Abstract
Elicitins are a family of small proteins secreted by Phytophthora species that have a high degree of homology and elicit defense reactions in tobacco (Nicotiana tabacum). They display acidic or basic characteristics, the acidic elicitins being less efficient in inducing plant necrosis. In this study we compared the binding properties of four elicitins (two basic and two acidic) and early-induced signal transduction events (Ca2+ influx, extracellular medium alkalinization, and active oxygen species production). The affinity for tobacco plasma membrane-binding sites and the number of binding sites were similar for all four elicitins. Furthermore, elicitins compete with one another for binding sites, suggesting that they interact with the same receptor. The four elicitins induced Ca2+ influx, extracellular medium alkalinization, and the production of active oxygen species in tobacco cell suspensions, but the intensity and kinetics of these effects were different from one elicitin to another. As a general observation the concentrations that induce similar levels of biological activities were lower for basic elicitins (with the exception of cinnamomin-induced Ca2+ uptake). The qualitative similarity of early events induced by elicitins indicates a common transduction scheme, whereas fine signal transduction tuning is different in each elicitin.
Elicitins are a family of proteins excreted by Phytophthora spp., a group of widespread and highly damaging pathogenic fungi (Ricci et al., 1993; Yu, 1995). These proteins have the property to elicit defense responses in tobacco (Nicotiana tabacum), triggering a hypersensitive response and systemic resistance to further inoculation (Bonnet et al., 1996; Keller et al., 1996). These 98 amino acid proteins show more than 60% sequence identity with each other. They are devoid of posttranslational modifications and possess six invariant Cys residues that account for three disulfide bridges, which were first established in capsicein, an elicitin secreted by Phytophthora capsici (Bouaziz et al., 1994).
Circular dichroism experiments performed on four elicitins, cryptogein, cinnamomin, parasiticein, and capsicein, together with capsicein structure analysis by NMR, led to the conclusion that the elicitin folding was mainly α-helical (Nespoulous et al., 1992; Bouaziz et al., 1994). A recent study (Boissy et al., 1996) determined the crystal structure of cryptogein, confirming the existence of three disulfide bridges. These authors proposed a globular structure composed of six α-helices and a beak-like motif built from invariant residues, forming an antiparallel, two-stranded β-sheet and an Ω-loop. However, beyond these general structural features, elicitins have long been classified into two groups according to their differences in net charge, the acidic or α-elicitins (pI between 3 and 5) and the basic or β-elicitins (pI between 7 and 9).
This classification correlates with the biological properties of elicitins in tobacco plants. The amount of basic elicitins required to induce leaf necrosis or protection against Phytophthora parasitica var nicotianae is 50- to 100-fold lower than for acidic elicitins (Ricci et al., 1989; Nespoulous et al., 1992; Pernollet et al., 1993; Bonnet et al., 1996). Necrotic activity was not the result of different migration rates or elicitin accumulation, since 125I-labeled elicitins (the basic cryptogein and the acidic capsicein) showed the same distribution within the plant (Devergne et al., 1992; Zanetti et al., 1992). Instead, this activity should depend on the high number of Lys residues present in basic elicitins, which contributed to their net charge. Particularly the mutation of Lys-13 resulted in a decrease in necrotic activity (O'Donohue et al., 1995).
The difference in biological activity between α- and β-elicitins, which is associated with their structural properties, might be correlated with one or several steps of the signal transduction pathway induced in the target cells. It is generally assumed that a putative receptor on the plasma membrane acts as the first component of the transduction pathway, and then secondary messengers, membrane proteins, or cytosolic proteins such as protein kinases transduce the signal to activate plant-defense responses (Staskawicz et al., 1995). Perception of elicitins by the target cell and transduction of this signal should direct the physiological effects observed at the plant level, emphasizing the importance of studying the initial steps of the transduction pathway.
Among elicitins cryptogein has been extensively studied, and early steps of its interaction with tobacco cells have been defined. Cryptogein high-affinity binding sites characterized on tobacco plasma membrane preparations had properties consistent with receptor sites (Wendehenne et al., 1995). Analysis of early events induced by this elicitin were performed on tobacco cell suspensions. Ca2+ influx (Tavernier et al., 1995b), modifications of ion fluxes (H+, K+, and Cl−; Blein et al., 1991; Pugin et al., 1997), depolarization of the plasma membrane (Pugin et al., 1997), production of AOS (Bottin et al., 1994), protein phosphorylation (Viard et al., 1994), cytosol acidification (Barbier-Brygoo et al., 1997; Pugin et al., 1997), and changes in gene expression (Suty et al., 1996; Petitot et al., 1997) were rapidly monitored after a few minutes of cryptogein treatment. Later, changes in lipid composition (Tavernier et al., 1995a) and the production of phytoalexin and ethylene were measured (Milat et al., 1991).
Protein phosphorylation and Ca2+ influx were the earliest effects monitored after cryptogein-tobacco cell interaction. Similar early effects were reported in other elicitor-plant cell interactions. Tomato cells treated with a yeast-extract-derived elicitor, a fungal xylanase elicitor, or chitin fragments produced an increase in extracellular pH and rapid changes in protein phosphorylation (Felix et al., 1991, 1994; Baureithel et al., 1994). Changes in the permeability of the plasma membrane to different ions (H+, Ca2+, K+, and Cl−) were among the earliest events detectable after treatment of parsley cells with a 42-kD glycoprotein from Phytophthora megasperma (Nürnberger et al., 1994) or after treatment of tobacco cells with Pseudomonas syringae pv syringae (Atkinson et al., 1990). Tobacco cells incubated with oligogalacturonides developed rapid cytoplasmic acidification and extracellular pH alkalinization, which depend on a phosphorylation process (Mathieu et al., 1996a, 1996b). AOS production, which is often correlated with plant defense reactions, is triggered by elicitors from different organisms (Medhy, 1994) and is probably coupled to ion flux modifications (Baker et al., 1993; Tavernier et al., 1995b). In cryptogein-treated tobacco cells, AOS production depends on Ca2+ influx. La3+, a Ca2+-channel blocker, inhibited extracellular alkalinization and AOS production (Tavernier et al., 1995b). Although the mechanisms linking ion fluxes and AOS generation to the elicitor-recognition step are not clearly defined, there was a tight and close connection between elicitor perception and physiological events.
In the present work we compared the binding properties of two acidic elicitins (capsicein and parasiticein) and two basic elicitins (cryptogein and cinnamomin) and studied their biological effects on tobacco cell suspensions (Ca2+ influx, alkalinization of the extracellular medium, and AOS production). We wanted to determine whether elicitins share different affinities for binding sites on tobacco plasma membranes, whether they have different activities according to their biophysical and structural properties, and whether the binding capacities may direct intensities of the early responses of tobacco cells involved in signal transduction after the recognition step.
MATERIALS AND METHODS
Plant Materials and Elicitors
Tobacco (Nicotiana tabacum cv Xanthi) plants were grown in a greenhouse for 60 d. Cell suspensions were cultivated in Chandler's medium (Chandler et al., 1972) on a rotary shaker (150 rpm, 25°C) under continuous light (photon flux rate 30–40 μmol m−2 s−1). Cells were maintained in the exponential phase and subcultured 1 d prior to utilization. Elicitins were purified according to the method of Bonnet et al. (1996).
Plasma Membrane Preparation
Purification of the plasma membrane was as previously described by Wendehenne et al. (1995) using the aqueous partitioning procedure of Widell et al. (1982). Tobacco microsomal fractions obtained from leaves (400 g) homogenized in 800 mL of grinding medium (50 mm Tris-Mes, pH 8.0, 0.5 m Suc, 20 mm EDTA, 10 mm DTT, and 1 mm PMSF) were added to an aqueous polymer two-phase system with final concentrations of 6.6% (w/w) dextran (Mr 500,000) and 6.6% (w/w) PEG (Mr 3,350) in a PSK buffer (5 mm phosphate buffer, pH 7.8, 0.3 m Suc, and 3 mm KCl). Plasma membrane-enriched fractions were suspended in 10 mm Tris-Mes, pH 6.5, 250 mm Suc, 1 mm PMSF, 2 mm MgCl2, and 20% glycerol and stored at −80°C. Protein content was measured according to the method of Lowry et al. (1951). The low level of contaminants in the plasma membrane preparations was confirmed using marker enzymes (Larsson et al., 1994).
Binding Experiments
Iodination of elicitins was performed as previously described (Wendehenne et al., 1995). One hundred micrograms of elicitin was incubated for 20 min at 20°C in 100 μL of 0.1 m phosphate buffer, pH 7.4, with 185 MBq Na125I (specific activity 625 MBq/μg iodine; Amersham) and Iodogen (Pierce) as the catalyst. Nonincorporated iodine was removed by gel filtration using a Sephadex G-25 column equilibrated with 50 mm Tris-HCl buffer, pH 7.4. Specific activity of radiolabeled elicitin was about 9.25 TBq/mmol. Binding experiments were carried out as previously described (Wendehenne et al., 1995), except for the binding medium (10 mm Hepes-KOH, pH 7.0, 5 mm MgCl2, and 0.1 m Suc). Binding of 125I-elicitins on plasma membrane preparations (50 μg of protein) was carried out on ice for 90 min after the addition of the appropriate concentration of radiolabeled elicitin (and 10 μm unlabeled elicitin in assays for nonspecific binding). Binding of 125I-elicitins was monitored by filtration under a vacuum using GF/B glass-fiber filters (Whatman). These filters were then washed three times with ice-cold binding buffer containing 0.1% BSA. Radioactivity remaining on the filters was measured in 5 mL of Ready-Safe cocktail (Beckman) with a gamma counter (model LS 600 TA, Beckman). Competition experiments were performed using 10−11 to 10−4 m unlabeled elicitins and 10 nm labeled elicitins.
Elicitor Treatment
Cells were collected during the exponential growth phase and washed by filtration in a suspension buffer containing 175 mm mannitol, 0.5 mm CaCl2, 0.5 mm K2SO4, and 2 mm Hepes adjusted to pH 5.75 with KOH (Keppler and Baker, 1989). Cells were resuspended at 0.1 g fresh weight mL−1 with suspension buffer and equilibrated for 2 h on a rotary shaker (150 rpm, 24°C). Tobacco cells were then used for determination of extracellular pH changes, Ca2+ influx measurements, and AOS production after treatment with 5 to 500 nm elicitin in aqueous solution. Control tobacco cells were incubated under the same conditions without elicitins.
Ca2+ Influx Measurements
Ca2+ influx measurements were performed as previously described (Tavernier et al., 1995b). Five minutes before the treatment with elicitins, cell suspensions were incubated with 45Ca2+ (0.033 MBq g−1 fresh weight cells; Amersham). After different periods of treatment, duplicate 2-mL aliquots were collected and filtered under a vacuum on GF/A glass-fiber filters, washed once for 1 min with 10 mL of ice-cold cell-suspension buffer containing 2 mm LaCl3, and then washed twice with 5 mL of the suspension buffer for 20 s. Cells remaining on filters were collected, dried overnight at 70°C, weighed, and placed in scintillation vials with 10 mL of Ready-Safe cocktail. Vials were shaken overnight before counting in a scintillation counter.
Extracellular pH Changes and AOS Production
Extracellular pH changes were measured at 10-min intervals in tobacco cell suspensions. AOS production was determined using chemiluminescence of luminol. Aliquots (250 μL) of cell suspensions were collected and mixed with 300 μL of 10 mm Hepes buffer, pH 6.5, 175 mm mannitol, 0.5 mm CaCl2, 0.5 mm K2SO4, and 50 μL of 0.3 mm luminol. Chemiluminescence measured within a 10-s period with a luminometer (EG&G Wallac, Gaithersburg, MD) was integrated and expressed in nanomoles of H2O2 per gram fresh weight of cells.
RESULTS
Binding Characteristics
Each elicitin was labeled with 125I with a specific activity of about 9.25 TBq/mmol, which corresponds to the radiolabeling of 12% of the molecules. This treatment did not modify elicitin activities such as alkalinization of the extracellular medium or AOS production (data not shown). Moreover, these activities were unaffected when elicitins were derivatized with a least one nonradioactive iodine per molecule (data not shown).
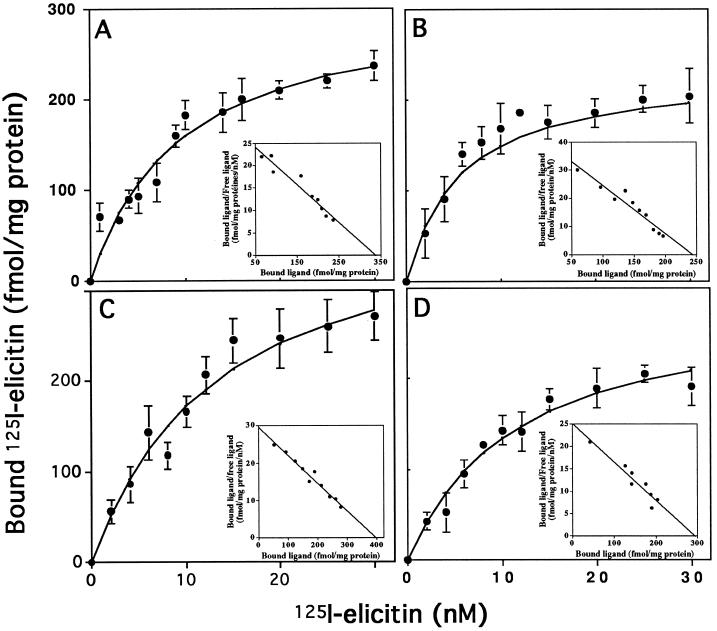
Binding experiments were performed by incubating 50 μg of plasma membrane proteins with increasing concentrations (1–30 nm) of each 125I-elicitin (Fig. 1). Nonspecific binding on the plasma membrane was similar for each elicitin, whereas when elicitins were used at 10 nm specific 125I-elicitin binding was between 2500 and 3500 cpm, depending on each 125I-elicitin specific activity. The saturation curves were hyperbolic, with half-maximal binding concentrations at about 10 nm. Scatchard plots gave straight lines, indicating the presence of a single class of specific binding sites for each elicitin (Fig. 1, insets). The Kd values and the number of binding sites deduced from Scatchard plots are shown in Table I. The Kd of cryptogein (10.3 nm) was similar to that previously obtained in a Tris buffer (Kd = 2 nm; Wendehenne et al., 1995).
Figure 1.
Saturation curves obtained with labeled elicitins and corresponding Scatchard plots (insets). Plasma membrane preparations were incubated with various concentrations of 125I-cryptogein (A), 125I-cinnamomin (B), 125I-capsicein (C), or 125I-parasiticein (D). Nonspecific binding was measured in the presence of a 10 μm concentration of the corresponding nonlabeled elicitin and was subtracted from total binding to give the saturation curves. Experiments were repeated at least three times. Results are means ± sd.
Table I.
125I-elicitin binding characteristics in tobacco plasma membranes
| 125I-Elicitin | Kd | Specific Binding Sites |
|---|---|---|
| nm | fmol mg−1 plasma membrane proteins | |
| Cryptogein | 10.3 ± 1.8 | 318 ± 37 |
| Cinnamomin | 5.8 ± 2.6 | 234 ± 25 |
| Capsicein | 13.5 ± 3.1 | 403 ± 60 |
| Parasiticein | 10.9 ± 4.4 | 280 ± 71 |
Specific binding was obtained by subtracting nonspecific binding from total binding. Data were obtained from Scatchard plots deduced from saturation curves and are the averages ± sd of three experiments.
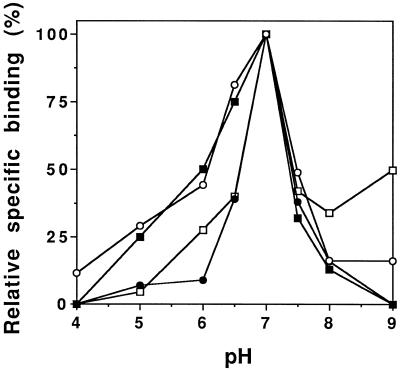
The Kd for the other elicitins ranged from 5.8 to 13.5 nm, showing that the four elicitins, independently of their acidic or basic properties, bound with the same affinity to plasma membrane-binding sites. Furthermore, the number of binding sites present on the plasma membrane was similar for each elicitin (Table I). Specific binding as a function of pH and tested at a pH range of 4.0 to 9.0 was optimum at pH 7.0 for each elicitin (Fig. 2). These results suggest that the four elicitins bind to common sites through invariant amino acids. Competition experiments were performed to verify this hypothesis.
Figure 2.
pH dependence of the specific binding of 125I-elicitins on tobacco plasma membrane preparations. Fifty micrograms of plasma membrane proteins was incubated with 10 nm 125I-cryptogein (○),125I-cinnamomin (•), 125I-capsicein (□), or 125I-parasiticein (▪) in the following buffers: citrate-KOH (pH 4.0–6.0), Hepes-KOH (pH 6.5–8.0), and Tris-HCl (pH > 8.0). Buffer strength was 10 mm. Specific binding was expressed as a percentage of the maximal specific binding measured for each elicitin. The experiment was repeated three times. Each data point represents the average of triplicate measurements.
Competition of Elicitins for Binding Sites
125I-cryptogein (10 nm) was incubated with plasma membrane preparations in the presence of increasing concentrations of unlabeled elicitins or elicitins derivatized with nonradioactive iodine. The corresponding IC50 values are reported in Tables II and III. Results indicated that the four unlabeled elicitins compete with labeled cryptogein (10 nm) for specific binding sites: the IC50 for unlabeled cryptogein, capsicein, or parasiticein was similar. The IC50 for unlabeled cinnamomin was 4 to 5 times higher than unlabeled cryptogein IC50 (Table II). The reverse-competition experiments using the four labeled elicitins (10 nm each) in competition with unlabeled cryptogein gave similar cryptogein IC50 values whatever the elicitin, with only a 2-fold variation (Table II). In this experiment the IC50 values were comparable to those obtained when unlabeled elicitins were competing with 125I-cryptogein.
Table II.
Inhibition of 125I-elicitin specific binding by noniodinated elicitins
| 125I-Elicitin | Competing Elicitin | IC50 |
|---|---|---|
| nm | ||
| 125I-Cryptogein | Cryptogein | 139 ± 18 |
| Cinnamomin | 660 ± 98 | |
| Capsicein | 195 ± 22 | |
| Parasiticein | 100 ± 27 | |
| Cryptogein | 139 ± 18 | |
| 125I-Cinnamomin | 240 ± 33 | |
| 125I-Capsicein | 251 ± 45 | |
| 125I-Parasiticein | 220 ± 20 |
IC50 values were determined from competition curves. Data are the averages ± sd of three experiments.
Table III.
Inhibition of 125I-cryptogein-specific binding by elicitins after derivatization with nonradioactive iodine calculated from competition curves
| IC50
|
||||
|---|---|---|---|---|
| I-Cryptogein | I-Cinnamomin | I-Capsicein | I-Parasiticein | |
| nm | ||||
| 125I-Cryptogein | 14.4 ± 6.2 | 4.8 ± 2.9 | 5.4 ± 1.8 | 20.1 ± 3.8 |
Data are from Figure 3 and represent the averages ± sd of three experiments.
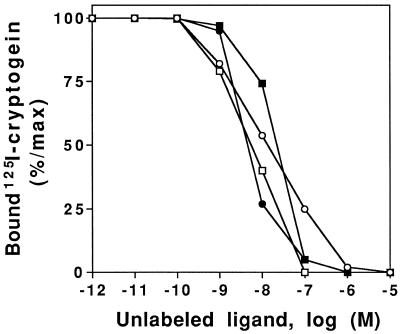
These results showed that elicitin-binding properties were comparable and that elicitins were acting as competitors; however, these IC50 values were higher than the expected values, which should reach the Kd values. This discrepancy could reflect different affinities of iodinated and noniodinated elicitins for binding sites. Therefore, competition experiments were also performed with increasing concentrations of elicitins derivatized with nonradioactive iodine (Fig. 3; Table III). In these conditions the IC50s for unlabeled iodinated elicitins were similar (close to the Kd of the labeled elicitin), suggesting that iodinated elicitins were better competitors for the plasma-binding sites than noniodinated elicitin. Consequently, Kd values should better reflect the iodinated elicitin affinities. However, all elicitins had a similar shifted IC50 value when iodinated; therefore, binding properties are comparable among the four elicitins, all of which bind to the same specific binding sites with a similar affinity.
Figure 3.
Inhibition of 125I-cryptogein binding to the plasma membrane by increasing concentrations of various elicitins (competitor). Fifty micrograms of plasma membrane proteins was incubated with 10 nm 125I-cryptogein and increasing concentrations of cryptogein (○), cinnamomin (•), capsicein (□), or parasiticein (▪). Data are expressed as percentages of the specific binding of 125I-cryptogein, each point being an average of triplicate measurements.
Extracellular Medium Alkalinization
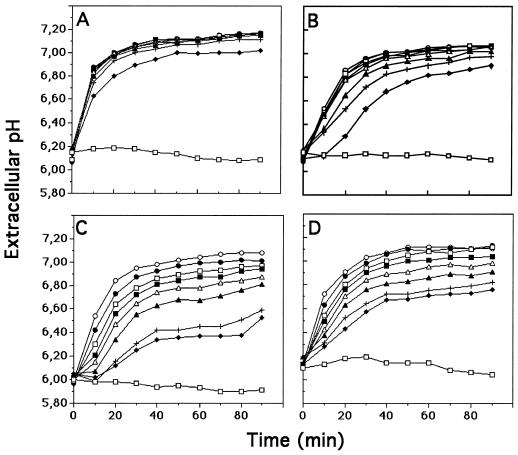
Cell suspensions were treated with various concentrations of elicitins (5–500 nm), and extracellular pH changes were measured in the cell-suspension medium for 90 min (Fig. 4). The four elicitins induced an extracellular pH increase. However, the kinetics and intensity of this response were specific for each elicitin and depended on their respective concentrations. For example, at a low concentration (10 nm) cryptogein induced a rapid and strong alkalinization of the extracellular medium, reaching the half-maximum pH increase in less than 10 min, whereas no significant or only slight pH changes were measured during the first 10 min with the other elicitins. The extracellular pH stabilized 40 to 60 min following elicitin treatment. However, the pH plateau depended on the elicitin used and gradually decreased in cinnamomin, parasiticein, and capsicein (in this order). When the four elicitins were used at higher concentrations (100 nm) the maximal medium alkalinizations were similar after treatment for 90 min, although initial rates for alkalinization were 0.085 pH unit min−1 for cryptogein, 0.047 pH unit min−1 for capsicein and parasiticein, and 0.020 pH unit min−1 for cinnamomin.
Figure 4.
Effects of increasing concentrations of elicitins on extracellular pH of tobacco cell suspensions. Cells were treated with cryptogein (A), cinnamomin (B), capsicein (C), or parasiticein (D). Elicitin concentrations were 5 nm (♦), 10 nm (+), 25 nm (▴), 50 nm (▵), 75 nm (▪), 100 nm (□), 250 nm (•), and 500 nm (○). Experiments were repeated three times. The figure corresponds to a representative experiment.
The lowest elicitin concentrations inducing the highest alkalinization were about 10 nm for cryptogein and 50 nm for cinnamomin, whereas those for the acidic elicitins were about 500 nm. These results show that basic elicitins were at least 10 times more efficient than acidic elicitins in inducing maximal extracellular pH alkalinization.
Ca2+ Influx
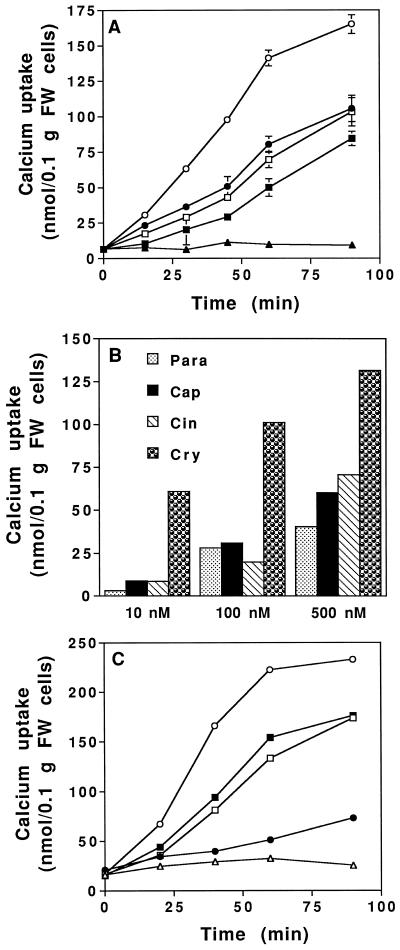
Using 45Ca2+ as a tracer we compared Ca2+ uptake induced by the four elicitins at three concentrations: 10, 100, and 500 nm. These assays were performed in the incubation medium used to monitor the alkalinization (low-buffered medium, pH 5.75). At 500 nm the four elicitins induced an uptake of 45Ca2+ (Fig. 5A). In the same experiments control cells did not show any 45Ca2+ uptake. Cryptogein was the most efficient elicitin, whereas the other elicitins induced a similar but lower Ca2+ uptake. In a second experiment Ca2+ influx was measured after treatment for 1 h with increasing concentrations of elicitins. Cryptogein was still the most efficient elicitin whatever the concentration (Fig. 5B); the other elicitins were less efficient in inducing 45Ca2+ uptake.
Figure 5.
Effect of various concentrations of elicitins on 45Ca2+ influx into tobacco cells. A, Time course of 45Ca2+ uptake by tobacco cells after treatment with 500 nm elicitins: cryptogein (○), cinnamomin (•), capsicein (□), and parasiticein (▪); control cells (▴) in a 2 mm Hepes low-buffered medium, pH 5.75. B, Effects of increasing concentrations of elicitins on 45Ca2+ uptake into tobacco cells after 1 h of incubation with cryptogein (Cry), cinnamomin (Cin), capsicein (Cap), or parasiticein (Para) in a 2 mm Hepes low-buffered medium, pH 5.75. C, Time course of 45Ca2+ uptake by tobacco cells after treatment with 100 nm elicitins: cryptogein (○), cinnamomin (•), capsicein (□), or parasiticein (▪); control cells (▴) in a 50 mm Hepes medium buffered at pH 7.0. Each point corresponds to a duplicate assay. Results are means ± sd. Specific activity of 45Ca2+ = 6.6 GBq/mol. FW, Fresh weight.
Because at pH 5.75 elicitins showed different relative specific binding, Ca2+ uptake induced by elicitins at 100 nm was also measured in a 50 mm Hepes medium buffered at pH 7.0, which corresponds to the optimum pH for the binding of the four elicitins. Under these conditions we obtained results comparable to those obtained at pH 5.75 (low-buffered medium; Fig. 5B), with the exception of cinnamomin, which triggered a lower Ca2+ uptake compared with the other elicitins. Therefore, even when used at their optimum binding pH, the elicitins triggered different Ca2+ uptakes (Fig. 5C).
AOS Production
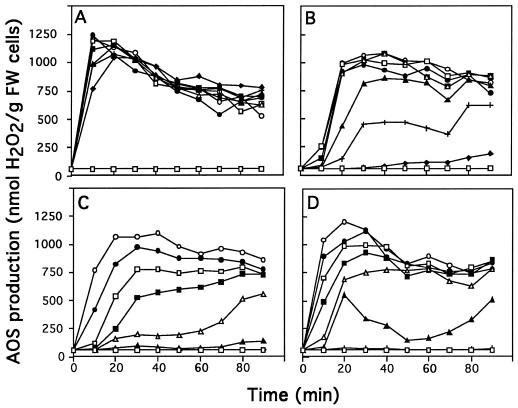
AOS production was measured in response to increasing concentrations of elicitins (5–500 nm). The four elicitins induced AOS production in tobacco cell suspensions. Nevertheless, the kinetics and the intensity of this production were specific for each elicitin and depended on their concentration (Fig. 6). For example, 100 nm cryptogein induced a rapid, strong burst of AOS after a 10-min treatment (about 1200 nmol H2O2 g−1 fresh weight cells), and then the production of H2O2 decreased slowly. When the other elicitins were used at 100 nm, the AOS production reached a similar maximal value, which was delayed (30–40 min) and did not decrease significantly in the following 1 h. When lower concentrations of elicitins were used (10 nm) the production of AOS induced by cryptogein reached almost the same maximum value (1000 nmol H2O2 g−1 fresh weight cells) after 10 min (Fig. 6). The oxidative burst induced by 10 nm cinnamomin appeared later (after 20 min) and had a lower intensity, whereas no significant burst was measured with 10 nm capsicein and parasiticein.
Figure 6.
AOS production in tobacco cell suspensions (nmol H2O2 g−1 fresh weight cells) treated with increasing concentrations of elicitins: 5 nm (♦), 10 nm (+), 25 nm (▴), 50 nm (▵), 75 nm (▪), 100 nm (□), 250 nm (•), and 500 nm (○). Cells were treated with cryptogein (A), cinnamomin (B), capsicein (C), or parasiticein (D). The experiment was repeated three times. The figure corresponds to a representative experiment. FW, Fresh weight.
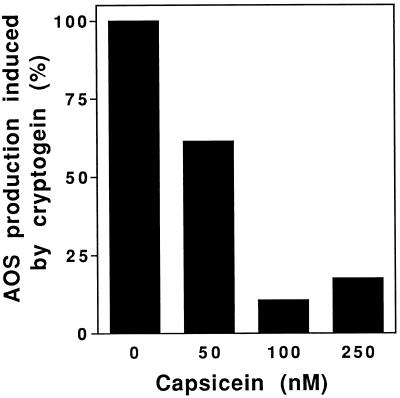
As previously observed with regard to the extracellular medium alkalinization or the Ca2+ influx, cryptogein was the most efficient elicitin: the highest AOS production was achieved with 25 nm cryptogein, whereas 50 nm cinnamomin or greater than 500 nm acidic elicitins were necessary to obtain a maximal AOS production. AOS production was also measured to verify whether competition between acidic and basic elicitins led to a decrease of the effects of the most efficient basic elicitin. In vivo competition experiments on tobacco cells were performed using 25 nm cryptogein and various concentrations of capsicein (Fig. 7). AOS production due to the cryptogein treatment in the competition assay was scored by subtracting the AOS production contributed by capsicein alone from the total AOS production, at the concentration corresponding to that of the competition experiment. Cryptogein-induced AOS production was decreased by 40% when tobacco cells were simultaneously treated with 50 nm capsicein. With higher capsicein concentrations (100 and 250 nm) the AOS production induced by cryptogein was reduced by about 80% to 90%, showing effective in vivo competition.
Figure 7.
In vivo competition experiment using 25 nm cryptogein and increasing capsicein concentrations. The AOS production induced by 25 nm cryptogein was referred to as 100%. AOS production due to cryptogein in competition experiments was deduced by subtracting from the total AOS production the AOS contribution of capsicein alone used at the concentration corresponding to that used in the competition experiment. The AOS production was measured after treatment for 15 min with elicitins.
DISCUSSION
In this study we compared the binding properties of four different elicitins on tobacco plasma membrane preparations and their effects on tobacco cells. The four elicitins produced by Phytophthora have a high degree of sequence conservation (74%) and should therefore have a similar structure (Bouaziz et al., 1994; Boissy et al., 1996). Nevertheless, they are classified into acidic and basic elicitins based on their pI. An elicitin necrotic index was established that correlated with pI, and basic elicitins had high necrotic activity (Pernollet et al., 1993). The different level of activities of elicitins should not depend on their stability: elicitin uptake experiments in tobacco with labeled cryptogein demonstrated high elicitin stability in vivo, because the protein could be recovered in a nondegradated form 8 d after the onset of treatment (Keller et al., 1996). Furthermore, the in vitro stability of elicitins is a general property of these proteins (resistance to boiling and to trypsin treatment; data not shown), probably because of their similar structure.
Using two basic elicitins (cryptogein and cinnamomin) and two acidic elicitins (capsicein and parasiticein), we examined several aspects of their structure-function relationship. We first looked at binding-affinity constants and found that all four 125I-labeled elicitins had a similar Kd, ranging from 5.8 to 13.5 nm, and that the number of binding sites on the plasma membrane was between 234 and 403 fmol/mg plasma membrane proteins (Table I). These binding constants were very close to those previously determined by Wendehenne et al. (1995) using tobacco plasma membrane preparations and 125I-labeled cryptogein: a Kd of 2 nm and 220 fmol/mg binding sites on plasma membrane proteins. Differences were expected because we used a different binding buffer suitable for cross-linking experiments. In the present study the data based on Scatchard plots suggest that elicitin-binding properties are equivalent. This assumption is further supported by experiments showing that the specific binding of each elicitin was optimal at pH 7.0, regardless of its acidic or basic nature (Fig. 2).
Competition experiments were performed to verify whether elicitins bound to the same high-affinity binding sites. Results demonstrated that each nonradioactive iodinated elicitin was able to compete with 125I-cryptogein, and the corresponding IC50 values were similar (between 4.8 and 20.1 nm) and close to the Kd of cryptogein (Table III). However, an apparent better affinity of iodinated versus noniodinated elicitins was suggested by the comparison of the IC50 values, which were higher for unlabeled iodinated elicitins (Tables II and III). Consequently, the affinity of noniodinated elicitins for binding sites might be lower than those determined for iodinated elicitins. At this stage we are unable to explain why iodination increases affinity and why this increase in affinity is not linked to an increase in activity. Elicitins possess four to five Tyr residues with various degrees of accessibility (based on the cryptogein structure; Boissy et al., 1996). Only two conserved Tyr residues (Tyr-47 and Tyr-87) are partly accessible to the solvent, and only one is fully accessible in basic elicitins (Tyr-12). The iodination of one of these residues could modify the binding properties if the residue lies within or in close vicinity to the interacting domain.
The fact that elicitins showed similar Kd constants and IC50 values regardless of the treatment (iodination or absence of iodination) demonstrated that they were able to interact with the same binding sites on plasma membranes. Binding experiments have also indicated that nonconserved, charged amino acids that confer acidic or basic pIs were probably not involved in binding. Therefore, binding relies on invariant amino acid interactions, which is in agreement with results of radiographic crystallography of cryptogein indicating that elicitins may share a very similar tertiary structure and a beak-like motif resulting from the arrangement of an Ω-loop and a β-sheet essentially composed of invariant residues. This conserved structure could be a major recognition site for a putative receptor (Boissy et al., 1996).
Elicitors of different chemical origin have been reported to interact with high affinity with plasma membrane components. Chemically pure carbohydrates and glycopeptides or proteinaceous elicitors bound with Kd values at or below the nanomolar range (Cheong and Hahn, 1991; Cosio et al., 1992; Basse et al., 1993; Shibuya et al., 1993; Baureithel et al., 1994; Nürnberger et al., 1994; Kooman-Gersmann et al., 1996). Evidence for high-affinity binding sites is usually correlated with biological activities, confirming a linkage with a cellular signal transduction chain. A strong relationship between competitor abilities of various elicitors and plant defense responses has been established previously (Cheong and Hahn, 1991; Basse et al., 1993; Baureithel et al., 1994; Nürnberger et al., 1994).
A positive binding/activity correlation has also been reported using β-glucan elicitors and six species of the same plant family (Cosio et al., 1996). However, since elicitor binding seems to be a prerequisite for the induction of the plant-defense response, this interaction is not sufficient for elicitor-induced plant-defense mechanisms. The race-specific AVR9 peptide elicitor from Cladosporium fulvum bound on plasma membrane preparations from both fungus-resistant and fungus-susceptible tomato cultivars and from other solanaceous plants (Kooman-Gersmann et al., 1996). It was suggested that in the susceptible cultivar binding properties were related to the presence of homologs of the resistance gene Cf-9, which characterized the resistant tomato cultivar. In tomato cells the same binding site was shown to bind a glycopeptide (elicitor), inducing ethylene production and the N-linked glycans released from this glycopeptide (Basse et al., 1993). The N-linked glycans act as suppressors of ethylene production.
A comparison of the previously described effects of elicitors on whole tobacco plants (see introduction) and of the elicitin-binding properties did not indicate a strict relationship between elicitin perception and plant response. Therefore, we looked at early physiological events in tobacco cells following elicitin perception, which would reflect a modulated signal transduction step dependent on an elicitor complex leading to different plant defense levels. We chose to measure the effects of the four elicitins on Ca2+ influx (which is, to our knowledge, the earliest event induced by cryptogein in tobacco cells after protein phosphorylation; Tavernier et al., 1995b) and on the subsequent Ca2+-dependent events extracellular medium alkalinization and AOS production.
Using 45Ca2+ as a tracer we observed that the four elicitins were able to induce Ca2+ influx. Nevertheless, cryptogein triggered a much more intense Ca2+ influx compared with the other elicitins (Fig. 5). The ability of elicitins to trigger different levels of Ca2+ influx was confirmed using pH conditions (buffered medium, pH 7.0) that allowed similar binding with the four elicitins (Fig. 5C). The intensity of the Ca2+ influx was not associated with the basic or acidic characters of elicitins, because the basic cinnamomin has an effect on Ca2+ influx similar to those of acidic elicitins.
Extracellular pH changes were also monitored in response to the four elicitins. The comparison of the effects of either 10 or 100 nm elicitin indicated that cryptogein was always the most efficient. At low concentrations all elicitins except cryptogein showed a lag phase of at least 10 min before the pH of the extracellular medium increased (Fig. 4). At higher concentrations the lag phase was shortened and the maximal pH variation was reached after 60 min of treatment with the four elicitins, although with different initial rates. The lowest concentrations necessary to achieve maximal pH increase with the highest rates were 10 and 50 nm for cryptogein and cinnamomin, respectively, and about 500 nm for capsicein and parasiticein. Basic elicitins appeared at least 10 times more efficient than acidic elicitins in inducing extracellular pH changes. The same conclusions were drawn when measuring the AOS production induced by the four elicitins (Fig. 6). The concentrations necessary to induce maximal AOS production were similar to those necessary to induce the maximal extracellular pH increase (25 and 50 nm for cryptogein and cinnamomin, respectively, and about 500 nm for the two acidic elicitins). The AOS burst increased concurrently with extracellular pH and was delayed for cinnamomin, capsicein, and parasiticein compared with cryptogein. However, at a high elicitin concentration (100 nm) AOS production reached the same level (approximately 1200 nmol H2O2 g−1 fresh weight cells) with the four elicitins, although with different kinetics.
Because the four elicitins bind to the same sites with the same affinity, one would expect that when mixed together an elicitin with low efficiency (acidic) would decrease the effects of a highly efficient (basic) elicitin. These competition assays required 20 nm basic elicitin and 10 to 50 times more acidic elicitin (200 nm to 1 μm). The acidic elicitin contributions at these concentrations were high and differences were particularly slight for extracellular pH measurements. Therefore, in vivo competition experiments were performed by measuring only the AOS production induced by cryptogein in competition with capsicein. Results showed that capsicein was also a competitor in vivo: 100 nm capsicein induced a 90% inhibition of the 25 nm cryptogein-induced AOS production, which supports an in vivo competition process.
Even when elicitins were tested on tobacco cell suspensions, differences in the intensity of the early effects of elicitin arose and could reflect the elicitin properties that were correlated with the electric charge of the elicitins. One explanation for the different biological activities of elicitin would be that the whole elicitin charge might restrict the acidic elicitin diffusion at the 5.8 physiological pH in the negatively charged cell wall, resulting in lower amounts of acidic elicitins interacting with binding sites. Alternatively, following binding between the elicitin conserved domain and the receptor, the amino acid electric charges that characterized the unconserved domain could trigger different conformational changes in the receptor, leading to variations in the intensities and kinetics of responses at the cellular level.
Conversely, the formation of various oligomeric forms of the receptor could be induced by elicitins, as has been reported for animal polypeptide hormones, cytokines, and growth factors (Heldin, 1995). For example, the different isoforms of platelet-derived growth factor induced different dimeric forms of receptors (homodimeric and heterodimeric forms), resulting in not only modulated response intensities but also additional signal transduction molecule interactions. Such mechanisms could also explain the behavior of the different elicitins at the cellular level. In addition to the beak-like motif, elicitins contain two other regions around residues 13 and 87 whose charge distribution could be correlated with the level of biological activity of elicitin (Boissy et al., 1996). These regions could modulate the intensity of tobacco cell responses after binding on the receptor. Presently, experiments are in progress to identify the receptor of elicitins. After this receptor has been identified, the interactions between ligand and receptor will be studied at the molecular level.
In conclusion, fine regulation of signal transduction is a topic of major interest, although many aspects remain obscure because of a lack of information about the many processes involved. Only recently was a β-glucan elicitor-binding site characterized at the molecular level (Umemoto et al., 1997). An integrated view of the transduction pathway includes the analysis of the environment of the receptor and knowledge of the molecules recruited to transduce signals such as protein kinases, protein phosphatases, or specific ion channels.
ACKNOWLEDGMENT
We wish to thank Annick Chiltz for excellent technical assistance.
Abbreviations:
- AOS
active oxygen species
- IC50
half-maximal inhibitor concentration
Footnotes
This work was supported by the Institut National de la Recherche Agronomique and by the Conseil Regional de Bourgogne. S.B. was supported by a grant from the Ministère de l'Enseignement Supérieur et de la Recherche.
LITERATURE CITED
- Atkinson MM, Keppler LD, Orlandi EW, Baker CJ, Mischke CF. Involvement of plasma membrane calcium influx in bacterial induction of the K+/H+ and hypersensitive responses in tobacco. Plant Physiol. 1990;92:215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW, Mock NM. Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 1993;102:1341–1344. doi: 10.1104/pp.102.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Joyard J, Pugin A, Ranjeva R. Intracellular compartmentation and plant cell signaling. Trends Plant Sci. 1997;2:214–222. [Google Scholar]
- Basse CW, Boller T. Glycopeptide elicitors of stress responses in tomato cells. Plant Physiol. 1992;98:1239–1247. doi: 10.1104/pp.98.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basse CW, Fath A, Boller T. High affinity binding of a glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993;268:14724–14731. [PubMed] [Google Scholar]
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Blein JP, Milat ML, Ricci P. Responses of cultured tobacco cells to cryptogein, a proteinaceous elicitor from Phytophthora cryptogea. Plant Physiol. 1991;95:486–491. doi: 10.1104/pp.95.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy G, de la Fortelle E, Kahn R, Huet JC, Bridogne G, Pernollet JC, Brunie S. Crystal structure of a fungal elicitor secreted by Phytophthora cryptogea, a member of a novel class of plant necrotic proteins. Structure. 1996;4:1429–1439. doi: 10.1016/s0969-2126(96)00150-5. [DOI] [PubMed] [Google Scholar]
- Bonnet P, Bourdon E, Ponchet M, Blein JP, Ricci P. Acquired resistance triggered by elicitins in tobacco and other plants. Eur J Plant Pathol. 1996;102:181–192. [Google Scholar]
- Bottin A, Véronesi C, Pontier D, Esquerré-Tugayé MT, Blein JP, Rustérucci C, Ricci P. Differential responses of tobacco cells to elicitors from two Phytophthora species. Plant Physiol Biochem. 1994;32:373–378. [Google Scholar]
- Bouaziz S, Van Heijenoort C, Guittet E, Huet JC, Pernollet JC. Resonance assignment, cysteine-pairing elucidation and secondary structure determination of capsicein, an α-elicitin, by three-dimensional 1H-NMR. Eur J Biochem. 1994;220:427–438. doi: 10.1111/j.1432-1033.1994.tb18640.x. [DOI] [PubMed] [Google Scholar]
- Chandler MT, Tandeau de Marsac N, Kouchkovsky Y. Photosynthetic growth of tobacco cells in liquid suspension. Can J Bot. 1972;50:2265–2270. [Google Scholar]
- Cheong JJ, Hahn MG. A specific, high-affinity binding site for the hepta-β-glucoside elicitor exists in soybean membranes. Plant Cell. 1991;3:137–147. doi: 10.1105/tpc.3.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio EG, Feger M, Miller CJ, Antelo L, Ebel J. High-affinity binding of a fugal β-glucan elicitors to cell membranes of species of the plant family Fabaceae. Planta. 1996;200:92–99. [Google Scholar]
- Cosio EG, Frey T, Ebel J. Identification of a high-affinity binding protein for a hepta-β-glucoside phytoalexin elicitor in soybean. Eur J Biochem. 1992;204:1115–1123. doi: 10.1111/j.1432-1033.1992.tb16736.x. [DOI] [PubMed] [Google Scholar]
- Devergne JC, Bonnet P, Panabières F, Blein JP, Ricci P. Migration of the fungal protein cryptogein within tobacco plants. Plant Physiol. 1992;99:843–847. doi: 10.1104/pp.99.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Grosskopf DG, Regenass M, Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci USA. 1991;88:8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [32P]phosphate. Proc Natl Acad Sci USA. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Keller H, Blein JP, Bonnet P, Ricci P. Physiological and molecular characteristics of elicitin-induced systemic acquired resistance in tobacco. Plant Physiol. 1996;110:365–376. doi: 10.1104/pp.110.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ. O2−-initiated lipid peroxidation in a bacteria-induced hypersensitive reaction in tobacco cell suspension. Phytopathology. 1989;79:555–562. [Google Scholar]
- Kooman-Gersmann M, Honée G, Bonnema G, De Wit PJGM. A high-affinity binding site for the AVR9 peptide elicitor of Cladosporium fulvum is present on plasma membranes of tomato and other solanaceous plants. Plant Cell. 1996;8:929–938. doi: 10.1105/tpc.8.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C, Sommarin M, Widell S. Isolation of a highly purified plant plasma membranes and separation of inside-out and right-side-out vesicles. Methods Enzymol. 1994;228:451–469. [Google Scholar]
- Lowry H, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mathieu Y, Lapous D, Thomine S, Laurière C, Guern J. Cytoplasmic acidification as an early phosphorylation-dependant response of tobacco cells to elicitors. Planta. 1996a;199:416–424. [Google Scholar]
- Mathieu Y, Sanchez FJ, Droillard MJ, Lapous D, Laurière C, Guern J. Involvement of protein phosphorylation in the early steps of transduction of the oligogalacturonide signal in tobacco cells. Plant Physiol Biochem. 1996b;34:399–408. [Google Scholar]
- Medhy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milat ML, Ricci P, Blein JP. Capsidiol and ethylene production by tobacco cells in response to cryptogein. Phytochemistry. 1991;30:2171–2173. [Google Scholar]
- Nespoulous C, Huet JC, Pernollet JC. Structure-function relationships of α and β elicitins, signal proteins involved in the plant-Phytophthora interaction. Planta. 1992;186:551–557. doi: 10.1007/BF00198035. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Nennstiel D, Jabs T, Sacks W, Halbrock K, Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- O'Donohue MJ, Gousseau H, Huet JC, Tepfer D, Pernollet JC . Chemical synthesis, expression and mutagenesis of a gene encoding a β cryptogein, an elicitin produced by Phytophthora cryptogea. Plant Mol Biol. 1995;27:577–586. doi: 10.1007/BF00019323. [DOI] [PubMed] [Google Scholar]
- Pernollet JC, Sallantin M, Salle-Tourne M, Huet JC. Elicitin isoforms from seven Phytophthora species: comparison of their physico-chemical properties and toxicity to tobacco and other plant species. Physiol Mol Plant Physiol. 1993;42:53–67. [Google Scholar]
- Petitot AS, Blein JP, Pugin A, Suty L. Cloning of two plant cDNAs encoding a β-type proteasome subunit and a transformer-2-like SR-related protein: early induction of the corresponding genes in tobacco cells treated with cryptogein. Plant Mol Biol. 1997;35:261–269. doi: 10.1023/a:1005833216479. [DOI] [PubMed] [Google Scholar]
- Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P, Bonnet P, Huet JC, Sallantin M, Beauvais-Cante F, Bruneteau M, Billard V, Michel G, Pernollet JC. Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur J Biochem. 1989;183:555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Ricci P, Panabières F, Bonnet P, Maia N, Ponchet M, Devergne JC, Marais A, Cardin L, Milat ML, Blein JP (1993) Proteinaceous elicitors of plant defense responses. In B. Fritig, M. Legrand, eds, Mechanisms of Plant Defense Responses. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 121–135
- Shibuya N, Kaku H, Kuchitsu K, Maliarik M. Identification of a novel high-affinity binding site for N-acetylchitooligosaccharide elicitor in the membrane fraction from suspension-cultured rice cells. FEBS Lett. 1993;329:75–78. doi: 10.1016/0014-5793(93)80197-3. [DOI] [PubMed] [Google Scholar]
- Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JDG. Molecular genetics of plant disease resistance. Science. 1995;268:661–667. doi: 10.1126/science.7732374. [DOI] [PubMed] [Google Scholar]
- Suty L, Petitot AS, Lecourieux D, Blein JP, Pugin A. Isolation of partial length cDNAs corresponding to early differentially expressed genes during elicitation of tobacco cells by cryptogein. Plant Physiol Biochem. 1996;34:443–451. [Google Scholar]
- Tavernier E, Stallaert V, Blein JP, Pugin A. Changes in lipid composition in tobacco cells treated with cryptogein, an elicitor from Phytophthora cryptogea. Plant Sci. 1995a;104:117–125. [Google Scholar]
- Tavernier E, Wendehenne D, Blein JP, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 1995b;109:1025–1031. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto N, Kakitani M, Iwamatsu A, Yoshikawa M, Yamaoka N, Ishida I. The structure and function of a soybean β-glucan-elicitor-binding protein. Proc Natl Acad Sci USA. 1997;94:1029–1034. doi: 10.1073/pnas.94.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard MP, Martin F, Pugin A, Ricci P, Blein JP. Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Binet MN, Blein JP, Ricci P, Pugin A. Evidence for specific, high-affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 1995;374:203–207. doi: 10.1016/0014-5793(95)01108-q. [DOI] [PubMed] [Google Scholar]
- Widell S, Lundberg T, Larsson C. Plasma membranes from oats prepared by partition in an aqueous polymer two-phase system. Plant Physiol. 1982;70:1429–1435. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LM. Elicitins from Phytophthora and basic resistance in tobacco. Proc Natl Acad Sci USA. 1995;92:4088–4094. doi: 10.1073/pnas.92.10.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti A, Beauvais F, Huet JC, Pernollet JC. Movement of elicitins, necrosis-inducing proteins secreted by Phytophthora sp., in tobacco. Planta. 1992;187:163–170. doi: 10.1007/BF00201933. [DOI] [PubMed] [Google Scholar]