2007;17:1784–90. doi: 10.1016/j.cub.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Ghanashyam C, Jain M. Role of auxin-responsive genes in biotic stress responses. Plant Signal Behav. 2009;4:846–8. doi: 10.4161/psb.4.9.9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An C, Mou Z. Salicylic acid and its function in plant immunity. J Integr Plant Biol. 2011;53:412–28. doi: 10.1111/j.1744-7909.2011.01043.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann M, Hentrich M, Pollmann S. Auxin-oxylipin crosstalk: relationship of antagonists. J Integr Plant Biol. 2011;53:429–45. doi: 10.1111/j.1744-7909.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 2006;45:1028–36. doi: 10.1111/j.1365-313X.2006.02656.x. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Kao CY, Cocciolone S, McCarty DR. Maize VP1 complements Arabidopsis abi3 and confers a novel ABA/auxin interaction in roots. Plant J. 2001;28:409–18. doi: 10.1046/j.1365-313X.2001.01165.x. [DOI] [PubMed] [Google Scholar]
- 29.Brady SM, Sarkar SF, Bonetta D, McCourt P. The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 2003;34:67–75. doi: 10.1046/j.1365-313X.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 30.Gazzarrini S, McCourt P. Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr Opin Plant Biol. 2001;4:387–91. doi: 10.1016/S1369-5266(00)00190-4. [DOI] [PubMed] [Google Scholar]
- 31.Cary AJ, Liu W, Howell SH. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995;107:1075–82. doi: 10.1104/pp.107.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert-Seilaniantz A, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–43. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- 33.Garg R, Sahoo A, Tyagi AK, Jain M. Validation of internal control genes for quantitative gene expression studies in chickpea (Cicer arietinum L.) Biochem Biophys Res Commun. 2010;396:283–8. doi: 10.1016/j.bbrc.2010.04.079. [DOI] [PubMed] [Google Scholar]
- 34.Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, Sharma P, et al. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007;143:1467–83. doi: 10.1104/pp.106.091900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Q, Wang XJ. GOEAST: a web-based software toolkit for Gene Ontology enrichment analysis. Nucleic Acids Res. 2008;36(Web Server issue):W358-63. doi: 10.1093/nar/gkn276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.