Abstract
Strigolactones (SL) and karrikins (KAR) both contain essential butenolide moieties, and both require the F-box protein MAX2 to control seed germination and photomorphogenesis in Arabidopsis thaliana. A new discovery that SL and KAR also require related α/β-hydrolase proteins for such activity suggests that they operate through a similar molecular mechanism. Based on structural similarity, a previously proposed mode of action for SL was also considered for KAR, but recent structure-activity studies suggest that this mechanism may not apply. Here we rationalise these observations into a hypothesis whereby different α/β-hydrolases distinguish SL and KAR by virtue of their non-butenolide moieties and catalyze nucleophilic attack on the butenolide. The products would be different for SL and KAR, and in the case of SL they have no biological activity. The inference is that nucleophilic attack on SL and KAR by α/β-hydrolases is required for their bioactivity, but the hydrolysis products are not.
Keywords: D14, KAI2, MAX2, butenolide, karrikin, strigolactone, α/β-hydrolase
KAR and SL Functions
Strigolactones (SL) exuded by roots stimulate the germination of seeds of parasitic plants and promote the formation of arbuscular mycorrhizae (AM).1,2 SL are also now recognized as important endogenous plant hormones that control seedling photomorphogenesis, shoot secondary thickening, shoot branching, lateral root growth and root hair formation.3-9 SL biosynthesis is stimulated by deficiency of soil phosphate and other nutrients, and therefore participates in the adaptation of root development and the formation of symbiotic associations to redress the deficiency.10,11 Karrikins (KAR) are found in smoke from burning vegetation.12,13 They contain a substituted butenolide moiety similar to SLs (Fig. 1), and they stimulate seed germination and control seedling photomorphogenesis, but they are not active in AM formation or shoot lateral bud outgrowth.14-16 Not only do SL and KAR exert similar effects on seeds and seedlings, but each acts through the same F-box protein (MAX2 in Arabidopsis) and members of a small α/β-hydrolase family.17,18 These observations tell us that SL and KAR have the same fundamental molecular mode of action. However, SL and KAR can be distinguished by different members of the α/β-hydrolase family in Arabidopsis,18 and seeds and seedlings of different species respond preferentially to either SL or KAR.15 These observations imply that specific proteins can discriminate between SL and KAR.
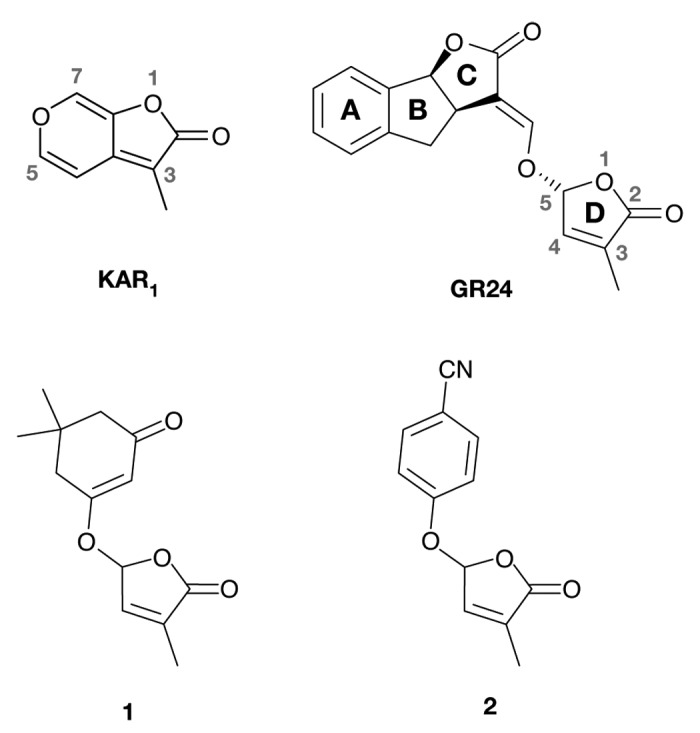
Figure 1. Karrikin (KAR1) and active strigolactone analogs. Natural SLs have a four-ring structure exemplified here by the artificial SL GR24. The D-ring (butenolide moiety) is essential for activity of all SL analogs. Small numbers identify atoms mentioned in the text. Compounds 1 and 2 were originally described by Mwakaboko and Zwanenburg24 and Fukai et al.23 respectively.
SL Structure-Activity Relationships
There have been several decades of research to elucidate the structure-activity relationships (SAR) of SL.10,19 This has been driven in large part by the goal of creating cheap and effective synthetic analogs that could be used in agriculture to stimulate the suicidal germination of seeds of parasitic species within the Orobanchaceae.10,19 This research has led to the invariant conclusion that the butenolide D-ring is essential for SL activity.19 In early research on the reactivity of the SL analog GR24, Zwanenburg and colleagues19-21 demonstrated addition of a nucleophile in a Michael fashion to the enol-ether moiety bridging the ABC and D rings, resulting in the attachment of the nucleophile to the ABC moiety while releasing the butenolide D-ring (Fig. 2, mechanism 1). They proposed that SLs could mediate their effects through such a reaction. However more recent studies of Zwanenberg and Mwakaboko22 and Fukui et al.23 show that the enol-ether functionality is not required for SL activity. Analogues 1 and 2 (Fig. 1) lack the α/β-unsaturated functionality, but 1 is active in seed germination of Striga hermonthica and Orobanche cernua,24 while 2 is active in repression of shoot branching in the rice d10–1 mutant.23 These new analogs, in common with other SL, are characterized by an effective leaving group at C5 of the D-ring. Zwanenburg and Mwakaboko22 therefore proposed an alternative Michael addition (Fig. 2, mechanism 2) based specifically on nucleophilic attack at C4 of the D-ring, resulting in the release of the leaving group at C5 (i.e., the ABC rings of natural SL).
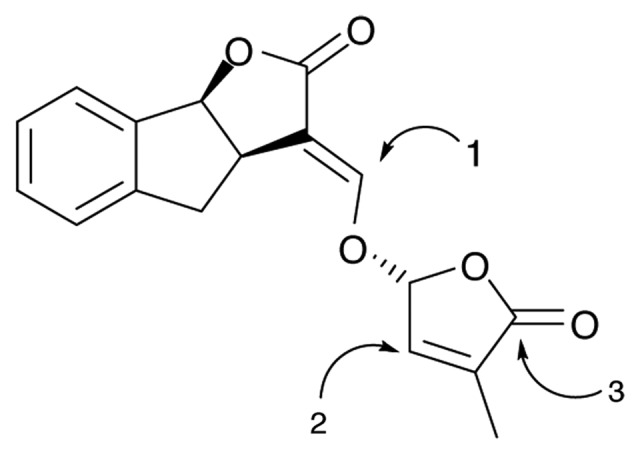
Figure 2. Potential nucleophilic attack on the artificial SL GR24. Mechanisms indicated by 1 and 2 were proposed by Zwanenburg and colleagues;22,32 mechanism 3 was proposed by Scaffidi et al.27 Attack at any of the three C atoms would be predicted to result in separation of the ABC rings from the D ring.
KAR Structure-Activity Relationships
In parallel there has been extensive research into SAR of KAR as a germination stimulant in smoke-responsive species including Solanum orbiculatum and Lactuca sativa (cv Grand Rapids). This work has led to the conclusion that while the pyran moiety is important, it can tolerate slight modification, particularly to the C5 position as well as conservative substitutions such as replacement of O6 with N.25,26 A new study now shows that while the pyran ring can undergo a Michael addition at C5, partial or complete saturation of the pyran moiety reduces but does not eliminate activity of the molecule, implying that KAR action does not proceed through a Michael addition at C5 of the pyran.27 Instead Scaffidi et al.27 propose that the pyran moiety creates an electronic effect that renders the butenolide susceptible to nucleophilic attack at the carbonyl group. Since a single α/β-hydrolase (KAI2) is active with both KAR and SL in Arabidopsis at least,18 we propose that the molecular mode of action of both types of molecule involves nucleophilic attack on the common carbonyl group of the butenolide (Fig. 2, mechanism 3), followed by hydrolysis of the resulting intermediate.27 Such a mechanism operating on SL will liberate 5-hydroxy-3-methyl-butenolide and release of the ABC rings (or other leaving group in the case of other active analogs). However, in the case of KAR, the pyran moiety does not function as a leaving group,27 and therefore it is proposed that the product of hydrolase activity on KAR will spontaneously eliminate water to recreate the original KAR molecule. Together with the fact that the D-ring structure alone is not active as a SL,14,19 we conclude that the process of nucleophilic attack on the butenolide of SL and KAR is essential for activity, rather than the products of the reaction.
The Role of α/β-Hydrolase Family Members
In Arabidopsis the α/β-hydrolase protein family participating in SL and KAR action comprises three members: AtD14, KAI2 and DLK2.18 The AtD14 gene is the ortholog of the rice OsD14 gene, and this gene is required for the SL-mediated inhibition of lateral shoot outgrowth in both species.17,18 KAR is not active in the control of shoot branching in Arabidopsis or pea,16 suggesting that AtD14 can distinguish KAR from SL. In contrast, the AtD14 paralogue KAI2 is preferentially expressed in seeds and seedlings where it functions with either SL or KAR to stimulate germination and photomorphogenesis.18 The function of DLK2, more closely related to D14 than KAI2, is currently unknown. While there is no evidence as yet that these α/β-hydrolases mediate SL responses in parasitic weeds, in Arabidopsis it appears that all responses to KAR and SL operate through AtD14 and/or KAI2, as the Atd14 kai2 double mutant phenocopies the max2 mutant and is insensitive to both classes of compound.18
The discovery that compound 2 is active in shoot repression in rice but not in germination of parasitic weed seeds23 led us to question if it is active in Arabidopsis seedlings and whether it acts through AtD14 or KAI2. We have developed a convenient bioassay for the activity of KAR, SL and their analogs based on the inhibition of hypocotyl elongation.7 This system has the advantage of comparing the relative contribution of AtD14 and KAI2 in the response to each compound in a single assay system, but future research will be needed to extend these findings to parasitic plant seed germination. Seedlings were grown for four days in red light in the presence of 1 µM KAR1, 1 µM GR24, or 1 µM 2, and hypocotyl lengths were measured (Fig. 3). GR24 and 2 were equally effective in repression of hypocotyl growth in Ler and Col ecotypes, but were completely inactive in max2 as expected (Fig. 3). GR24 and 2 were both partially active in kai2 and d14 mutants although 2 was much less effective than GR24 in kai2 seedlings. All molecules were inactive in a kai2 d14 double mutant. We conclude that the SL mimic 2 is very active in Arabidopsis seedlings and, like KAR, acts preferentially through KAI2 rather than D14. However, unlike KAR it can also operate through either protein in a partially redundant manner, as does GR24.18 The fact that 2 is a potent shoot branching inhibitor in rice suggests that 2 is an effective SL analog that operates via D14; however, at the same time, 2 is a relatively weak germination stimulant compared with GR24 in Striga hermonthica.23 This apparent contradiction may indicate that parasitic weeds have evolved highly specific responses to natural SLs, while D14-dependent inhibition of branching in rice is a less discriminatory process. Accordingly, direct comparisons between species and experimental systems of how each protein may mediate activity are not straightforward. Nevertheless, in Arabidopsis seedlings at least, KAR, SL and their analogs such as 2 and GR24 are all active via KAI2. This protein now provides a focal point for the elucidation of the mode of action of these butenolides.
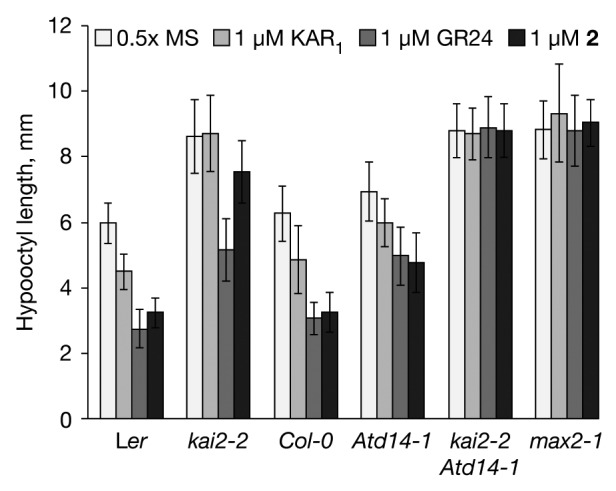
Figure 3. Butenolide-mediated inhibition of Arabidopsis hypocotyl growth is dependent on KAI2 and AtD14. Seedlings were grown under red light for four days on 0.5x MS medium supplemented with 1 µM KAR1, 1 µM GR24 or 1 µM 2. Seedlings were photographed and hypocotyls measured using ImageJ software. Data are means ± st. dev. (n = 20 seedlings per sample).
Proposed Mode of Action
The action mechanism of α/β-hydrolases typically involves an Asp-His-Ser catalytic triad that creates an electron relay system to establish the serine oxygen as a strong nucleophile.28,29 We therefore hypothesize that SL and KAR mode of action involves nucleophilic attack by the serine oxygen on the butenolide carbonyl group. Modeling of the KAI2 protein structure against that of RsbQ30 suggests that KAR is readily accommodated in its active site with the butenolide and serine moieties juxtaposed.27 In order to trigger an intracellular signal we speculate that this reaction could induce a conformational change in KAI2, analogous to the conformational change induced by gibberellin binding to its receptor GID1, also an α/β-hydrolase-like protein.31 This hypothesis is now amenable to testing. A striking conclusion from this proposed mode of action is that SL molecules would be destroyed by hydrolysis whereas KAR molecules would not.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Rohan Bythell-Douglas, Kelly Sun, Emilio Ghisalberti and Charlie Bond (UWA) for valuable discussion. We thank two reviewers for constructive suggestions on improving the manuscript. This project was supported by the Australian Research Council [DP0880484, LP0882775 and DP1096717].
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20977
References
- 1.Akiyama K, Matsuzaki K-i, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–7. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 2.Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science. 1966;154:1189–90. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- 3.Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci U S A. 2011;108:20242–7. doi: 10.1073/pnas.1111902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–94. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 5.Kapulnik Y, Delaux P-M, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011;233:209–16. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- 6.Koltai H. Strigolactones are regulators of root development. New Phytol. 2011;190:545–9. doi: 10.1111/j.1469-8137.2011.03678.x. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DC, Flematti GR, Riseborough J-A, Ghisalberti EL, Dixon KW, Smith SM. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2010;107:7095–100. doi: 10.1073/pnas.0911635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen A, Mason MG, De Cuyper C, Brewer PB, Herold S, Agusti J, et al. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol. 2012;158:1976–87. doi: 10.1104/pp.111.187104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 10.Xie X, Yoneyama K, Yoneyama K. The strigolactone story. Annu Rev Phytopathol. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- 11.Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007;225:1031–8. doi: 10.1007/s00425-006-0410-1. [DOI] [PubMed] [Google Scholar]
- 12.Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. Identification of alkyl substituted 2H-furo[2,3-c]pyran-2-ones as germination stimulants present in smoke. J Agric Food Chem. 2009;57:9475–80. doi: 10.1021/jf9028128. [DOI] [PubMed] [Google Scholar]
- 13.Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. A compound from smoke that promotes seed germination. Science. 2004;305:977. doi: 10.1126/science.1099944. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama K, Ogasawara S, Ito S, Hayashi H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010;51:1104–17. doi: 10.1093/pcp/pcq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson DC, Flematti GR, Ghisalberti EL, Dixon KW, Smith SM. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu Rev Plant Biol. 2012;63:107–30. doi: 10.1146/annurev-arplant-042811-105545. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DC, Scaffidi A, Dun EA, Waters MT, Flematti GR, Dixon KW, et al. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2011;108:8897–902. doi: 10.1073/pnas.1100987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, et al. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50:1416–24. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- 18.Waters MT, Nelson DC, Scaffidi A, Flematti GR, Sun YK, Dixon KW, et al. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development. 2012;139:1285–95. doi: 10.1242/dev.074567. [DOI] [PubMed] [Google Scholar]
- 19.Zwanenburg B, Mwakaboko AS, Reizelman A, Anilkumar G, Sethumadhavan D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag Sci. 2009;65:478–91. doi: 10.1002/ps.1706. [DOI] [PubMed] [Google Scholar]
- 20.Mangnus E, Zwanenburg B. Tentative molecular mechanism for germination stimulation of striga and orobanche seeds by strigol and its synthetic analogs. J Agric Food Chem. 1992;40:1066–70. doi: 10.1021/jf00018a032. [DOI] [Google Scholar]
- 21.Wigchert SC, Zwanenburg B. A critical account on the inception of Striga seed germination. J Agric Food Chem. 1999;47:1320–5. doi: 10.1021/jf980926e. [DOI] [PubMed] [Google Scholar]
- 22.Zwanenburg B, Mwakaboko AS. Strigolactone analogues and mimics derived from phthalimide, saccharine, p-tolylmalondialdehyde, benzoic and salicylic acid as scaffolds. Bioorg Med Chem. 2011;19:7394–400. doi: 10.1016/j.bmc.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 23.Fukui K, Ito S, Ueno K, Yamaguchi S, Kyozuka J, Asami T. New branching inhibitors and their potential as strigolactone mimics in rice. Bioorg Med Chem Lett. 2011;21:4905–8. doi: 10.1016/j.bmcl.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Mwakaboko AS, Zwanenburg B. Single step synthesis of strigolactone analogues from cyclic keto enols, germination stimulants for seeds of parasitic weeds. Bioorg Med Chem. 2011;19:5006–11. doi: 10.1016/j.bmc.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 25.Flematti GR, Goddard-Borger ED, Merritt DJ, Ghisalberti EL, Dixon KW, Trengove RD. Preparation of 2H-furo[2,3-c]pyran-2-one derivatives and evaluation of their germination-promoting activity. J Agric Food Chem. 2007;55:2189–94. doi: 10.1021/jf0633241. [DOI] [PubMed] [Google Scholar]
- 26.Flematti GR, Scaffidi A, Goddard-Borger ED, Heath CH, Nelson DC, Commander LE, et al. Structure-activity relationship of karrikin germination stimulants. J Agric Food Chem. 2010;58:8612–7. doi: 10.1021/jf101690a. [DOI] [PubMed] [Google Scholar]
- 27.Scaffidi A, Waters MT, Bond CS, Dixon KW, Smith SM, Ghisalberti EL, et al. Exploring the molecular mechanism of karrikins and strigolactones. Bioorg Med Chem Lett. 2012;22:3743–6. doi: 10.1016/j.bmcl.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, et al. The α/β hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 29.Bugg TDH. Diverse catalytic activities in the alphabeta-hydrolase family of enzymes: activation of H2O, HCN, H2O2, and O2. Bioorg Chem. 2004;32:367–75. doi: 10.1016/j.bioorg.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko T, Tanaka N, Kumasaka T. Crystal structures of RsbQ, a stress-response regulator in Bacillus subtilis. Protein Sci. 2005;14:558–65. doi: 10.1110/ps.041170005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–8. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 32.Mangnus E. LA V, Vandenput D, Zwanenburg B. Structural modifications of strigol analogs - influence of the B and C rings on the bioactivity of the germination stimulant GR24. J Agric Food Chem. 1992;40:1222–9. doi: 10.1021/jf00019a030. [DOI] [Google Scholar]


