Abstract
Recent experiments from our laboratory are consistent with the idea that hypothalamic astrocytes are critical components of the central nervous system (CNS) mediated estrogen positive feedback mechanism. The “astrocrine hypothesis” maintains that ovarian estradiol rapidly increases free cytoplasmic calcium concentrations ([Ca2+]i) that facilitate progesterone synthesis in astrocytes. This hypothalamic neuroprogesterone along with the elevated estrogen from the ovaries allows for the surge release of gonadotropin-releasing hormone (GnRH) that triggers the pituitary luteinizing hormone (LH) surge. A narrow range of estradiol stimulated progesterone production supports an “off-on-off” mechanism regulating the transition from estrogen negative feedback to estrogen positive feedback, and back again. The rapidity of the [Ca2+]i response and progesterone synthesis support a non-genomic, membrane-initiated signaling mechanism. In hypothalamic astrocytes, membrane-associated estrogen receptors (mERs) signal through transactivation of the metabotropic glutamate receptor type 1a (mGluR1a), implying that astrocytic function is influenced by surrounding glutamatergic nerve terminals. Although other putative mERs, such as mERβ, STX-activated mER-Gαq, and G protein-coupled receptor 30 (GPR30), are present and participate in membrane-mediated signaling, their influence in reproduction is still obscure since female reproduction be it estrogen positive feedback or lordosis behavior requires mERα. The astrocrine hypothesis is also consistent with the well-known sexual dimorphism of estrogen positive feedback. In rodents, only post-pubertal females exhibit this positive feedback. Hypothalamic astrocytes cultured from females, but not males, responded to estradiol by increasing progesterone synthesis. Estrogen autoregulates its own signaling by regulating levels of mERα in the plasma membrane of female astrocytes. In male astrocytes, the estradiol-induced increase in mERα was attenuated, suggesting that membrane-initiated estradiol signaling (MIES) would also be blunted. Indeed, estradiol induced [Ca2+]i release in male astrocytes, but not to levels required to stimulate progesterone synthesis. Investigation of this sexual differentiation was performed using hypothalamic astrocytes from post-pubertal four core genotype (FCG) mice. In this model, genetic sex is uncoupled from gonadal sex. We demonstrated that animals that developed testes (XYM and XXM) lacked estrogen positive feedback, strongly suggesting that the sexual differentiation of progesterone synthesis is driven by the sex steroid environment during early development.
Keywords: Estrogen, Progesterone, LH surge, Positive feedback, Estrogen receptor, Astrocyte
1. Role of neuroprogesterone in female reproduction
Ovulation is a critical event in mammalian female reproduction. In rodents and primates, maturing ovarian follicles synthesize and secrete estrogens. Circulating estrogen levels increase until they activate the hypothalamic-pituitary axis producing a surge release of luteinizing hormone (LH). This is the estrogen positive feedback that triggers ovulation. During positive feedback, the same estrogens that inhibited the hypothalamus and pituitary gland now stimulate these cells [1]. Many of the steps in the positive feedback cascade have been elucidated. In particular, rising estrogen levels induce the synthesis of hypothalamic progesterone receptors (PRs), which are required for the LH surge [2–6]. Specifically, Chappel and Levine demonstrated that both transcription and activation of PRs in the hypothalamus are obligatory events in the stimulation of the gonadotropin-releasing hormone (GnRH) and LH surges in estradiol-primed, ovariectomized (OVX) rats [7]. Studies with PR knockout mice in vivo demonstrated that PR-A in the hypothalamus, but not PR-B, mediates the LH surge and sexual receptivity in estrogen-primed female mice [8]. Therefore, not only is a pre-ovulatory increase in peripheral estradiol required, but an increase in progesterone synthesis and activation of PRs are all essential for inducing the LH surge.
In the intact rat, both the ovary and the adrenal cortex are highly steroidogenic organs capable of producing the pre-ovulatory rise in progesterone needed for the LH surge [9]. However, no significant rise in progesterone has been detected in the systemic circulation prior to the LH surge, indicating that the progesterone required for the LH surge may not be synthesized peripherally [10–12]. Consistent with this idea is that neither the adrenals nor the ovaries are necessary for an estrogen-induced LH surge [13,14]. Indeed, OVX and adrenalectomized (ADX) rats injected with 17β-estradiol, but not progesterone, have been shown to produce a robust LH surge [13,15].
The source of this progesterone appears to be from the brain. The steroidogenic capacity of the brain has been well established [16–26]. Neuroprogesterone, progesterone synthesized de novo in the brain, can be induced by estradiol. Neurons, astrocytes, and oligodendrocytes have been demonstrated to possess all the steroidogenic enzymes and associated proteins required to convert cholesterol directly to progesterone within the brain, including cytochrome P-450 side-chain cleavage (P450scc), 3β-hydroxysteroid dehydrogenase (3β-HSD), steroidogenic acute regulatory protein (StAR), and 18 kDa translocator protein (TSPO), formerly known as peripheral-type benzodiazepine receptor (PTBR) [27,28]. Estradiol treatment of OVX/ADX female rats increased hypothalamic neuroprogesterone levels and induce a physiological relevant LH surge, indicating that the source of progesterone was neither the ovary nor adrenal gland [15]. Furthermore, treatment with trilostane, a blocker of the enzyme 3β-HSD that catalyzes the conversion of pregnenolone to progesterone, inhibited the LH surge, indicating that neuroprogesterone synthesis is critical for estrogen positive feedback in OVX/ADX female rats [15]. In gonadally intact rats with normal four-day estrous cycles, blocking hypothalamic steroidogenesis with aminoglutethemide (AGT), a P450scc enzyme inhibitor, on the morning of proestrus eliminated the LH surge, ovulation and luteinization [29]. After several days, the effects of AGT wore off, hypothalamic progesterone synthesis recovered, and the treated rats resumed their estrous cycles. These data strongly suggest that estrogen stimulates neuroprogesterone synthesis locally within the hypothalamus, which is essential (directly or through its metabolites) in mediating the positive feedback regulation of the LH surge.
2. Estrogen effects on astrocytes
Our understanding of astrocytes in regulating nervous system function has evolved from providing structural support to regulating metabolic events [30] and synaptic function in adjacent neurons [31,32]. Astrocytes respond to numerous transmitters, peptides, and steroids [33–36]. It is now well accepted that estradiol acts on astrocytes [37]. Similar to granulosa cells of the ovary, astrocytes have been shown to express estrogen receptor-alpha (ERα) and estrogen receptor-beta (ERβ), which provides a mechanism for estradiol regulation [33,38–42]. Estradiol profoundly influences astrocyte morphology and function [43], glial fibrillary acidic protein (GFAP) distribution [44], sexual differentiation [45], and steroidogenesis [46,47]. The presence of surrounding neurons further enhances the changes in astrocytic shape induced by estradiol [48]. Astrocytes have been described to play an important role in estrogen-mediated neuroprotection [49]. Astrocytes also regulate numerous hypothalamic processes including regulation of releasing factors [50–54] and synthesis of neurosteroids [54–57].
Although enriched cultures of neurons and oligodendrocytes are capable of synthesizing progesterone, enzymatic activity studies indicate that astrocytes are the most steroidogenically active cells in the brain [57]. Not only do astrocytes contain ERs and interact with neurons in response to estradiol, but astrocytes are the main source of the essential neuroprogesterone produced within the hypothalamus [15,37,46,57]. Thus, hypothalamic astrocytes are critical for the central nervous system (CNS) response mediating estrogen positive feedback [58,59]. This increase in hypothalamic neuroprogesterone activates the progesterone receptors in the neuronal circuit that regulates the activity of GnRH neurons, resulting in greater release of GnRH that triggers the pituitary LH surge leading to ovulation – the critical event in female reproduction [6,7,15,29,37].
3. mER signaling
As in neurons, estradiol can influence cell signaling in astrocytes, which express ERα and ERβ both intracellularly and in the plasma membrane [33,40–42]. Therefore, estradiol can activate nuclear-initiated and/or membrane-initiated signaling mechanisms. Classic nuclear-initiated estradiol action is well established and mediated through activation of ERα and ERβ located in the nucleus to behave as ligand-activated transcription factors. Evidence suggests that these same receptors can mediate both nuclear- and membrane-initiated signaling. Although long-studied, it is only more recently that membrane-initiated estradiol action has been widely accepted [33,42,60–76].
Activation of membrane-associated estrogen receptors (mERs) with estradiol or a membrane impermeable construct E-6–BSA (estradiol-coupled to bovine serum albumin) initiates a rapid [Ca2+]i increase via activation of the phospholipase C/inositol trisphosphate (PLC/IP3) pathway that releases intracellular stores of calcium from the smooth endoplasmic reticulum in neurons and astrocytes [33,46,77]. This rise in [Ca2+]i stimulates the de novo synthesis of progesterone in post-pubertal female hypothalamic astrocytes within 5 min [15,46,78]. Confirmation of this idea was obtained through the use of thapsigargin, a potent Ca2+-ATPase inhibitor that rapidly releases IP3-sensitive Ca2+ stores from the smooth endoplasmic reticulum. This massive release of Ca2+, which was similar in magnitude to estradiol stimulation, resulted in progesterone synthesis by itself [46]. Although these studies were done with nanomolar doses of estradiol, subsequent experiments demonstrated that subnanomolar doses of estradiol were sufficient to induce [Ca2+]i release in cultured hypothalamic astrocytes [62]. Estradiol induction of progesterone synthesis had a half-maximal effector concentration (EC50) of 0.82 nM, which may be related to the extent of the [Ca2+]i increase [78]. Thus, the estradiol facilitation of progesterone synthesis appears to be a “step function” responding to physiological levels of estradiol that are reached during the proestrus surge [79–81]. Both estradiol and progesterone stimulation of the hypothalamus are essential for estrogen positive feedback, ultimately leading to the LH surge [7,15,37,59]. The threshold response to estradiol is consistent with the idea that stimulation of neuroprogesterone synthesis is part of an “off-on-off” mechanism regulating the transition from estrogen negative feedback to estrogen positive feedback, and back again [78]. For example, as estradiol rises with developing ovarian follicles, gradually increasing levels of [Ca2+]i release will be stimulated by the estradiol. However, only with physiologically peak estradiol levels, consistent with mature follicles ready for ovulation, does the [Ca2+]i release reach a critical threshold allowing for progesterone synthesis. Otherwise, hypothalamic progesterone may rise too early, resulting in a premature LH surge before ovarian follicles are fully mature and ready to ovulate.
Membrane-impermeable E-6–BSA–FITC (estradiol–bovine serum albumin–fluorescein isothiocyanate conjugate) and E-6–biotin (estradiol–biotin conjugate) constructs also bind to and demonstrate membrane ERs as well as their internalization in neurons and astrocytes [58,82,83]. The estradiol effects were blocked by the ER inhibitor ICI 182,780, confirming non-genomic estradiol signaling through a mER [33,46]; however, how a membrane-associated nuclear receptor activates intracellular signaling cascades was not immediately clear. Membrane-initiated estradiol signaling (MIES) has been shown to activate G protein-dependent cell signaling cascades [84], including activation of the mitogen-activated protein kinase (MAPK) pathway, increasing [Ca2+]i, activation of protein kinase A (PKA) and protein kinase C (PKC), and phosphorylation of cAMP-responsive element binding protein (CREB) [85,86] reviewed by [87]. A mechanism for MIES was proposed in which ligand-bound mERα or mERβ transactivated metabotropic glutamate receptors (mGluRs) to stimulate PLC/IP3–MAPK pathways leading to the activation of CREB [85,88]. These membrane-initiated signaling cascades have been implicated in the estradiol activation of lordosis behavior through receptor activation and neuropeptide expression [89]. For steroid synthesis, activation of PKA and/or PKC is required for the phosphorylation and activation of StAR, the rate-limiting step in steroid biosynthesis [90–92]. In mammalian astrocytes, TSPO is another possible regulator of intramitochondrial cholesterol transfer [28,93]. Activation of the TSPO in whole animals increased brain levels of allopregnanolone, pregnenolone, and progesterone [94]. In astrocytes, TSPO agonists stimulated the synthesis of pregnenolone, the immediate precursor of progesterone [95].
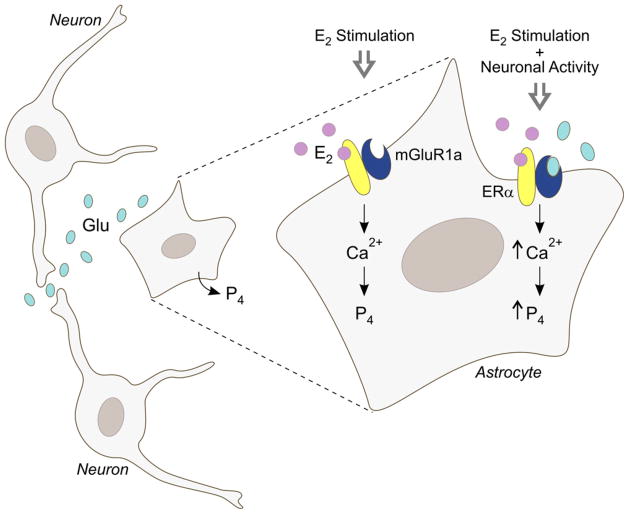
In hypothalamic astrocytes, both the estradiol-induced [Ca2+]i release and progesterone synthesis are blocked by the metabotropic glutamate receptor type 1a (mGluR1a) antagonist LY 367385 [62,78]. Co-immunoprecipitation of ERα and mGluR1a is consistent with a mER-mGluR interaction. We observed an interesting phenomenon. Even though estradiol would transactivate the mGluR1a in the absence of glutamate, if the mGluR1a was selectively activated with DHPG, a mGluR1a agonist, the estradiol response was augmented (Fig. 1) [62,78]. High dose DHPG can be used to accurately predict the maximal effect on astrocytes from the glutamate released by surrounding neurons that would be present in vivo. Furthermore, surface biotinylation studies in hypothalamic astrocytes demonstrated that mERα trafficking is dependant upon the mGluR1a, such that mGluR1a antagonism with LY 367385 blocked estradiol-dependant mERα insertion and its internalization [60]. In hypothalamic neurons, estradiol treatment significantly increased the internalization of mGluR1a in parallel with ERα, further supporting an mERα-mGluR1a signaling unit [96]. Thus, trafficking and internalization of mERα likely occurs together as a complex with mGluR1a. These studies also suggested that membrane-initiated ER signaling may be required for the initiation of mERα-mGluR1a trafficking to the membrane. The membrane impermeable E-6–BSA construct demonstrated increased mERα trafficking similar to estradiol [97]. Internalization, a measure of receptor activation, closely mimicked the mERα-mGluR1a trafficking. As expected, the more receptor complexes on the membrane, the more activation and internalization. In astrocytes, this is supported by the augmentation of the [Ca2+]i response during maximal mERα insertion and internalization at 30–60 min of estradiol exposure [60]. Overall, maximal signaling in hypothalamic astrocytes requires the presence of both glutamate and estradiol, implying that estradiol signaling is augmented by excitatory neural activity involving glutamate and astrocytes are a site of neural–hormonal integration (Fig. 1). Indeed, middle-aged female rats exhibit reduced excitation of GnRH neurons and attenuated LH surges compared to young females under estrogen positive feedback conditions, in part, due to decreased glutamate neurotransmission in the medial preoptic area, demonstrating the importance of local modulatory effects on estrogen positive feedback, the LH surge, and reproductive function [98,99].
Fig. 1.
An illustration of the additive effects of glutamate released from surrounding neurons on the estradiol response in hypothalamic astrocytes. Estradiol acting upon mERα induces [Ca2+]i release that stimulates progesterone synthesis essential for estrogen positive feedback and the LH surge. Local neuronal activity modulates hypothalamic astrocyte function through the release of glutamate, which activates the mGluR1a to augment both the estradiol-induced [Ca2+]i response and progesterone synthesis. Only when estradiol bound mERα interacts with glutamate bound mGluR1a does maximal intracellular signaling take place in hypothalamic astrocytes, suggesting that astrocytes are a site of neural–hormonal integration.
4. One ligand, many receptors
In terms of cell signaling, the classic nuclear receptors ERα and ERβ have been shown to associate with the plasma membrane with a variety of methods. Overexpression of ERα and ERβ demonstrated that a percentage of the nuclear proteins are targeted to the plasma membrane [73] where they have been localized with immunohistochemistry, western blotting, and surface biotinylation [33,42,60–63,65,68,70,71,73,74,82,96,100,101]. In terms of reproduction, ER knockout mice in vivo demonstrated that ERα, but not ERβ, was critical for estrogen positive feedback and the LH surge [102]. In our laboratory, OVX ERα knockout mice (ERKO) mice in vivo treated with 17β-estradiol benzoate (10 μg) failed to significantly increase hypothalamic progesterone levels that were seen in OVX wild type mice [78]. Similar results were seen with in vitro studies using ERKO mouse astrocytes cultures such that the estradiol-induced [Ca2+]i response was significantly attenuated [78]. In addition, several other membrane-associated ERs that mediate rapid estrogen effects have been reported: ER-X [103,104], STX-activated protein called mER-Gαq [69,105] and G protein-coupled receptor 30 (GPR30) [66,67,72,106].
We examined several of these putative mERs in our astrocrine model in which estradiol elicits an increase in [Ca2+]i stimulating progesterone synthesis [78]. Since we have demonstrated both ERα and ERβ in the plasma membrane fractions of astrocytes [33], we selectively activated them with PPT, an ERα agonist [107], and DPN, an ERβ agonist [108,109]. As expected, PPT mimicked the estradiol actions and was antagonized by the mGluR1a antagonist LY367385 [78]. Conversely, equimolar DPN did not increase [Ca2+]i nor progesterone synthesis in our hypothalamic astrocyte cultures [78]. Furthermore, mERα co-immunoprecipitated with mGluR1a indicating a potential interaction, but mERβ did not co-immunoprecipitate with mGluR1a [62,78]. These results support the idea that, of the two classic ERs, ERα appears to be the mER mediating estradiol actions for progesterone synthesis in hypothalamic astrocytes, consistent with estrogen receptor knockout studies demonstrating that ERα, not ERβ, is essential for estrogen positive feedback and the LH surge [102].
The location of mERs had been a subject of controversy with possible cytoplasmic localization associated with the inner phospholipid bilayer. However, surface biotinylation with a membrane impermeable reagent tags proteins that protrude from the plasma membrane and has recently been used to confirm the status of ERα as a membrane protein with an extracellular portion, possibly containing the ligand binding domain in both hypothalamic astrocytes and neurons [60,96,110]. Hydropathicity analysis of ERα suggests a potential transmembrane domain near the amino-terminal domain (SOSUI, TMpred program), which support a potential extracellular ERα binding site. Unfortunately, the biotinylation experiments do not indicate which part of the ERα extends through the membrane. Interestingly, a 52–55 kDa ERα protein was also labeled by surface biotinylation. This protein is an alternatively spliced ERα that is missing exon 4 (ERαΔ 4) [97], which has been reported in the brain and breast [111–114]. Coincidentally, exon 4 of ERα codes for the hinge region directing nuclear localization, which may explain the preferential trafficking of ERαΔ 4 to the plasma membrane. However, only the full-length 66 kDa ERα co-immunoprecipitated with mGluR1a, which in astrocytes is needed for the estradiol-induced [Ca2+]i release and progesterone synthesis [62,78].
A significant observation from these experiments was the autoregulation of estradiol signaling. ERα trafficking to and from the plasma membrane was rapid, such that 5 min of estradiol treatment significantly increased mERα insertion and internalization, suggesting that de novo synthesis of ERα is probably not occurring [60]. Insertion is most likely through estradiol-induced exocytosis of vesicles containing mERα. Such ERα-immunoreactive vesicles have been observed in hippocampal neurons [115,116] and pituitary cells [117]. Internalization, a common mechanism for regulating membrane signaling, is usually through the formation of endocytic vesicles [83]. Once receptors are internalized and release their ligand, they can either be recycled to the cell surface or degraded. This mERα trafficking and internalization is blocked by the ER antagonist ICI 182,780 and dependant upon the presence of estradiol, with estradiol-induced [Ca2+]i response correlating with maximal insertion and internalization of mERα [60]. Continuous exposure to estradiol eventually reduced levels of mERα and its internalization to basal levels, suggesting a down-regulation of the receptor that temporally limits membrane-initiated cell signaling [60,96]. Membrane levels of ERα remained low for 24–48 h after estradiol exposure [60]. At some point after this down-regulation, mERα levels are partially restored. Currently, the time course for recovery of mERα levels is not known. If mERα-mGluR1a is a signaling unit, then blockade of the mGluR1a should prevent both the insertion of ERα into the membrane and its internalization. This is exactly what was observed [60]. Therefore, levels of mERα are autoregulated by the concentration of estradiol in the surrounding extracellular environment, which determines the magnitude of the MIES response and its duration.
In addition to mERα and mERβ, several other candidate mERs have been proposed [69,72,103]. One putative mER is GPR30, a G protein-coupled receptor (GPCR) that activates adenylyl cyclase in breast cancer cells lacking both ERα and ERβ [66,67,72,106]. Although FLAG- and hemagglutinin-tagged GPR30 have been reported at the plasma membrane [118,119], GPR30 could not be identified at the plasma membrane or labeled with surface biotinylation in native cells [60,78,110]. The GPR30 agonist G-1, a substituted dihydroquinoline [120], stimulated [Ca2+]i release and progesterone synthesis [78]. However, G-1 seemed to signal through a different mechanism compared to estradiol since G-1 was not blocked by antagonizing the mGluR1a [78]. The lack of interaction between GPR30 and mGluR1a was confirmed by the absence of co-immunoprecipitation between these proteins [78]. While it is difficult to understand the discrepancy with GPR30 localization in the plasma membrane, our results can be interpreted to support the observation of estradiol activation of intracellular GPR30 on the endoplasmic reticulum [67]. Activation of GPR30 may directly induce the release of intracellular stores of Ca2+, which in turn stimulates progesterone synthesis. However, only very large molar concentrations of G-1 activated [Ca2+]i release and progesterone synthesis [78]. Such a response was reminiscent of DPN, an ERβ agonist, which is not thought to be physiologically important in the regulation of the LH surge.
Another candidate receptor is a membrane-associated binding protein that is Gαq-coupled and activated by estrogen as well as STX, a diphenylacrylamide selective estrogen receptor modulator (SERM) [69]. STX remains efficacious in double ERα/ERβ knockout mice, but blocked with the ER antagonist ICI 182,780 [105]. STX does not activate ERα or ERβ having a million-fold lower binding affinity compared with estradiol for these receptors [69]. However, this STX-activated mER-Gαq also activates PLC [69], a signaling pathway similar to that activated by mERα-mGluR1a. In hypothalamic astrocytes, STX increased [Ca2+]i and progesterone synthesis through transactivation of mGluR1a [78]. It has been suggested that STX signals through GPR30, such that small interfering RNA directed against GPR30 abolished the STX-induced transcription [121]. However, estradiol has been proposed to signal through the STX-activated mER-Gαq pathway in GPR30 knockout mice [122]. In our hands, STX and G-1 produced distinctly different responses in hypothalamic astrocytes [78]. Although estradiol and STX responses are blocked by mGluR1a antagonism and activate the same PLC pathway, these actions are mediated through different receptors since STX stimulation of Ca2+ release remains in astrocytes from ERKO mice, where estradiol was ineffective [78]. Since ERKO mice do not demonstrate estrogen positive feedback and lack sexual receptivity due to the lack of estradiol effect, the STX response in ERKO mice is not consistent with a STX-related signaling mechanism for induction of the LH surge or receptivity in female reproduction [78,102,123,124]. Furthermore, this mER-Gαq has much lower affinity for estradiol (~20-fold) compared with STX [105]. Although STX was extremely potent compared with estradiol at stimulating the mER-Gαq in ERKO mouse astrocytes, STX does not exist in vivo and mER-Gαq activation by physiological estradiol levels seems too weak for any measurable signaling actions in parameters of reproduction. Therefore, the physiological relevance of the STX-activated mER-Gαq in reproductive function remains to be elucidated.
Lastly, ER-X has been proposed as an estrogen receptor during development and following injury, especially in the cortex [103,104]. This mER is not inhibited by ICI 182,780, but is activated by 17β-estradiol. Interestingly, ER-X is unique in that it is preferentially activated by 17α-estradiol [104]. However, in astrocytes, the estradiol action on [Ca2+]i is stereospecific [33] and the 17β-estradiol-induced [Ca2+]i release and progesterone synthesis was inhibited by ICI 182,780, which are not consistent with an ER-X mediated action [46,62].
In summary, several putative mERs are involved in regulation of [Ca2+]i release and progesterone synthesis in hypothalamic astrocytes [78]. The evidence was strongest for ERα and the STX-activated mER-Gαq, both of which required transactivation of mGluR1a to initiate cell signaling. From a reproductive vantage point as well as evidence from wild-type and ERKO hypothalamic astrocytes, ERαis the primary mER responsible for the rapid cell signaling that leads to an increase in hypothalamic neuroprogesterone [46,60,62,78].
5. Sex differences in estradiol stimulated progesterone release
Although both male and female rodents have a well-developed negative feedback mechanism regulating the release of GnRH and LH, one of the most robust sexually differentiated physiological responses is estrogen positive feedback, which induces the surge release of LH in response to estradiol stimulation. This phenomenon of estrogen positive feedback is a hallmark of various female animal species. Males, especially male rodents, do not exhibit this phenomenon. For rodents, once the ability to produce estrogen positive feedback is lost during development, this loss is permanent. In primates, including humans, many years of continuous estrogen exposure in males can result in an estrogen positive feedback response, although it is quite attenuated [125]. According to the epigenetic theory of sexual differentiation of the brain, the sex difference in estrogen positive feedback arises from the action of estradiol (aromatized from testosterone) during organization of the neural circuit(s) controlling the GnRH neurons [126–131]. Several mechanisms have been proposed to account for this differentiation, including the lack of estrogen-induced synaptic plasticity in the male arcuate nucleus [132] and an attenuated distribution of kisspeptin neurons in males [133]. Various structural sex differences that result from perinatal exposure to estradiol have been identified. In terms of regulating GnRH, males have greater postnatal apoptosis in the developing anteroventral periventricular nucleus (AVPV), a region crucial for estrogen positive feedback in females [134–136]. Although it is not clear whether such a sex difference in apoptosis is an important mechanism for elimination of estrogen positive feedback, it does support a role for postnatal sex steroids in organizing brain mechanisms involved in reproduction [137].
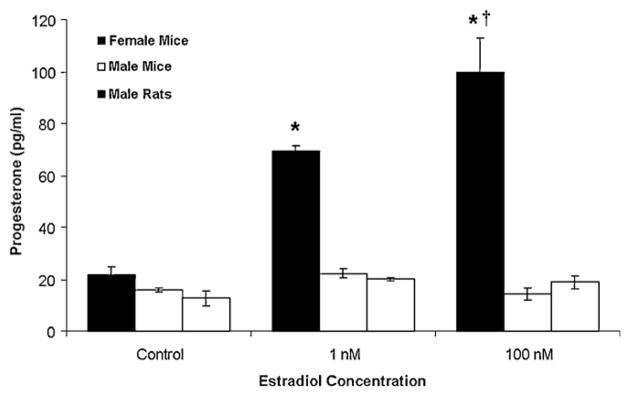
A mechanism for mediating estrogen positive feedback, the LH surge, and transition from proestrus to estrus involves the synthesis of neuroprogesterone by astrocytes in the hypothalamus [58,59]. Significantly, estradiol treatment in vivo increased progesterone levels in the female, but not male, hypothalamus [15,58]. In other words, males and reproductive senescent females, which do not have increased hypothalamic progesterone synthesis in response to estradiol, fail to demonstrate an estrogen positive feedback mechanism. Investigation of astrocytic sex differences in the post-pubertal hypothalamus of rodents further confirmed astrocytes and neuroprogesterone as a critical feature in the neurosteroid regulation of the LH surge. Male astrocytes had a significantly attenuated estradiol-induced [Ca2+]i response and failed to synthesize progesterone in contrast to female astrocytes (Fig. 2) [37,46,78,138]. This was consistent with previous observations that only post-pubertal female rodents have increased levels of progesterone in the hypothalamus before the LH surge [15]. There appears to be a crucial concentration of [Ca2+]i required for neuroprogesteone synthesis. For example, male astrocytes had an attenuated Ca2+ response that was unable to facilitate progesterone synthesis [138], which is consistent with previous reports in neonatal astrocytes [46] and in post-pubertal astrocytes, in which 0.1 nM estradiol stimulated [Ca2+]i release [62], but not progesterone synthesis [78]. One possible mechanism that could produce the decreased [Ca2+]i response is the attenuated insertion of mERα into male astrocytes compared with female astrocytes [138].
Fig. 2.
Sex differences in estradiol stimulated progesterone synthesis in post-pubertal hypothalamic astrocytes. Estradiol at 1 nM and 100 nM stimulated significant progesterone synthesis in female astrocytes (p < 0.05 vs. control). Conversely, male astrocytes did not synthesize progesterone (p > 0.05 vs. controls) when stimulated by estradiol at 1 nM or 100 nM. *Significantly different compared to female control and all male groups (p < 0.05, one-way ANOVA with Student–Newman–Keuls post hoc test). †Significantly different compared to female astrocytes stimulated with 1 nM estradiol (p < 0.05, one-way ANOVA with Student–Newman–Keuls post hoc test).
This Figure was originally published by BioMed Central and is reproduced from: Kuo et al. [138]. Biology of Sex Differences adheres to the BioMed Central Open Access license agreement.
Biological differences between males and females can result genetically from direct sex chromosome differences, developmentally through differential exposure to sex steroids during developmental “organization”, or functionally from acute “activational” effects of gonadal steroids operating at many life stages, which can be controlled through gonadectomy [139]. Perinatal gonadal hormone secretions have been shown to have powerful and permanent actions on physiology, including pituitary function, gene expression in the brain and sexual behavior [140–143]. In spite of these epigenetic effects, several chromosomal dependent sex differences have been demonstrated in the brain. Specifically, the four core genotype (FCG) mice model has demonstrated purely chromosomal XX versus XY differences in behaviors, including aggression, parenting, habit formation, nociception and social interactions [144]. In addition, Sry is expressed in the brain, and it has been shown to directly influence the biochemical properties of the dopaminergic neurons of the nigrostriatal system and the specific motor behaviors they control [145]. Although a stark difference between male and female astrocytic response to estradiol was demonstrated, it was not clear whether the differences result from the sex chromosome complement or to the presence of the Sry gene with its influence on gonadal development and the early sex steroid environment. Using FCG mice, animals with ovaries (XXF and XYF) had astrocytes in which estradiol facilitated progesterone synthesis, regardless of whether they had one or two X chromosomes [138]. Conversely, mice with testes (XYM and XXM) were unresponsive to estradiol and did not increase progesterone synthesis [138]. These results suggest a Sry transgene effect and not a sex chromosome effect on hypothalamic astrocyte response to estradiol. The effects from the Sry transgene could be due to direct effects of the Sry gene itself or its influence on gonadal differentiation and the sex steroid environment during early development. Interestingly, the XYM from FCG mice synthesized little or no progesterone [138], which could suggest a potential chromosomal effect. However, male wild type astrocytes, without Sry translocation to an autosome, synthesized basal progesterone levels similar to female wild type astrocytes [138]. Therefore, this difference could potentially be caused by the deletion and transgenic insertion of Sry, resulting in the inactivation of surrounding gene(s), positional effects or differential expression of the Sry transgene. Differences between wild type XY males and FCG XYM have been previously reported for mounting behavior, social exploration and concentration of tyrosine hydroxylase-immunoreactive neurons within the AVPV [146]. Unfortunately, the steroid profile of XYM in FCG mice has not yet been characterized.
6. Conclusion
Membrane ERs are clearly important for signaling in astrocytes as well as in neurons. Our studies have addressed several key questions: can ERα be a membrane receptor, how does it signal, what regulates its signaling, and is it sexually differentiated. Estradiol binds to mERα to transactivate mGluR1a, which activates the PLC/IP3 pathway leading to the release of IP3 receptor-sensitive stores of intracellular Ca2+. The rapid increase in [Ca2+]i activates various kinases resulting in the phosphorylation of enzymes that facilitate neuroprogesterone synthesis essential for estrogen positive feedback, the LH surge, and ovulation. In contrast to female astrocytes, male astrocytes had an attenuated [Ca2+]i release that was unable to facilitate progesterone synthesis. One possible mechanism for this sexually differentiated response is the attenuated trafficking of mERα to the plasma membrane in male astrocytes that may result from differential sex steroid exposure during early development.
We have also demonstrated that other putative ERs are present in astrocytes and participate in cell signaling, but their influence on reproductive function is still obscure. Female reproduction be it lordosis behavior or estrogen positive feedback requires ERα. In hypothalamic astrocytes, high doses of DPN were required to induced estradiol-like [Ca2+]i responses, most probably through weak non-selective mERα activation rather than mERβ activation, but even high doses of DPN failed to stimulate progesterone synthesis. Our studies do not resolve the role of GPR30 in MIES. We and others have not been able to demonstrate native GPR30 in the plasma membrane. GPR30 may uniquely mediate estradiol-like responses exclusively through an intracellular receptor on the endoplasmic reticulum. This is supported by the high concentration of G-1 required to stimulate a [Ca2+]i response and progesterone synthesis. Its dose response resembles that of DPN, which does not seem to play a critical role in the regulation of estrogen positive feedback. Unfortunately, the STX-activated mER-Gαq has yet to be identified. STX was extremely potent compared with estradiol at stimulating the mER-Gαq in ERKO mouse astrocytes, but physiological estradiol levels seem insufficient to stimulate any significant reproductive signaling effects through this receptor. To fully elucidate the role of GPR30 and the STX-activated mER-Gαq, additional experiments will be required. Overall, mERα in post-pubertal female hypothalamic astrocytes seems to play an essential role in sensing the rising levels of estradiol during proestrus to regulate neuroprogesterone levels critical for regulating estrogen positive feedback and the LH surge.
Acknowledgments
The research from our laboratory presented here was supported by National Institutes of Health (NIH) grants DA013185, HD042635 and HD001281 as well as the Reproductive Scientist Development Program through NIH grant HD00849-23 and Bayer HealthCare Pharmaceuticals.
References
- 1.Chazal G, Faudon M, Gogan F, Laplante E. Negative and positive effects of oestradiol upon luteinizing hormone secretion in the female rat. J Endocrinol. 1974;61(3):511–512. doi: 10.1677/joe.0.0610511. [DOI] [PubMed] [Google Scholar]
- 2.Brom GM, Schwartz NB. Acute changes in the estrous cycle following ovariectomy in the golden hamster. Neuroendocrinology. 1968;3(6):366–377. doi: 10.1159/000121725. [DOI] [PubMed] [Google Scholar]
- 3.Mahesh VB, Brann DW. Regulation of the preovulatory gonadotropin surge by endogenous steroids. Steroids. 1998;63(12):616–629. doi: 10.1016/s0039-128x(98)00075-0. [DOI] [PubMed] [Google Scholar]
- 4.Ferin M, Tempone A, Zimmering PE, Van de Wiele RL. Effect of antibodies to 17beta-estradiol and progesterone on the estrous cycle of the rat. Endocrinology. 1969;85(6):1070–1078. doi: 10.1210/endo-85-6-1070. [DOI] [PubMed] [Google Scholar]
- 5.Labhsetwar AP. Role of estrogens in ovulation: a study using the estrogen-antagonist, I.C.I. 46,474. Endocrinology. 1970;87(3):542–551. doi: 10.1210/endo-87-3-542. [DOI] [PubMed] [Google Scholar]
- 6.Rao IM, Mahesh VB. Role of progesterone in the modulation of the preovulatory surge of gonadotropins and ovulation in the pregnant mare’s serum gonadotropin-primed immature rat and the adult rat. Biol Reprod. 1986;35(5):1154–1161. doi: 10.1095/biolreprod35.5.1154. [DOI] [PubMed] [Google Scholar]
- 7.Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141(4):1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- 8.White MM, Sheffer I, Teeter J, Apostolakis EM. Hypothalamic progesterone receptor-A mediates gonadotropin surges, self priming and receptivity in estrogen-primed female mice. J Mol Endocrinol. 2007;38(1–2):35–50. doi: 10.1677/jme.1.02058. [DOI] [PubMed] [Google Scholar]
- 9.Mahesh VB, Brann DW. Neuroendocrine mechanisms underlying the control of gonadotropin secretion by steroids. Steroids. 1998;63(5–6):252–256. doi: 10.1016/s0039-128x(98)00031-2. [DOI] [PubMed] [Google Scholar]
- 10.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 11.Kalra SP, Kalra PS. Temporal interrelationships among circulating levels of estradiol, progesterone and LH during the rat estrous cycle: effects of exogenous progesterone. Endocrinology. 1974;95(6):1711–1718. doi: 10.1210/endo-95-6-1711. [DOI] [PubMed] [Google Scholar]
- 12.Feder HH, Brown-Grant K, Corker CS. Pre-ovulatory progesterone, the adrenal cortex and the ‘critical period’ for luteinizing hormone release in rats. J Endocrinol. 1971;50(1):29–39. doi: 10.1677/joe.0.0500029. [DOI] [PubMed] [Google Scholar]
- 13.Mann DR, Korowitz CD, Macfarland LA, Cost MG. Interactions of the light–dark cycle, adrenal glands and time of steroid administration in determining the temporal sequence of LH and prolactin release in female rats. Endocrinology. 1976;99(5):1252–1262. doi: 10.1210/endo-99-5-1252. [DOI] [PubMed] [Google Scholar]
- 14.Sridaran R, Blake CA. Effects of long-term adrenalectomy on periovulatory increases in serum gonadotrophins and ovulation in rats. J Endocrinol. 1980;84(1):75–82. doi: 10.1677/joe.0.0840075. [DOI] [PubMed] [Google Scholar]
- 15.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78(1):29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- 16.Le Goascogne C, Robel P, Gouezou M, Sananes N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987;237(4819):1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- 17.Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71(1–2):3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- 18.Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- 19.Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268(5216):1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- 20.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA. 1981;78(8):4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanne JL, Krueger KE. Expression of cytochrome P450 side-chain cleavage enzyme and 3 beta-hydroxysteroid dehydrogenase in the rat central nervous system: a study by polymerase chain reaction and in situ hybridization. J Neurochem. 1995;65(2):528–536. doi: 10.1046/j.1471-4159.1995.65020528.x. [DOI] [PubMed] [Google Scholar]
- 22.Jung-Testas I, Alliot F, Pessac B, Robel P, Baulieu EE. Immunocytochemical localization of cytochrome P-450scc in cultured rat oligodendrocytes. C R Acad Sci III. 1989;308(6):165–170. [PubMed] [Google Scholar]
- 23.Jung-Testas I, Hu ZY, Baulieu EE, Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology. 1989;125(4):2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- 24.Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629(2):283–292. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- 25.Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydroge-nase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30(2):287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- 26.Baulieu EE. Steroid hormones in the brain: several mechanisms? In: Fuxe K, Gustafsson JA, editors. Steroid Hormone Regulation of the Brain. Pergamon; Oxford: 1981. pp. 3–14. [Google Scholar]
- 27.Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination Growth Horm. IGF Res. 2004;14(Suppl A):S18–S33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Kugler W, Veenman L, Shandalov Y, Leschiner S, Spanier I, Lakomek M, Gavish M. Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomo-choline. Cell Oncol. 2008;30(5):435–450. doi: 10.3233/CLO-2008-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290(1–2):44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magistretti PJ. Neuronglia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 31.Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27(2):331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317(5841):1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 33.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145(8):3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 34.Hirst WD, Price GW, Rattray M, Wilkin GP. Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles. Neurochem Int. 1998;33(1):11–22. doi: 10.1016/s0197-0186(05)80003-8. [DOI] [PubMed] [Google Scholar]
- 35.Hosli E, Hosli L. Autoradiographic localization of binding sites for arginine vasopressin and atrial natriuretic peptide on astrocytes and neurons of cultured rat central nervous system. Neuroscience. 1992;51(1):159–166. doi: 10.1016/0306-4522(92)90480-p. [DOI] [PubMed] [Google Scholar]
- 36.Oka M, Wada M, Wu Q, Yamamoto A, Fujita T. Functional expression of metabotropic GABAB receptors in primary cultures of astrocytes from rat cerebral cortex. Biochem Biophys Res Commun. 2006;341(3):874–881. doi: 10.1016/j.bbrc.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 37.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev. 2008;57(2):470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchanan CD, Mahesh VB, Brann DW. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: a role for transforming growth factor-beta(1) Biol Reprod. 2000;62(6):1710–1721. doi: 10.1095/biolreprod62.6.1710. [DOI] [PubMed] [Google Scholar]
- 39.Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26(3):260–267. [PubMed] [Google Scholar]
- 40.Garcia-Segura LM, Naftolin F, Hutchison JB, Azcoitia I, Chowen JA. Role of astroglia in estrogen regulation of synaptic plasticity and brain repair. J Neurobiol. 1999;40(4):574–584. [PubMed] [Google Scholar]
- 41.Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra. J Comp Neurol. 2007;503(1):198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawlak J, Karolczak M, Krust A, Chambon P, Beyer C. Estrogen receptor-alpha is associated with the plasma membrane of astrocytes and coupled to the MAP/Src-kinase pathway. Glia. 2005;50(3):270–275. doi: 10.1002/glia.20162. [DOI] [PubMed] [Google Scholar]
- 43.Mong JA, Blutstein T. Estradiol modulation of astrocytic form and function: implications for hormonal control of synaptic communication. Neuroscience. 2006;138(3):967–975. doi: 10.1016/j.neuroscience.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Segura LM, Torres-Aleman I, Naftolin F. Astrocytic shape and glial fibrillary acidic protein immunoreactivity are modified by estradiol in primary rat hypothalamic cultures. Brain Res Dev Brain Res. 1989;47(2):298–302. doi: 10.1016/0165-3806(89)90186-7. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy MM, Amateau SK, Mong JA. Steroid modulation of astrocytes in the neonatal brain: implications for adult reproductive function. Biol Reprod. 2002;67(3):691–698. doi: 10.1095/biolreprod.102.003251. [DOI] [PubMed] [Google Scholar]
- 46.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148(2):782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 47.Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;25(5):343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- 48.Torres-Aleman I, Rejas MT, Pons S, Garcia-Segura LM. Estradiol promotes cell shape changes and glial fibrillary acidic protein redistribution in hypothalamic astrocytes in vitro: a neuronal-mediated effect. Glia. 1992;6(3):180–187. doi: 10.1002/glia.440060305. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48(2):273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Cashion AB, Smith MJ, Wise PM. The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology. 2003;144(1):274–280. doi: 10.1210/en.2002-220711. [DOI] [PubMed] [Google Scholar]
- 51.Cavarretta I, Magnaghi V, Ferraboschi P, Martini L, Melcangi RC. Interactions between type 1 astrocytes and LHRH-secreting neurons (GT1-1 cells): modification of steroid metabolism and possible role of TGFbeta1. J Steroid Biochem Mol Biol. 1999;71(1–2):41–47. doi: 10.1016/s0960-0760(99)00121-1. [DOI] [PubMed] [Google Scholar]
- 52.Galbiati M, Martini L, Melcangi RC. Oestrogens, via transforming growth factor alpha, modulate basic fibroblast growth factor synthesis in hypothalamic astrocytes: in vitro observations. J Neuroendocrinol. 2002;14(10):829–835. doi: 10.1046/j.1365-2826.2002.00852.x. [DOI] [PubMed] [Google Scholar]
- 53.Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246(1–2):1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Zwain IH, Arroyo A, Amato P, Yen SS. A role for hypothalamic astrocytes in dehydroepiandrosterone and estradiol regulation of gonadotropin-releasing hormone (GnRH) release by GnRH neurons. Neuroendocrinology. 2002;75(6):375–383. doi: 10.1159/000059434. [DOI] [PubMed] [Google Scholar]
- 55.Akwa Y, Sananes N, Gouezou M, Robel P, Baulieu EE, Le Goascogne C. Astrocytes and neurosteroids: metabolism of pregnenolone and dehydroepiandrosterone. Regulation by cell density. J Cell Biol. 1993;121(1):135–143. doi: 10.1083/jcb.121.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung-Testas I, Do Thi A, Koenig H, Desarnaud F, Shazand K, Schumacher M, Baulieu EE. Progesterone as a neurosteroid: synthesis and actions in rat glial cells. J Steroid Biochem Mol Biol. 1999;69(1–6):97–107. doi: 10.1016/s0960-0760(98)00149-6. [DOI] [PubMed] [Google Scholar]
- 57.Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140(8):3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- 58.Micevych P, Bondar G, Kuo J. Estrogen actions on neuroendocrine glia. Neuroendocrinology. 2010;91(3):211–222. doi: 10.1159/000289568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Micevych P, Sinchak K. Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology. 2008;149(6):2739–2742. doi: 10.1210/en.2008-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29(48):15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirahara Y, Matsuda K, Gao W, Arvanitis DN, Kawata M, Boggs JM. The localization and non-genomic function of the membrane-associated estrogen receptor in oligodendrocytes. Glia. 2009;57(2):153–165. doi: 10.1002/glia.20742. [DOI] [PubMed] [Google Scholar]
- 62.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150(3):1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 64.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 65.Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138(3):851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 67.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A trans-membrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 68.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16(1):231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23(29):9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142(6):2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 71.Ivanova T, Karolczak M, Beyer C. Estrogen stimulates the mitogen-activated protein kinase pathway in midbrain astroglia. Brain Res. 2001;889(1–2):264–269. doi: 10.1016/s0006-8993(00)03149-8. [DOI] [PubMed] [Google Scholar]
- 72.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 73.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13(2):307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 74.Lagrange AH, Ronnekleiv OK, Kelly MJ. Estradiol-17 beta and muopioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136(5):2341–2344. doi: 10.1210/endo.136.5.7720682. [DOI] [PubMed] [Google Scholar]
- 75.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114(1):152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 76.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58(4):1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beyer C, Raab H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. Eur J Neurosci. 1998;10(1):255–262. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- 78.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30(39):12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 80.Hawkins RA, Freedman B, Marshall A, Killen E. Oestradiol-17 beta and prolactin levels in rat peripheral plasma. Br J Cancer. 1975;32(2):179–185. doi: 10.1038/bjc.1975.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaikh AA, Shaikh SA. Adrenal and ovarian steroid secretion in the rat estrous cycle temporally related to gonadotropins and steroid levels found in peripheral plasma. Endocrinology. 1975;96(1):37–44. doi: 10.1210/endo-96-1-37. [DOI] [PubMed] [Google Scholar]
- 82.Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuro-protection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29(13):4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30(3):315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 85.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermel-stein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27(35):9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149(12):5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290(1–2):14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25(20):5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watters JJ, Dorsa DM. Transcriptional effects of estrogen on neuronal neu-rotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci. 1998;18(17):6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manna PR, Jo Y, Stocco DM. Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling. J Endocrinol. 2007;193(1):53–63. doi: 10.1677/JOE-06-0201. [DOI] [PubMed] [Google Scholar]
- 91.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 92.Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19(11):2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- 93.Itzhak Y, Baker L, Norenberg MD. Characterization of the peripheral-type benzodiazepine receptors in cultured astrocytes: evidence for multiplicity. Glia. 1993;9(3):211–218. doi: 10.1002/glia.440090306. [DOI] [PubMed] [Google Scholar]
- 94.Serra M, Madau P, Chessa MF, Caddeo M, Sanna E, Trapani G, Franco M, Liso G, Purdy RH, Barbaccia ML, Biggio G. 2-Phenylimidazo[1,2-a]pyridine derivatives as ligands for peripheral benzodiazepine receptors: stimulation of neurosteroid synthesis and anticonflict action in rats. Br J Pharmacol. 1999;127(1):177–187. doi: 10.1038/sj.bjp.0702530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCauley LD, Park CH, Lan NC, Tomich JM, Shively JE, Gee KW. Benzodiazepines and peptides stimulate pregnenolone synthesis in brain mitochondria. Eur J Pharmacol. 1995;276(1–2):145–153. doi: 10.1016/0014-2999(95)00036-k. [DOI] [PubMed] [Google Scholar]
- 96.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30(38):12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dominguez R, Kuo J, Dewing P, Micevych P. Trafficking of membrane ER involves membrane-initiated estrogen signaling in immortalized hypothalamic N-38 neurons. Neuroendocrinology. doi: 10.1016/j.steroids.2012.12.008. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology. 2009;150(8):3699–3708. doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146(10):4331–4339. doi: 10.1210/en.2005-0575. [DOI] [PubMed] [Google Scholar]
- 100.Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor alpha and beta in reactive astrocytes at the male rat hippocampus after status epilepticus. Neuropathology. 2009;29(1):55–62. doi: 10.1111/j.1440-1789.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- 101.Lagrange AH, Wagner EJ, Ronnekleiv OK, Kelly MJ. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64(2):114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- 102.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toran-Allerand CD. Novel sites and mechanisms of oestrogen action in the brain. Novartis Found Symp. 2000;230:56–69. doi: 10.1002/0470870818.ch6. (discussion 69–73) [DOI] [PubMed] [Google Scholar]
- 104.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22(19):8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26(21):5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16(1):70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 107.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure–affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 108.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206(1–2):13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 109.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure–activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 110.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuro-science. 2008;154(4):1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 111.Fuqua SA, Fitzgerald SD, Allred DC, Elledge RM, Nawaz Z, McDonnell DP, O’Malley BW, Greene GL, McGuire WL. Inhibition of estrogen receptor action by a naturally occurring variant in human breast tumors. Cancer Res. 1992;52(2):483–486. [PubMed] [Google Scholar]
- 112.Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol Endocrinol. 2000;14(5):634–649. doi: 10.1210/mend.14.5.0460. [DOI] [PubMed] [Google Scholar]
- 113.Deecher DC, Swiggard P, Frail DE, O’Connor LT. Characterization of a membrane-associated estrogen receptor in a rat hypothalamic cell line (D12) Endocrine. 2003;22(3):211–223. doi: 10.1385/ENDO:22:3:211. [DOI] [PubMed] [Google Scholar]
- 114.Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134(1):81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 115.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultra-structural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429(3):355–371. [PubMed] [Google Scholar]
- 116.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27(8):2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gonzalez M, Reyes R, Damas C, Alonso R, Bello AR. Oestrogen receptor alpha and beta in female rat pituitary cells: an immunochemical study. Gen Comp Endocrinol. 2008;155(3):857–868. doi: 10.1016/j.ygcen.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 118.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346(3):904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 119.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148(7):3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 120.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 121.Lin BC, Suzawa M, Blind RD, Tobias SC, Bulun SE, Scanlan TS, Ingraham HA. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res. 2009;69(13):5415–5423. doi: 10.1158/0008-5472.CAN-08-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73(9–10):985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138(1):507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 124.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 125.Goh HH, Ratnam SS. The LH surge in humans: its mechanism and sex difference. Gynecol Endocrinol. 1988;2(2):165–182. doi: 10.3109/09513598809023624. [DOI] [PubMed] [Google Scholar]
- 126.Neill JD. Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology. 1972;90(5):1154–1159. doi: 10.1210/endo-90-5-1154. [DOI] [PubMed] [Google Scholar]
- 127.Rudd CD, Short RV, McFarlane JR, Renfree MB. Sexual differentiation of oestradiol-LH positive feedback in a marsupial. J Reprod Fertil. 1999;115(2):269–274. doi: 10.1530/jrf.0.1150269. [DOI] [PubMed] [Google Scholar]
- 128.Gorski RA. Sexual differentiation of the endocrine brain and its control. In: Motta M, editor. Brain Endocrinology. Raven Press; New York: 1991. pp. 71–104. [Google Scholar]
- 129.Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;61(Suppl 3):38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- 130.Booth JE. Sexual differentiation of the brain. In: Finn CA, editor. Oxford Reviews of Reproductive Biology. Clarendon Press; Oxford: 1979. pp. 58–158. [Google Scholar]
- 131.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 132.Horvath TL, Garcia-Segura LM, Naftolin F. Lack of gonadotropin-positive feedback in the male rat is associated with lack of estrogen-induced synaptic plasticity in the arcuate nucleus. Neuroendocrinology. 1997;65(2):136–140. doi: 10.1159/000127173. [DOI] [PubMed] [Google Scholar]
- 133.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19(3):302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 135.Yoshida M, Yuri K, Kizaki Z, Sawada T, Kawata M. The distributions of apoptotic cells in the medial preoptic areas of male and female neonatal rats. Neurosci Res. 2000;36(1):1–7. doi: 10.1016/s0168-0102(99)00100-5. [DOI] [PubMed] [Google Scholar]
- 136.Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol. 2006;66(13):1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- 137.Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol. 2009;21(4):370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- 138.Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol Sex Differ. 2010;1(1):7. doi: 10.1186/2042-6410-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arnold AP, van Nas A, Lusis AJ. Systems biology asks new questions about sex differences. Trends Endocrinol Metab. 2009;20(10):471–476. doi: 10.1016/j.tem.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Micevych PE, Abelson L, Fok H, Ulibarri C, Priest CA. Gonadal steroid control of preprocholecystokinin mRNA expression in the limbic–hypothalamic circuit: comparison of adult with neonatal steroid treatments. J Neurosci Res. 1994;38(4):386–398. doi: 10.1002/jnr.490380404. [DOI] [PubMed] [Google Scholar]
- 141.Ward I. Differential effect of pre- and postnatal androgen on the sexual behavior of intact and spayed rats. Horm Behav. 1969;1:25–36. [Google Scholar]
- 142.Gorski RA. Gonadal hormones and the perinatal development of neuroendocrine function. In: Martini L, Ganong WF, editors. Frontiers in Neuroendocrinology. Oxford University Press; New York: 1971. pp. 237–290. [Google Scholar]
- 143.Pfeiffer CA. Sexual differences of the hyphophyses and their determination by the gonads. Am J Anat. 1936;58:195–225. [Google Scholar]
- 144.Arnold AP, Chen X. What does the four core genotypes mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, Ches-selet MF, Micevych PE, Albrecht KH, Harley VR, Vilain E. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006;16(4):415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 146.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22(20):9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]