Abstract
The V2 protein of Tomato yellow leaf curl geminivirus (TYLCV) is an RNA-silencing suppressor that counteracts the innate immune response of the host plant. However, this anti-host defense function of V2 may include targeting of other defensive mechanisms of the plant. Specifically, we show that V2 recognizes and directly binds the tomato CYP1 protein, a member of the family of papain-like cysteine proteases which are involved in plant defense against diverse pathogens. This binding occurred both in vitro and in vivo, within living plant cells. The V2 binding site within mCYP1 was identified in the direct proximity to the papain-like cysteine protease active site.
Keywords: CYP1, papain-like cysteine protease, protein interaction, TYLCV, V2 protein
Tomato yellow leaf curl virus (TYLCV), a whitefly-transmitted geminivirus, is a major tomato pathogen world-wide1,2 that causes extensive (up to 100%) crop losses.3 Geminiviridae family of plant viruses characterized by their ssDNA genomes, which replicate and transcribe in the host-cell nucleus. Although TYLCV is a DNA virus that replicates by a dsDNA intermediate, it is capable of inducing RNA silencing in infected plants.4 This anti-TYLCV reaction is counteracted by RNA-silencing suppressor proteins encoded by the virus. One of such suppressor protein is V2, identified in two TYLCV isolates, Israeli (TYLCV-Is)5,6 and Chinese (TYLCV-China).7 The TYLCV-Is V2 most likely suppresses RNA silencing via its interaction with the host RNA silencing machinery component SGS3.6
Intriguingly, the counter-defense function of TYLCV-Is V2 may not be limited to silencing suppression. Our present data suggest that V2 interacts with a member of the family of papain-like cysteine proteases (PLCPs) which are involved in plant defense against pathogens via hypersensitive response (HR).8-11 This PLCP of tomato (CYP1) belongs to an intriguing class of papain-like Cys proteases (PLCPs) typified by the presence of a C-terminal granulin domain which is removed during the subsequent maturation step, resulting in mature CYP1 (mCYP1). Indeed, TYLCV induces HR in some plants (e.g., Nicotiana benthamiana),12,13 but not in others (e.g., tomato), suggesting its ability to suppress this defense in the latter host species. This role of PLCPs in plant anti-pathogen response and their targeting by pathogen-derived inhibitors is well documented for pathogenic fungi and bacteria. For example, RCR3, a tomato PLCP, triggers HR in plants carrying the Cf-2 resistance gene upon infection by the fungal pathogen Cladosporium fulvum.14 Similarly, the Arabidopsis PLCP RD19 is required for RRS1-R-mediated resistance against the bacterial pathogen Ralstonia solanacearum.15
Consistent with the key role of PLCPs in disease immunity, many pathogens have evolved effectors that target these proteases. For instance, the PopP2 effector of R. solanacearum interacts with RD19 and relocalizes it from vesicles to the nucleus.15 RCR3 is inhibited by the Avr2 effector of C. fulvum16 as well as by EPIC1 and EPIC2B, two closely related cystatine-like proteins of the oomycete pathogen Phytophthora infestans.17,18 Unlike pathogenic bacteria and fungi or oomycetes, plant viruses have not been known to target host PLCPs. Here, we identify the first plant viral protein, the V2 of TYLCV-Is, that interacts with the tomato PLCP CYP1 within living plant cells.
To produce a bait construct, the PCR-amplified ORF of TYLCV V25 was inserted into the EcoRI-XhoI sites of pEG202,19 resulting in pEG202-V2.
For subcellular localization studies, TYLCV-Is V2 was tagged at its C terminus with CFP by cloning a PCR-amplified V2 ORF into the BglII-EcoRI sites of pSAT6A-ECFP-N1,20 resulting in pSAT6A-V2-ECFP. mCYP1 was tagged at its N terminus with YFP by cloning PCR-amplified mCYP1 cDNAs into the XhoI-BamHI sites, of pSAT6-EYFP-C1,20 resulting in pSAT6-EYFP-mCYP1. All PCRs were performed using a high-fidelity Pfu DNA polymerase (Promega) and their products were verified by DNA sequencing.
A tomato cDNA library19 was screened with pEG202-V2 as bait in Saccharomyces cerevisiae strain EGY48 as previously described,6,19 and positive clones were selected on a leucine-deficient medium, confirmed by β-galactosidase assay21 and isolated as described.6
CelluSpotsTM CYP1-specific peptide arrays immobilized on cellulose sheets were custom-made by INTAVIS (www.intavis.com/en/). The coding sequence of TYLCV-Is V2 was subcloned into the pHIS-parallel1 bacterial expression vector in frame with an N-terminal six-histidine tag,22 overexpressed in E. coli (BL21) and purified to near homogeneity (95–98% pure) by an ion metal affinity chromatography on a Ni–sepharose matrix following manufacturer [Adar Biotech Israel (www.adarbiotech.com/)] instructions .
2.65 mg (in 300 μl buffer) of the purified protein were incubated with the peptide array membrane in 8 ml of 5% skim milk blocking solution as described,23 and V2 binding to the membrane was detected by western blot analysis using anti-His antibodies.
Leaf mesophyll protoplasts were isolated from Nicotiana tabacum L. cv Samsun NN,24 and a mixture of 5 µg plasmid DNA and 15 µg calf thymus DNA was used for electroporation of 0.5 ml of protoplast solution.25 Transformed protoplasts were incubated in the dark for 24 h at 27°C prior to imaging.
For confocal imaging, we used an Olympus IX 81 inverted laser scanning confocal microscope (Fluoview 500) equipped with an argon ion laser and a 60x1.0 N.A. PlanApo water-immersion objective. CFP and YFP were excited at 458 nm and 515 nm and imaged using BA480–495 nm and BA535–565 nm emission filters, respectively. For chlorophyll autofluorescence, a BA 660 nm IF emission filter was used. Transmitted light images were obtained using Nomarski differential interference contrast (DIC).
The FRET procedure was performed using the acceptor photobleaching method.26 CFP (donor) and YFP (acceptor) were excited at 70% and 3% laser power, respectively; all other conditions were as described for confocal imaging. The microscope was configured with a 458/515-nm dichroic mirror for dual excitation, and with a 515-nm beam-splitter to help separate CFP and YFP fluorescence. Acceptor was bleached by scanning a region of interest (ROI) at 100% laser power, resulting in photobleaching of at least 90% of the original fluorescence. The pre- and post-bleach images were collected and ROI fluorescence intensity was measured by using Fluoview 500 software. Each measurement was conducted on a set of 10 different cells. The percentage of FRET efficiency (EF) was calculated as EF = (In+1-In)x100/In+1, where In and In+1 are the CFP intensities at the time points between which the bleaching occurred.27
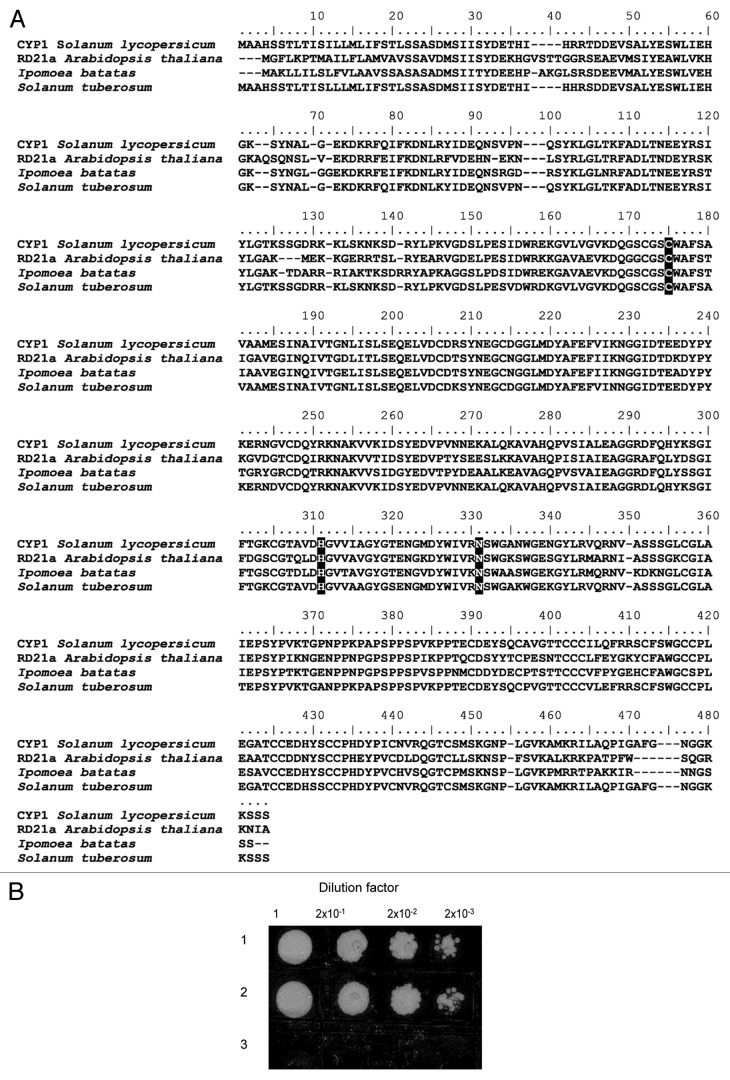
To better understand the involvement of V2 in suppression of the host defenses, we used a yeast two-hybrid system (Y2H) and our tomato two-hybrid cDNA library28 to identify host proteins, in addition to previously identified SGS3,6 with which V2 interacts within the host cell. These experiments isolated a cDNA clone encoding a protein product that interacted with V2. Amino-acid sequence analysis identified the V2 interactor as tomato PLCP designated CYP1/C1429,30 (GenBank accession number AJ003137, CAA05894) and revealed significant homology to PLCPs from Arabidopsis thaliana (RD21a, NP_564497), sweet potato (Ipomoea batatas, AAK48495), and potato (Solanum tuberosum, CAB53515.1) (Fig. 1A). CYP1 is encoded by a single gene located on chromosome 1230 and contains several distinct domains that include a cysteine-protease domain, a proline-rich domain, and a C-terminal granulin domain (Fig. 2A). Similar to RD21,31 CYP1 accumulates in two active isoforms; the immature isoform, iCYP1, contains a granulin domain which is cleaved off (not self-catalytically, but by another, yet unidentified protease), producing the mature isoform, mCYP1.31
Figure 1. Identification of tomato CYP1 and its interaction with TYLCV V2 in yeast. (A) Amino acid sequence alignment of the tomato (Solanum lycopersicum) CYP1 (CAA05894) with its papain-like cysteine protease homologs from Arabidopsis thaliana (RD21a, NP_564497), sweet potato (Ipomoea batatas, AAK48495), and potato (Solanum tuberosum, CAB53515.1). Conserved residues of the cysteine protease active site are indicated by black boxes, and gaps introduced for alignment are indicated by dashes. Alignment was performed using the ClustalW algorithm (www.genebee.msu.su/clustal/advanced.html). (B) Interaction in the Y2H system. The indicated cell inocula were plated on a leucine-deficient growth medium. In this assay system, growth in the absence of leucine represents selective conditions for protein-protein interaction. Lane 1, TYLCV V2 – iCYP1; Lane 2, TYLCV V2 – mCYP1; Lane 3, control plasmid pRFHM1.
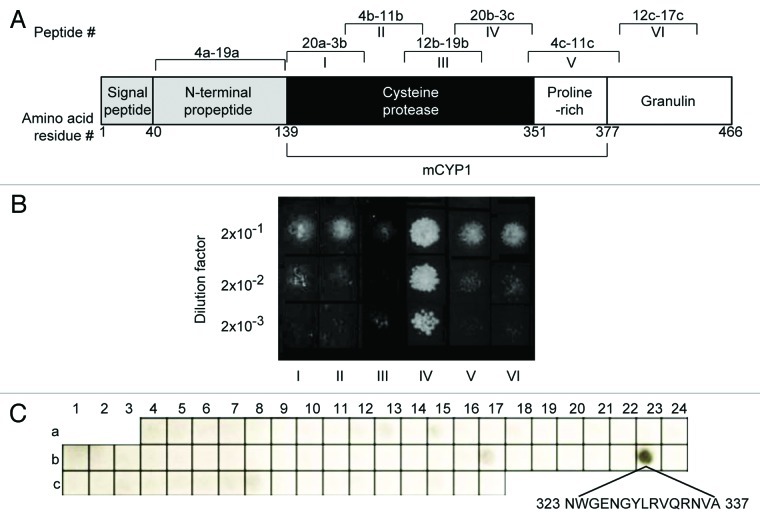
Figure 2. Identification of the V2 binding site within mCYP1. (A) Schematic representation of the CYP1 protein domains. Positions of amino acid residues delimiting each of the indicated domains are shown. Roman numbers I-VI indicate locations of the CYP1 fragments assayed in panel B; these fragments are delimited by the following amino acid residue positions: I, 139–198; II, 188–248; III, 238–301; IV, 292–351; V, 335–393; VI, 377–433. Peptide numbers indicate the positions of each of the tested CYP1 peptides on the array shown in panel C. (B) V2 binding to CYP1 fragments in the Y2H system. (C) V2 binding to CYP1 peptide array. The amino acid sequence of peptide 23b, exhibiting binding to V2, is indicated.
CYP1 Interacts with TYLCV V2 Protein in Yeast Two Hybrid Assay
Interestingly, our Y2H experiments demonstrated that V2 recognized and interacted with both the mature and the immature forms of CYP1, activating the LEU2 reporter gene and enabling yeast transformants to grow on a leucine dropout medium (Fig. 1B, lanes 1 and 2). In contrast, V2 did not interact with the granulin domain of CYP1 (Fig. 2B, lane VI). The interaction between V2 and CYP1 was specific because it did not occur with Bicoid homeodomain (pRFHM1) (Fig. 1B lane 3) or with topoisomerase I (not shown), known to eliminate false positives in yeast Y2H assays.32,33 Furthermore, no interaction was detected between mCYP1 and several other TYLCV proteins, including V1/CP, C3, and C4 (not shown). Under non-selective conditions, i.e., in the presence of leucine, cells expressing all combinations of proteins grew to the same extent (not shown), indicating that none of the tested proteins adversely and nonspecifically affected yeast-cell physiology.
V2 Interacts with the Cysteine Protease Domain of CYP1
We then separated iCYP1 into six fragments that spanned its cysteine protease, proline-rich, and granulin domains (fragments I-VI, Figure 2A) and examined their ability to bind V2 in the Y2H assay. Figure 2B shows that only fragment IV, corresponding to the C-terminal 25 of the cysteine protease domain exhibited interaction with V2. Next, we further defined the V2-binding site of CYP1 with the help of peptide arrays, which allow to narrow down large interaction interfaces to relatively short and specific binding sequences.23 Specifically, we used the CelluSpotsTM peptide array that represented the CYP1 amino acid sequence in 15-amino acid-long peptides overlapping each other by 7 residues (Fig. 2A). This array was probed with the recombinant V2 and detected by western blot analysis. Figure 2C shows that from all peptides that saturated the entire length of the CYP1 protein, except its signal peptide domain, only one, peptide 23b, was able to bind V2. Consistent with our Y2H data in Figure 2B, peptide 23b was situated within the fragment IV of the cysteine protease domain. Thus, the amino acid sequence NWGENGYLRVQRNVA, corresponding to this peptide, most likely represents a major binding site for V2 within mCYP1.
CYP1 is Localized in the Cytoplasm of Tobacco Protoplasts
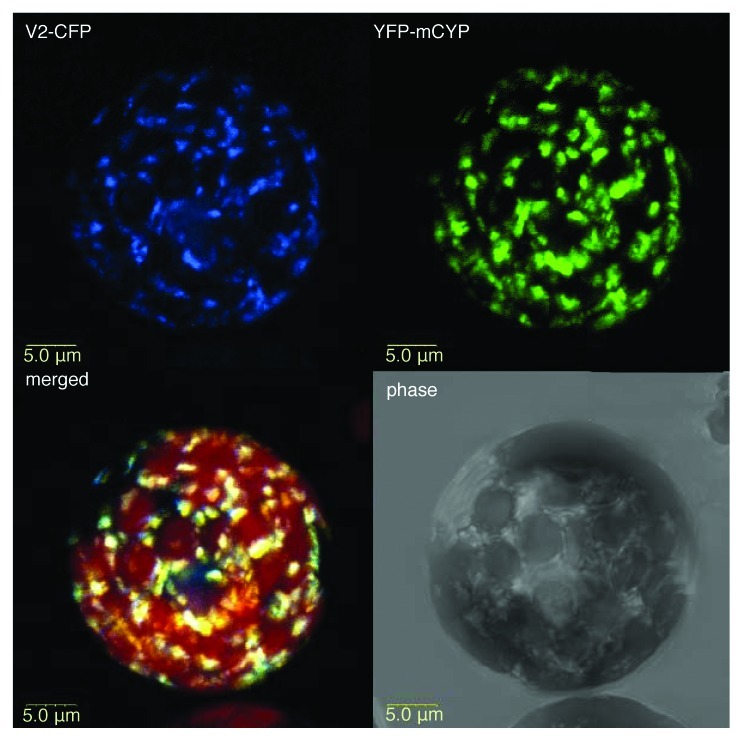
Next, we examined the subcellular localization patterns of YFP-tagged mCYP1 coexpressed with CFP-tagged V2. Figure 3 shows that, in tobacco protoplasts, mCYP1 was cytoplasmic, accumulating in distinct microbodies. This finding is important because it differentiates the tomato CYP1 from its Arabidopsis homolog RD21, which is a vacuolar protein in both its mature and immature forms.31 V2 also exhibited a cytoplasmic localization6 and largely colocalized with mCYP1 (Fig. 3). This colocalization of mCYP1 and V2 is consistent with their ability to interact with each another.
Figure 3. Subcellular colocalization of V2 with mCYP1. V2-CFP was coexpressed with YFP-mCYP1 in N. tabacum protoplasts. CFP signal is in blue. YFP signal is in green. Overlapping signal produced by the colocalizing proteins in the merged image is in yellow; plastid autofluorescence in the merged image is in red, and it was filtered out in other images. All images are projections of several confocal sections.
CYP1 and TYLCV V2 Protein Physically Interact in planta
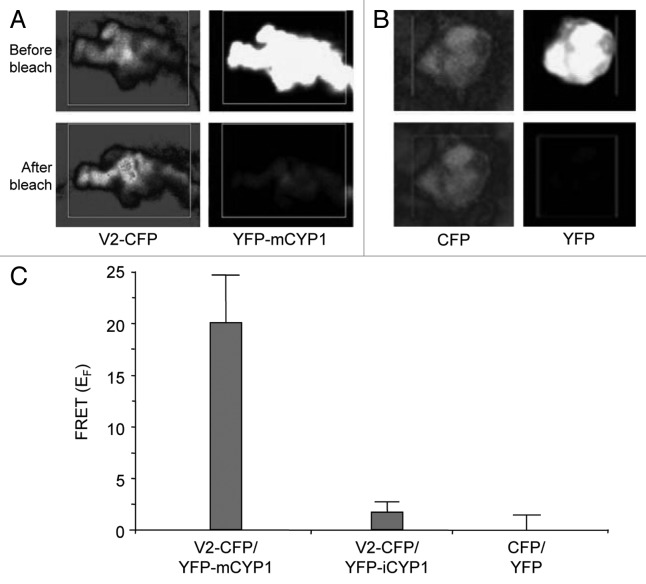
Finally, the interaction of V2 with mCYP1 was demonstrated directly in planta by fluorescence resonance energy transfer (FRET) microscopy, which allows detection of protein interactions within living cells.34,35 V2-CFP was transiently coexpressed with YFP-mCYP1 in tobacco protoplasts. FRET between these proteins was assayed by focusing on the colocalizing microbodies and photobleaching them as described.6,26,27Figure 4A shows energy transfer between V2-CFP and YFP-mCYP1, i.e., reduction in acceptor (YFP) signal and increase in the donor (CFP) signal, which is indicative of FRET.6,26,27 Note that these images represent magnified signal foci in the protoplast cytoplasm, which correspond to microbodies within the V2-CFP/YFP-mCYP1-expressing protoplasts shown at lower magnification in Figure 3. As expected, free coexpressed YFP and CFP did not generate detectable FRET (Fig. 4B).
Figure 4. Interaction between V2 and mCYP1 in planta. Protein-protein interaction was monitored by FRET microscopy of living N. tabacum protoplasts coexpressing either V2-CFP and YFP-mCYP1 (A) or free CFP and YFP (B). Representative acceptor photobleaching images show CFP (donor) and YFP (acceptor) channels before and after bleaching. After bleaching, CFP fluorescence increases and YFP fluorescence decreases in the bleached areas indicated by rectangles. All images are projections of several confocal sections. (C) Quantification of donor fluorescent intensity in representative samples of protoplasts coexpressing V2-CFP and YFP-mCYP1, V2-CFP and YFP-iCYP1, or CFP and YFP. Data represent average values of three independent experiments, with 10 protoplasts each, with indicated standard deviation values.
Quantification of the CFP signal after photobleaching of YFP revealed an increase in the intensity of the donor fluorescence with a FRET efficiency (EF) of 19.29 ± 4.22% for YFP-mCYP1/V2-CFP, which is indicative of FRET26,27 (Fig. 4C). Confirming the Y2H in Figure 2, practically no FRET was observed for YFP-iCYP1/V2-CFP (EF = 1.28 ± 1.15%). As expected, free coexpressed YFP and CFP generated no detectable FRET (Fig. 3C).
In summary, we described the first case of interaction between a plant viral pathogenesis protein and a host defense-related PLCP. Specifically, we showed that TYLCV V2 directly interacts with tomato mCYP1 in vitro and in vivo. Although the exact role of this interaction during the infection process remains to be elucidated, it is tempting to speculate that V2 interferes with the defensive activity of mCYP1, facilitating viral invasion and/or spread. Consistent with this idea, V2, a known anti-host defense factor,5,6 specifically targets the mCYP1 amino acid sequence, located between positions 323 to 337, which is directly adjacent to H298 and N318, the two out of three conserved residues of the cysteine protease active site of CYP1. Conceptually, therefore, V2 may function similar to plant serpins, which are known to associate with and negatively regulate PLCPs.36-38 Thus, V2 may represent a viral functional analog of cellular serpins, acting to downregulate the CYP1 activity. This is by analogy to many other plant and animal pathogen-encoded proteins which have acquired (potentially by convergent evolution) functional features of host proteins required for infection.39-41
Acknowledgments
This work was supported by the US-Israel Binational Agricultural Research and Development (BARD) grant IS-4237 to V.C. and Y.G., and by the US-Israel Binational Science Foundation (BSF) grant 2007140 to Y.G. The work in V.C.’s lab was supported by grants from NIH, NSF, and NIFA/USDA. This paper is a contribution from the Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel, No. 106/2012
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20935
References
- 1.Gafni Y. Tomato yellow leaf curl virus, the intracellular dynamics of a plant DNA virus. Mol Plant Pathol. 2003;4:9–15. doi: 10.1046/j.1364-3703.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 2.Moriones E, Navas-Castillo J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2000;71:123–34. doi: 10.1016/S0168-1702(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 3.Navot N, Pichersky E, Zeidan M, Zamir D, Czosnek H. Tomato yellow leaf curl virus: a whitefly-transmitted geminivirus with a single genomic component. Virology. 1991;185:151–61. doi: 10.1016/0042-6822(91)90763-2. [DOI] [PubMed] [Google Scholar]
- 4.Vanitharani R, Chellappan P, Fauquet CM. Short interfering RNA-mediated interference of gene expression and viral DNA accumulation in cultured plant cells. Proc Natl Acad Sci U S A. 2003;100:9632–6. doi: 10.1073/pnas.1733874100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zrachya A, Glick E, Levy Y, Arazi T, Citovsky V, Gafni Y. Suppressor of RNA silencing encoded by Tomato yellow leaf curl virus-Israel. Virology. 2007;358:159–65. doi: 10.1016/j.virol.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Glick E, Zrachya A, Levy Y, Mett A, Gidoni D, Belausov E, et al. Interaction with host SGS3 is required for suppression of RNA silencing by tomato yellow leaf curl virus V2 protein. Proc Natl Acad Sci U S A. 2008;105:157–61. doi: 10.1073/pnas.0709036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Dong J, Xu Y, Wu J. V2 protein encoded by Tomato yellow leaf curl China virus is an RNA silencing suppressor. Virus Res. 2012;163:51–8. doi: 10.1016/j.virusres.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 8.van der Hoorn RA. Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol. 2008;59:191–223. doi: 10.1146/annurev.arplant.59.032607.092835. [DOI] [PubMed] [Google Scholar]
- 9.Avrova AO, Stewart HE, De Jong WD, Heilbronn J, Lyon GD, Birch PR. A cysteine protease gene is expressed early in resistant potato interactions with Phytophthora infestans. Mol Plant Microbe Interact. 1999;12:1114–9. doi: 10.1094/MPMI.1999.12.12.1114. [DOI] [PubMed] [Google Scholar]
- 10.Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–53. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- 11.Gilroy EM, Hein I, van der Hoorn R, Boevink PC, Venter E, McLellan H, et al. Involvement of cathepsin B in the plant disease resistance hypersensitive response. Plant J. 2007;52:1–13. doi: 10.1111/j.1365-313X.2007.03226.x. [DOI] [PubMed] [Google Scholar]
- 12.Glick E, Levy Y, Gafni Y. The viral etiology of tomato yellow leaf curl disease - a review. Plant Prot Sci. 2009;45:81–97. [Google Scholar]
- 13.van Wezel R, Dong X, Blake P, Stanley J, Hong Y. Differential roles of geminivirus Rep and AC4 (C4) in the induction of necrosis in Nicotiana benthamiana. Mol Plant Pathol. 2002;3:461–71. doi: 10.1046/j.1364-3703.2002.00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Krüger J, Thomas CM, Golstein C, Dixon MS, Smoker M, Tang S, et al. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science. 2002;296:744–7. doi: 10.1126/science.1069288. [DOI] [PubMed] [Google Scholar]
- 15.Bernoux M, Timmers T, Jauneau A, Brière C, de Wit PJ, Marco Y, et al. RD19, an Arabidopsis cysteine protease required for RRS1-R-mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector. Plant Cell. 2008;20:2252–64. doi: 10.1105/tpc.108.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooney HC, Van’t Klooster JW, van der Hoorn RA, Joosten MH, Jones JD, de Wit PJ. Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance. Science. 2005;308:1783–6. doi: 10.1126/science.1111404. [DOI] [PubMed] [Google Scholar]
- 17.Tian M, Win J, Song J, van der Hoorn R, van der Knaap E, Kamoun S. A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease. Plant Physiol. 2007;143:364–77. doi: 10.1104/pp.106.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J, Win J, Tian M, Schornack S, Kaschani F, Ilyas M, et al. Apoplastic effectors secreted by two unrelated eukaryotic plant pathogens target the tomato defense protease Rcr3. Proc Natl Acad Sci U S A. 2009;106:1654–9. doi: 10.1073/pnas.0809201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Loh YT, Bressan RA, Martin GB. The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell. 1995;83:925–35. doi: 10.1016/0092-8674(95)90208-2. [DOI] [PubMed] [Google Scholar]
- 20.Tzfira T, Tian GW, Lacroix B, Vyas S, Li J, Leitner-Dagan Y, et al. pSAT vectors: a modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol Biol. 2005;57:503–16. doi: 10.1007/s11103-005-0340-5. [DOI] [PubMed] [Google Scholar]
- 21.Durfee T, Becherer K, Chen PL, Yeh SH, Yang Y, Kilburn AE, et al. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–69. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 22.Sheffield P, Garrard S, Derewenda Z. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr Purif. 1999;15:34–9. doi: 10.1006/prep.1998.1003. [DOI] [PubMed] [Google Scholar]
- 23.Katz C, Levy-Beladev L, Rotem-Bamberger S, Rito T, Rüdiger SG, Friedler A. Studying protein-protein interactions using peptide arrays. Chem Soc Rev. 2011;40:2131–45. doi: 10.1039/c0cs00029a. [DOI] [PubMed] [Google Scholar]
- 24.Draper J, Scott R, Armitage P, Walden R. Plant Genetic Transformation and Gene Expression, A Laboratory Manual. Blackwell Scientific Publications, Oxford, 1988. [Google Scholar]
- 25.Fromm M, Taylor LP, Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985;82:5824–8. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenworthy AK. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods. 2001;24:289–96. doi: 10.1006/meth.2001.1189. [DOI] [PubMed] [Google Scholar]
- 27.Karpova TS, Baumann CT, He L, Wu X, Grammer A, Lipsky P, et al. Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J Microsc. 2003;209:56–70. doi: 10.1046/j.1365-2818.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 28.Kunik T, Mizrachy L, Citovsky V, Gafni Y. Characterization of a tomato karyopherin alpha that interacts with the tomato yellow leaf curl virus (TYLCV) capsid protein. J Exp Bot. 1999;50:731–2. [Google Scholar]
- 29.Lers A, Burd S, Sonego L, Khalchitski A, Lomaniec E. Nucleotide sequence of a full-length C14 cDNA clone (Accession No. AJ003137) encoding for cysteine protease CYP1 from tomato. Plant Physiol. 1998;116:1193. [Google Scholar]
- 30.Kaschani F, Shabab M, Bozkurt T, Shindo T, Schornack S, Gu C, et al. An effector-targeted protease contributes to defense against Phytophthora infestans and is under diversifying selection in natural hosts. Plant Physiol. 2010;154:1794–804. doi: 10.1104/pp.110.158030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada K, Matsushima R, Nishimura M, Hara-Nishimura I. A slow maturation of a cysteine protease with a granulin domain in the vacuoles of senescing Arabidopsis leaves. Plant Physiol. 2001;127:1626–34. doi: 10.1104/pp.010551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartel P, Chien CT, Sternglanz R, Fields S. Elimination of false positives that arise in using the two-hybrid system. Biotechniques. 1993;14:920–4. [PubMed] [Google Scholar]
- 33.Hollenberg SM, Sternglanz R, Cheng PF, Weintraub H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15:3813–22. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hink MA, Bisselin T, Visser AJ. Imaging protein-protein interactions in living cells. Plant Mol Biol. 2002;50:871–83. doi: 10.1023/A:1021282619035. [DOI] [PubMed] [Google Scholar]
- 35.Jares-Erijman EA, Jovin TM. FRET imaging. Nat Biotechnol. 2003;21:1387–95. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- 36.Lampl N, Budai-Hadrian O, Davydov O, Joss TV, Harrop SJ, Curmi PM, et al. Arabidopsis AtSerpin1, crystal structure and in vivo interaction with its target protease RESPONSIVE TO DESICCATION-21 (RD21) J Biol Chem. 2010;285:13550–60. doi: 10.1074/jbc.M109.095075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts TH, Marttila S, Rasmussen SK, Hejgaard J. Differential gene expression for suicide-substrate serine proteinase inhibitors (serpins) in vegetative and grain tissues of barley. J Exp Bot. 2003;54:2251–63. doi: 10.1093/jxb/erg248. [DOI] [PubMed] [Google Scholar]
- 38.Roberts TH, Hejgaard J. Serpins in plants and green algae. Funct Integr Genomics. 2008;8:1–27. doi: 10.1007/s10142-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 39.Zaltsman A, Krichevsky A, Loyter A, Citovsky V. Agrobacterium induces expression of a host F-box protein required for tumorigenicity. Cell Host Microbe. 2010;7:197–209. doi: 10.1016/j.chom.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacroix B, Vaidya M, Tzfira T, Citovsky V. The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J. 2005;24:428–37. doi: 10.1038/sj.emboj.7600524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai H, Roy CR. Show me the substrates: modulation of host cell function by type IV secretion systems. Cell Microbiol. 2003;5:373–83. doi: 10.1046/j.1462-5822.2003.00285.x. [DOI] [PubMed] [Google Scholar]