Abstract
Background
Three first-line antituberculosis drugs, isoniazid, rifampicin and pyrazinamide, may induce liver injury, especially isoniazid. This antituberculosis drug-induced liver injury (ATLI) ranges from a mild to severe form, and the associated mortality cases are not rare. In the past decade, many investigations have focused the association between drug-metabolising enzyme (DME) gene polymorphisms and risk for ATLI; however, these studies have yielded contradictory results.
Methods
PubMed, EMBASE, ISI web of science and the Chinese National Knowledge Infrastructure databases were systematically searched to identify relevant studies. A meta-analysis was performed to examine the association between polymorphisms from 4 DME genes (NAT2, CYP2E1, GSTM1 and GSTT1) and susceptibility to ATLI. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Heterogeneity among articles and their publication bias were also tested.
Results
38 studies involving 2,225 patients and 4,906 controls were included. Overall, significantly increased ATLI risk was associated with slow NAT2 genotype and GSTM1 null genotype when all studies were pooled into the meta-analysis. Significantly increased risk was also found for CYP2E1*1A in East Asians when stratified by ethnicity. However, no significant results were observed for GSTT1.
Conclusions
Our results demonstrated that slow NAT2 genotype, CYP2E1*1A and GSTM1 null have a modest effect on genetic susceptibility to ATLI.
Introduction
Tuberculosis (TB) is an important infectious disease plaguing the developed and developing countries worldwide, with more than nine million new cases of active tuberculosis reported annually. Isoniazid (INH), rifampicin (RIF) and pyrazinamide (PZA) are all widely used as first-line multidrug therapy for TB. Anti-tuberculosis drug induced liver injury (ATLI), a common serious adverse drug reaction, is one of the most challenging clinical problems, cause of hospitalization and life-threatening events. ATLI can be fatal if therapy is not interrupted on time, and the subsequent adherence problem may cause treatment failure and, relapse or drug resistance [1], [2].
Of the various antituberculosis regimens, isoniazid is the main drug to induce hepatotoxicity [3]. Metabolic intermediates of isoniazid are incriminated to be the cause of hepatotoxicity. In the liver, isoniazid is first metabolized into acetylisoniazid via N-acetyltransferase (NAT) [4], followed by hydrolysis to acetylhydrazine. Acetylhydrazine is proposed to be oxidized into hepatotoxic intermediates by cytochrome P450 2E1 (CYP2E1) [5] and some hepatotoxic intermediates can be detoxified by glutathione S-transferase (GST) enzyme [6]. Drug-metabolizing enzymes (DME) have critical effects on both the synthesis and detoxification of reactive metabolites [7]. Therefore, studies on genetic predisposition for anti-TB drug-induced liver injury have focused on a few metabolizing enzymes including N-acetyltransferase 2 (NAT2), CYP2E1, GSTM1 and GSTT1.
Over the past few years, considerable efforts have been devoted to exploring the relationships between the DME polymorphisms and ATLI among various populations. However, existing studies have yielded inconsistent results. These disparate findings may be due partly to insufficient power, false-positive results, and publication biases. Therefore, we performed a meta-analysis of the published studies to clarify this inconsistency and obtain summary risk estimates for the association of specific polymorphism in DME and risk of ATLI.
Materials and Methods
Literature Search Strategy
Eligible literature published before the end of May 2012 were identified through a search of PubMed, EMBASE, ISI Web of Science and CNKI (Chinese National Knowledge Infrastructure) without language restriction. Search term combinations were as follows: drug-metabolising enzymes, antitubercular agents, drug-induced hepatotoxicity, drug-induced liver injury, genetic polymorphism, arylamine N-acetyltransferase, glutathione-S-transferase, cytochrome 2E1, acetylator phenotype, genetic susceptibility. All reference lists from the main reports and relevant reviews were hand searched for additional eligible studies.
Eligible Studies and Data Extraction
Eligible studies had to meet all of the following criteria: (i) the studies were published in peer-reviewed journals and were independent studies using original data; (ii) the studies provided genotype distribution information of polymorphism in both cases and controls or odds ratio (OR) with its 95% confidence interval and P value; (iii) the studies investigated the DME polymorphism using either case-control or cohort design; (iv) the studies described the genotyping method, equipment, and protocols used or provided reference to them and (v) the studies used TB patients without ATLI as controls.
For each study, the following data were extracted independently by two authors: first author’s surname, year of publication, diagnosis criterion, age, sex, ethnicity, genotyping method, total number of cases and controls and genotype frequency in cases and controls. The results were compared, and disagreements were discussed and resolved with consensus.
Statistical Methods
The proposed risk genotypes are NAT2 slow acetylator (without wild-type NAT2*4 allele), CYP2E1*1A/*1A (homozygous wild type c1/c1) and homozygous null GST genotype (GSTM1 null/null or GSTT1 null/null). Our primary analysis for measuring the overall effects of every DME was to compare the genotype distribution for ATLI patients against controls by the contrast of the above risk genotypes vs. other combined genotypes.
Odds ratio (OR) with 95% confidence intervals (CIs) was used to assess the strength of association between gene polymorphism and ATLI risk. Cochran’s chi-square-based Q statistic test was performed in order to assess possible heterogeneity between the individual studies and thus to ensure that each group of studies was suitable for meta-analysis. ORs were pooled according to the method of DerSimonian and Laird that takes into account the variation between studies, and 95% CI were constructed using Woolf’s method [8], [9]. The Z test was used to determine the significance of the pooled OR. Sensitivity analyses were performed to assess the stability of the results, namely, a single study in the meta-analysis was deleted each time to reflect the influence of the individual data set to the overall OR. Publication bias was assessed using Egger’s test [10] and Begg’s funnel plots [11]. All P values are two-sided, and P<0.05 were considered statistically significant. Statistical analyses were done with Stata (version 10.0).
Results
Characteristics of Studies
The combined search yielded 412 references. Study selection process was shown in Figure S1. A total of 38 studies were finally included with 2,225 patients and 4,906 controls [12]–[42]. For the CYP2E1, 13 studies were available, including a total of 674 cases and 1,990 controls. For the NAT2, 24 studies involved a total of 1,116 cases and 2,655 controls. For the GST M1, 11 studies involved a total of 896 cases and 1,604 controls. For the GST T1, 10 studies involved a total of 792 cases and 1,493 controls. Of the cases, 80% were East Asian, 8% were Indian, 7% were Caucasian, and 5% were of other ethnic populations. The detailed characteristics of the studies included in this meta-analysis are shown in Table 1.
Table 1. Characteristics of the studies included in the meta-analysis.
| Study | Year | Ethnicity | Gene | No. of case/control | Mean age of case/control | Gender component in caseand control (% male) | Genotyping method |
| Tang [12] | 2012 | Chinese | CYP2E1; GSTM1; GSTT1 | 89/356 | 32.7/43.6 | 73.0/73.0 | RFLP |
| An [13] | 2012 | Chinese | NAT 2; CYP2E1 | 101/107 | 36.0/33.4 | 55.4/70.0 | Sequencing |
| Rana [14] | 2012 | Indian | NAT2 | 50/201 | 45.3/43.8 | 24.8/75.2 | RFLP |
| Mahmoud [15] | 2011 | Tunisian | NAT2 | 14/52 | 42.4/42.1 | 42.8/48.1 | RFLP |
| Zhu [16] | 2011 | Chinese | GSTM1; GSTT1 | 228/300 | 39.8/37.5 | 57.9/53.3 | PCR |
| Fernandez [17] | 2011 | Spanish | NAT2 | 50/67 | 34/30.5 | 54.0/56.7 | RFLP |
| Sotsuka [18] | 2011 | Japanese | NAT2; CYP2E1; GSTM1; GSTT1 | 52/92 | 54.9/50.4 | 92.3/73.9 | RFLP; PCR |
| Sistanizad [19] | 2011 | Iranian | NAT2 | 14/36 | 43.1/49.6 | 57.1/55.5 | RFLP |
| Huang [20] | 2011 | Chinese | NAT2 | 119/198 | 41.0/44.0 | 66.4/64.1 | Sequencing |
| Teixeira [21] | 2011 | Brazilian | CYP2E1; GSTM1; GSTT1 | 26/141 | 47.6/43.0 | 61.5/52.5 | Sequencing; PCR |
| Yimer [22] | 2011 | European | NAT2 | 41/160 | NA/NA | NA/NA | Sequencing |
| Bose [23] | 2011 | Indian | NAT2; CYP2E1 | 41/177 | 38.0/36.0 | 43.9/47.4 | RFLP |
| Chatterjee [24] | 2010 | Indian | GSTM1; GSTT1 | 51/100 | 37.2/33.2 | 49.0/63.0 | PCR |
| Wang [25] | 2010 | Chinese | GSTM1; CYP2E1 | 104/111 | 48.6/44.7 | 67.3/67.6 | PCR |
| Lee [26] | 2010 | Chinese | NAT2; CYP2E1 | 45/95 | 58.4/54.9 | 60.0/66.3 | Taqman |
| Kim [27] | 2010 | Korean | GSTM1; GSTT1 | 57/190 | 47.3/42.4 | 59.6/67.9 | PCR |
| Wu [28] | 2010 | Chinese | NAT2 | 155/162 | NA/NA | 58.0/60.0 | Taqman |
| Guo [29] | 2010 | Chinese | NAT2 | 106/106 | 48.8/48.6 | 68.9/68.9 | RFLP |
| Chen [30] | 2010 | Chinese | CYP2E1 | 103/236 | 45.0/45.7 | 78.6/66.1 | RFLP |
| Wang [31] | 2009 | Chinese | CYP2E1 | 104/111 | 47.7/45.5 | 61.1/64.0 | RFLP |
| Guo [32] | 2009 | Chinese | GSTM1; GSTT1 | 106/106 | 48.8/48.6 | 68.9/68.9 | PCR |
| Kim [33] | 2009 | Korean | NAT2 | 67/159 | 42.1/42.8 | 65.7/65.4 | SNPstream |
| Yamada [34] | 2009 | Japanese | NAT2; CYP2E1 | 23/147 | NA/NA | 13.0/42.8 | Sequencing, RFLP |
| Wang [35] | 2009 | Chinese | NAT2 | 36/36 | NA/NA | NA/NA | RFLP |
| Leiro [36] | 2008 | Spanish | GSTM1; GSTT1 | 35/60 | 34.0/31.0 | 40.0/41.7 | PCR |
| Bozok [37] | 2008 | Turkish | NAT2 | 30/70 | 39.8/37.3 | 50.0/72.8 | PCR |
| Possuelo [38] | 2008 | Brazilian | NAT2 | 14/240 | 38.9/36.5 | 50.0/67.9 | Sequencing |
| Huang [39] | 2007 | Chinese | GSTM1; GSTT1 | 115/115 | 60.3/59.1 | 63.5/63.5 | PCR |
| Cho [40] | 2007 | Korean | NAT2; CYP2E1 | 18/114 | 51.2/46.7 | 66.7/55.3 | Sequencing |
| Higuchi [41] | 2007 | Japanese | NAT2 | 18/82 | 60.8/64.7 | 50.0/57.3 | RFLP |
| Vuilleumier [42] | 2006 | Swiss | CYP2E1 | 34/55 | NA/NA | NA/NA | RFLP |
| Shimizu [43] | 2006 | Japanese | NAT2 | 10/32 | 60.5/64.9 | 70.0/46.9 | RFLP |
| Roy [44] | 2006 | Indian | CYP2E1 | 8/101 | NA/NA | NA/NA | RFLP |
| Wang [45] | 2004 | Chinese | NAT2 | 32/35 | NA/NA | 53.1/45.7 | RFLP |
| Huang [46] | 2003 | Chinese | NAT2; CYP2E1 | 49/269 | 70.0/59.0 | 18.4/14.9 | RFLP |
| Huang [47] | 2002 | Chinese | NAT2 | 33/191 | 73.3/63.7 | 87.9/88.5 | RFLP |
| Roy [48] | 2001 | Indian | NAT2; GSTM1; GSTT1 | 33/33 | NA/NA | NA/NA | PCR |
| Ohno [49] | 2000 | Japanese | NAT2 | 14/63 | NA/NA | NA/NA | RFLP |
Association of CYP2E1 Gene with ATLI
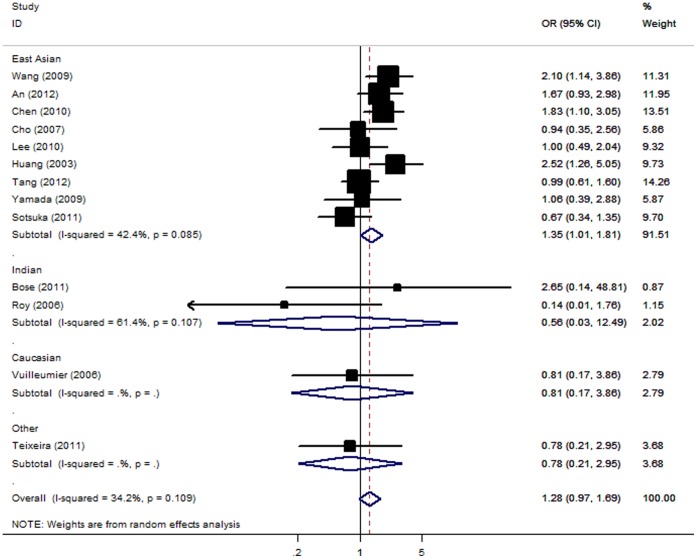
Meta-analysis revealed no statistically significant association between ATLI and CYP2E1 c1/c1 genotype [OR = 1.28; 95% CI: 0.97–1.69; P(Z) = 0.08; P(Q) = 0.11]. When studies were stratified for ethnicity, significant risks were found among East Asians [OR = 1.35; 95% CI: 1.01–1.81; P(Z) = 0.04; P(Q) = 0.08]. However, no significant associations were detected among Indian, Caucasian and other ethnic populations (Figure 1). In the subgroup analyses by sample size, the summary OR for big studies of the c1/c1 genotype was 1.36 [95% CI: 0.92–2.00; P(Z) = 0.12; P(Q) = 0.05] and for small studies was 1.17 [95% CI: 0.75–1.81; P(Z) = 0.49; P(Q) = 0.28].
Figure 1. Forest plot from the meta-analysis of ATLI and CYP2E1.
Association of NAT2 Gene with ATLI
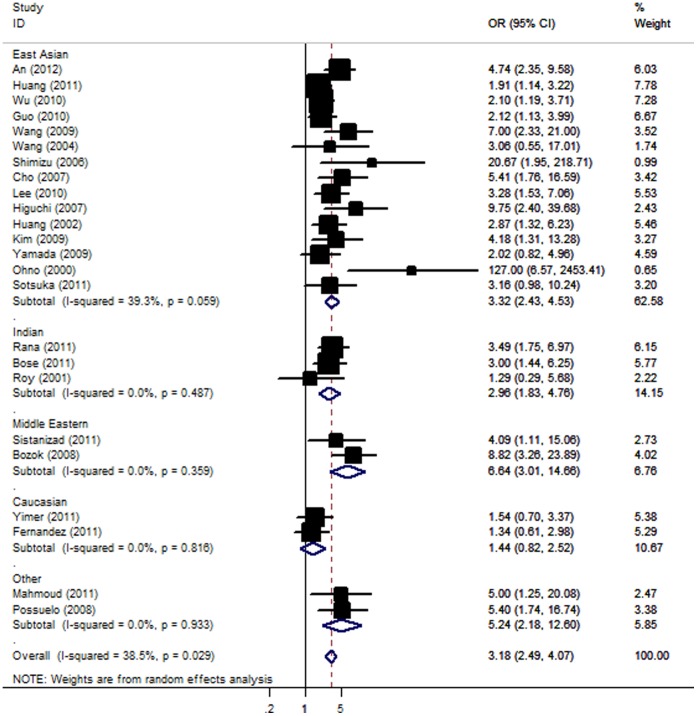
Overall, there was evidence of an association between the increased risk of ATLI and the variant when all eligible studies were pooled into the meta-analysis. Using random effect model, the summary OR of the NAT2 slow acetylator genotype for ATLI was 3.18 [95% CI: 2.49–4.07; P(Z) <10−5; P(Q) = 0.03]. When stratifying for ethnicity, an OR of 3.32 [95% CI: 2.43–4.53; P(Z) <10−5; P(Q) = 0.06], 2.96 [95% CI: 1.83–4.76; P(Z) <10−4; P(Q) = 0.49], 6.64 [95% CI: 3.01–14.66; P(Z) <10−4; P(Q) = 0.36] and 5.24 [95% CI: 2.18–12.60; P(Z) <10−4; P(Q) = 0.93] resulted for the slow acetylator genotype, among East Asian, Indian, Middle Eastern and other ethnic population, respectively (Figure 2). Unfortunately, we failed to detect any association to ATLI risk for Caucasians. Subsidiary analyses of control source yielded an overall OR for big studies of 2.48 [95% CI: 1.87–3.30; P(Z) <10−5; P(Q) = 0.26] and for small studies of 3.92 [95% CI: 2.75–5.58; P(Z) <10−5; P(Q) = 0.06].
Figure 2. Forest plot from the meta-analysis of ATLI and NAT2.
Association of GSTM1 and GSTT1 Gene with ATLI
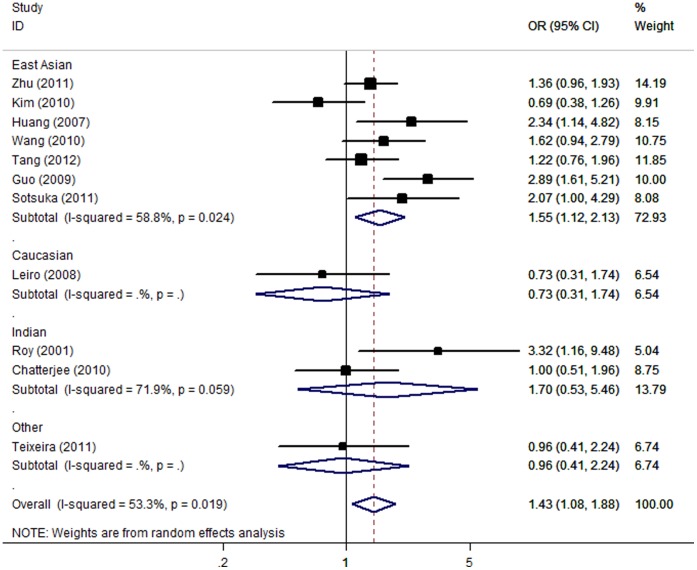
For ATLI risk and the null genotype of GSTM1, our meta-analysis gave an overall OR of 1.43 [95% CI: 1.08–1.88; P(Z) = 0.01; P(Q) = 0.02] with statistically significant between-study heterogeneity. This analysis is based on pooling of data from a number of different ethnic populations. When stratifying for ethnicity, an OR of 1.55 [95% CI: 1.12–2.13; P(Z) = 0.008; P(Q) = 0.02] resulted for null genotype among East Asians; while no significant association was found among Caucasian and Indian populations (Figure 3). By considering sample size subgroups, the OR was 1.61 [95% CI: 1.18–2.19; P(Z) = 0.003; P(Q) = 0.005] in big studies, compared to 1.26 [95% CI: 0.54–2.95; P(Z) = 0.59; P(Q) = 0.08] in small studies.
Figure 3. Forest plot from the meta-analysis of ATLI and GST M1.
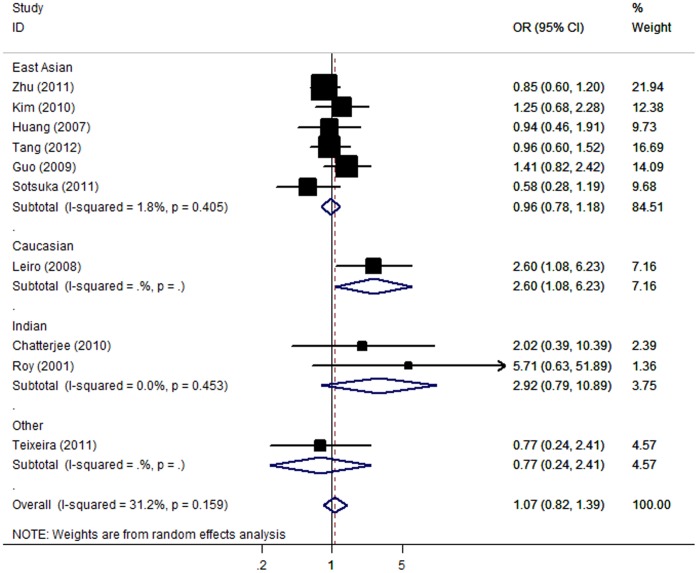
The meta-analysis resulted in a statistically non-significant association between GSTT1 deficiency and ATLI. The overall OR was 1.07 [95% CI: 0.82–1.39; P(Z) = 0.61; P(Q) = 0.16]. When stratifying for ethnicity, an OR of 0.96 [95% CI: 0.78–1.18; P(Z) = 0.69; P(Q) = 0.40] and 2.92 [95% CI: 0.79–10.89; P(Z) = 0.11; P(Q) = 0.45] resulted for null genotype, among East Asian and Indian populations, respectively (Figure 4). No significant association was found in stratified analyses according to sample size. The OR was 0.97 [95% CI: 0.79–1.19; P(Z) = 0.76; P(Q) = 0.44] in big studies, 1.88 [95% CI: 0.68–5.20; P(Z) = 0.23; P(Q) = 0.15] in small studies.
Figure 4. Forest plot from the meta-analysis of ATLI and GST T1.
The effect of each genotype of GSTs was independently assessed. The data on both null genotype of GSTs among cases and controls were available in five studies, which included 460 cases and 1006 controls. The interaction between GSTM1 null and GSTT1 null, for which an OR of 1.12 [95% CI: 0.86–1.48; P(Z) = 0.40; P(Q) = 0.69] for ATLI appeared in compared with individuals with the positive genotypes.
Sensitivity Analyses and Publication Bias
Sensitivity analyses were performed to assess the influence of each individual study on the pooled OR by sequential removal of individual studies. The results suggested that no individual study significantly affected the pooled OR, thus suggesting that the results of this meta-analysis are stable.
A funnel plot of these 24 included studies concerning NAT2 and ATLI suggested a possibility of the preferential publication of positive findings in smaller studies (t = 3.72, P = 0.001; Figure S2). The shape of the funnel plots was symmetrical (Figures S3, S4 and S5) for CYP2E1, GSTM1 and GSTT1. The statistical results still did not show publication bias in these studies for CYP2E1 (t = −1.27, P = 0.23), GSTM1 (t = 0.32, P = 0.76) and GSTT1 polymorphism (t = 1.65, P = 0.14).
Discussion
This is the most comprehensive meta-analysis concerning the relationship between polymorphisms of three DME genes and ATLI risk. Its strength was based on the accumulation of published data giving greater information to detect significant differences. In total, the meta-analysis involved 38 studies for ATLI that provided 2,225 patients and 4,906 controls. Our results demonstrated that the slow NAT2 genotype and GSTT1 null polymorphism is a risk factor for developing ATLI. Although no significant association between CYP2E1 *1A polymorphism and ATLI susceptibility was detected in the overall comparison, we found that the CYP2E1 *1A polymorphism was associated with increased risk of ATLI among East Asian populations when stratified by ethnicity. Ethnic differences may contribute to these different results, since the CYP2E1 c2 allele distribution of the polymorphism varies between East Asian and other ethnic populations [50]. However, we failed to detect any positive relationship between ATLI and GSTT1 null polymorphism.
As the pathogenic mechanism of ATLI is poorly understood, most studies were based on INH metabolic pathway. For predicting ATLI, the NAT2 genotype is seemingly more important than other genotypes, because this genotype possesses high susceptibility to ATLI with OR of 3.18 compared with CYP2E1 (OR = 1.28) and GSTM1 (OR = 1.43). Slow acetylators not only acetylate isoniazid more slowly but also monoacetylhydrazine, the immediate precursor of the toxic intermediates, to the harmless diacetylhydrazine [51]. This protective acetylation is further suppressed by isoniazid. Therefore, slow acetylators may critically increase the accumulation of toxic metabolites indirectly. The action of NAT in the disposition of isoniazid is followed by CYP2E1. Earlier reports demonstrated CYP2E1 activity was less inhibited by isoniazid in subjects with CYP2E1 *1A/*1A genotype than in those with other genotypes [46]. Therefore, under the administration of isoniazid, subjects with CYP2E1 *1A/*1A genotype have higher CYP2E1 activity than those with other genotypes, and, hence, may produce more hepatotoxins and finally increase the risk of liver injury.
GST, as an important phase II detoxification enzyme, was correlated to the susceptibility of alcoholic liver disease and many cancers [6]. Subjects with homozygous ‘null’ mutant genotype of GSTM1 or GSTT1 have been found to lose enzymatic activity [6]. It is speculated that people with null GSTM1 or GSTT1 genotypes could not detoxify the toxic reactive metabolites efficiently, and thus have higher risk of drug-induced liver injury and many cancers. However, failing to identify the association between GSTT1 polymorphism and ATLI may be due partly to the small sample size and low frequency of patients with ATLI; additional studies with large sample size are therefore needed to confirm our finds.
In interpreting the results, some limitations of this meta-analysis should be addressed. Firstly, lack of clarification regarding the exact criteria used for the diagnosis of ATLI, and the Hardy-Weinberg equilibrium status among controls in several studies may over inflate our results. Secondly, variation in the anti-TB drugs administered in the studies we analyzed limited the possibility of examining the association of DME with any specific drug. In these studies, multiple anti-TB drugs were utilized in some research, while some other adopts the single-drug therapy. Thirdly, our meta-analysis is based on unadjusted estimates, whereas a more precise analysis could be performed if individual data were available, which would allow for an adjustment estimate (by age, sex, alcohol consumption, cigarette smoking and other lifestyle). Additionally, it should be noted that the studies included patients from several different ethnic groups, with an overrepresentation of East Asian patients (e.g., Chinese, Korean, and Japanese populations) and an underrepresentation of individuals of Caucasian descent. Since allele frequencies may vary considerably between ethnic groups, careful consideration of the potential effect of population genetics on genotypic and phenotypic distribution is warranted, but the limited samples currently available have hampered this effort.
As a result of the heterogeneity of medication used, the duration of illness in different samples, and the different racial groups, it is possible that we have underestimated the effect size of the gene-drug response association. Furthermore, none of the studies formally accounted for medication noncompliance, which is prevalent in patients with TB. Put simply, when a patient does not take the prescribed anti-TB drug, the measured effect size of gene-drug response association is assessed as zero, whereas the true effect of genotype on the phenotype is perhaps larger. Nevertheless, despite the potential underestimation of effect size produced by these uncontrolled factors, we were still able to detect a significant association between the NAT2, GSTM1 and CYP2E1 polymorphism and anti-TB drug response. It is suggested that patients with high-risk genotypes should have regular liver biochemical tests in the first few months following administration of anti-TB drugs. Tailoring the anti-TB regimen to patients according to individual genetic profiles is expected in the coming years.
In summary, our meta-analysis indicates that CYP2E1, NAT2 and GSTM1 genetic variation is significantly associated with anti-tuberculosis drug-induced liver injury. Polymorphisms in these DME, such as NAT2*4, may be particularly important in predicting clinical response to anti-TB drug treatment. Furthermore, interaction between candidate genes and other risk factors, such as diet, alcohol consumption, smoking, existing liver disease and other comorbid diseases, should be explored to realize the modification effect of these extrinsic factors to the expression of different genotypes.
Supporting Information
The flow chart of the included studies.
(TIF)
Begg’s funnel plot of NAT2 polymorphism and ATLI risk.
(TIF)
Begg’s funnel plot of CYP2E1 polymorphism and ATLI risk.
(TIF)
Begg’s funnel plot of GST M1 polymorphism and ATLI risk.
(TIF)
Begg’s funnel plot of GST T1 polymorphism and ATLI risk.
(TIF)
(DOC)
Funding Statement
This work was supported by Shanghai Natural Science Foundation (12ZR1405300) and Shanghai Science and Technology Commission (10410709400; 10411950100). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, et al. (2008) Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 23: 192–202. [DOI] [PubMed] [Google Scholar]
- 2. Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, et al. (2006) An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 174: 935–952. [DOI] [PubMed] [Google Scholar]
- 3. Lee WM (1995) Drug-induced hepatotoxicity. N Engl J Med 333: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell JR, Zimmerman HJ, Ishak G, et al. (1976) Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med 84: 181–192. [DOI] [PubMed] [Google Scholar]
- 5. Runge-Morris M, Feng Y, Zangar R C, Novak R F (1996) Effects of hydrazine, phenelzine, and hydralazine treatmenton rat hepatic and renal drug-metabolizing enzyme expression. Drug Metab Dispos 24: 734. [PubMed] [Google Scholar]
- 6. Strange RC, Jones PW, Fryer AA (2000) Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett 112–113: 357–363. [DOI] [PubMed] [Google Scholar]
- 7. Naisbitt DJ, Williams DP, Pirmohamed M, Kitteringham NR, Park BK (2001) Reactive metabolites and their role in drug reactions. Curr Opin Allergy Clin Immunol 1: 317–325. [DOI] [PubMed] [Google Scholar]
- 8. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 9. Woolf B (1955) On estimating the relation between blood group and disease. Ann Hum Genet 19: 251–253. [DOI] [PubMed] [Google Scholar]
- 10. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 12. Tang SW, Lv XZ, Zhang Y, Wu SS, Yang ZR, et al. (2012) CYP2E1, GSTM1 and GSTT1 genetic polymorphisms and susceptibility to antituberculosis drug-induced hepatotoxicity: a nested case-control study. J Clin Pharm Ther 37: 588–593. [DOI] [PubMed] [Google Scholar]
- 13. An HR, Wu UQ, Wang ZY, Zhang JX, Liang Y (2012) NAT 2 and CYP2E1 polymorphisms associated with antituberculosis drug-induced hepatotoxicity in Chinese patients. Clin Exp Pharmacol Physiol 39: 535–543. [DOI] [PubMed] [Google Scholar]
- 14. Rana SV, Ola RP, Sharma SK, Arora SK, Sinha SK, et al. (2012) Comparison between acetylator phenotype and genotype polymorphism of n-acetyltransferase-2 in tuberculosis patients. Hepatol Int 6: 397–402. [DOI] [PubMed] [Google Scholar]
- 15.Ben Mahmoud L, Ghozzi H, Kamoun A, Hakim A, Hachicha H, et al. (2011) Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatotoxicity in Tunisian patients with tuberculosis. Pathol Biol (Paris) doi:10.1016/j.patbio.2011.07.001. [DOI] [PubMed]
- 16. Zhu DL, Xi Y, Wu XQ (2011) [Relationship between genetic polymorphisms of GSTM1, GSTT1 and the susceptibility to antituberculosis drug-induced liver injury]. Zhongguo Kang Sheng Su Za Zhi 36: 864–868. [Google Scholar]
- 17. Leiro-Fernandez V, Valverde D, Vazquez-Gallardo R, Botana-Rial M, Constenla L, et al. (2011) N-acetyltransferase 2 polymorphisms and risk of anti-tuberculosis drug-induced hepatotoxicity in Caucasians. Int J Tuberc Lung Dis 15: 1403–1408. [DOI] [PubMed] [Google Scholar]
- 18. Sotsuka T, Sasaki Y, Hirai S, Yamagishi F, Ueno K (2011) Association of isoniazid-metabolizing enzyme genotypes and isoniazid-induced hepatotoxicity in tuberculosis patients. In Vivo 25: 803–812. [PubMed] [Google Scholar]
- 19. Sistanizad M, Azizi E, Khalili H, Hajiabdolbaghi M, Gholami K, et al. (2011) Antituberculosis Drug-Induced Hepatotoxicity in Iranian Tuberculosis Patients: Role of Isoniazid Metabolic Polymorphism. Iranian Journal of Pharmaceutical Research 10: 633–639. [PMC free article] [PubMed] [Google Scholar]
- 20. Huang DS, Zou YH, He G, LV JC, Wang YN (2011) [Association between polymorphism of N-acetyltransferase 2 gene and development of antituberculosis drug-induced liver injury]. ZhongHua Sheng Wu Yi Xue Gong Cheng Za Zhi 17: 444–447. [Google Scholar]
- 21. Teixeira RL, Morato RG, Cabello PH, Muniz LM, Moreira Ada S, et al. (2011) Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz 106: 716–724. [DOI] [PubMed] [Google Scholar]
- 22. Yimer G, Ueda N, Habtewold A, Amogne W, Suda A, et al. (2011) Pharmacogenetic & pharmacokinetic biomarker for efavirenz based ARV and rifampicin based anti-TB drug induced liver injury in TB-HIV infected patients. PLoS One 6: e27810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bose PD, Sarma MP, Medhi S, Das BC, Husain SA, et al. (2011) Role of polymorphic N-acetyl transferase2 and cytochrome P4502E1 gene in antituberculosis treatment-induced hepatitis. J Gastroenterol Hepatol 26: 312–318. [DOI] [PubMed] [Google Scholar]
- 24. Chatterjee S, Lyle N, Mandal A, Kundu S (2010) GSTT1 and GSTM1 gene deletions are not associated with hepatotoxicity caused by antitubercular drugs. J Clin Pharm Ther 35: 465–470. [DOI] [PubMed] [Google Scholar]
- 25. Wang T, Yu HT, Wang W, Pan YY, He LX, et al. (2010) Genetic polymorphisms of cytochrome P450 and glutathione S-transferase associated with antituberculosis drug-induced hepatotoxicity in Chinese tuberculosis patients. J Int Med Res 38: 977–986. [DOI] [PubMed] [Google Scholar]
- 26. Lee SW, Chung LS, Huang HH, Chuang TY, Liou YH, et al. (2010) NAT2 and CYP2E1 polymorphisms and susceptibility to first-line anti-tuberculosis drug-induced hepatitis. Int J Tuberc Lung Dis 14: 622–626. [PubMed] [Google Scholar]
- 27. Kim SH, Yoon HJ, Shin DH, Park SS, Kim YS, et al. (2010) GSTT1 and GSTM1 null mutations and adverse reactions induced by antituberculosis drugs in Koreans. Tuberculosis (Edinb) 90: 39–43. [DOI] [PubMed] [Google Scholar]
- 28. Wu YM, Luo ZY, Zhang HM, Peng JF, Liu SY, et al. (2010) [The association between NAT2 polymorphism and anti-tuberculosis drug-induced hepatitis]. Zhonghua Gan Zang Bing Za Zhi 18: 467–469. [DOI] [PubMed] [Google Scholar]
- 29. Guo M, Guo YH, Li SM, Wang D, Liu Q, et al. (2010) [The relationship between polymorphisms of N-acetyltransferase 2 genes and anti-tuberculosis drug induced hepatic-injury]. Zhonghua Chuan Ran Bing Za Zhi 28: 99–102. [Google Scholar]
- 30. Chen Y, Guo M, Li SM, Zhang P, Hao JQ, et al. (2010) [Study of the relationship between polymorphisms of cytochrome P450 2E1 and antituberculosis drug-induced hepatic injury]. Zhonghua Chuan Ran Bing Za Zhi 28: 748–752. [Google Scholar]
- 31. Wang T, Wang W, Wang ZY, Pan YY, Su QQ, et al. (2009) [Association of P450–2E1 and GSTM1 genetic polymorphisms with susceptibility to antituberculosis drug-induced hepatotoxicity]. Zhonghua Jie He He Hu Xi Za Zhi 32: 585–587. [PubMed] [Google Scholar]
- 32. Guo M, Sun YH, Li SM, Wang D, Liu Q, et al. (2009) [The effect of GST M1 and GST T1 gene mutations on anti-tuberculous drug induced hepatic injury]. Zhonghua Jie He He Hu Xi Za Zhi 32: 266–269. [PubMed] [Google Scholar]
- 33. Kim SH, Bahn JW, Kim YK, Chang YS, Shin ES, et al. (2009) Genetic polymorphisms of drug-metabolizing enzymes and anti-TB drug-induced hepatitis. Pharmacogenomics 10: 1767–1779. [DOI] [PubMed] [Google Scholar]
- 34. Yamada S, Tang M, Richardson K, Halaschek-Wiener J, Chan M, et al. (2009) Genetic variations of NAT2 and CYP2E1 and isoniazid hepatotoxicity in a diverse population. Pharmacogenomics 10: 1433–1445. [DOI] [PubMed] [Google Scholar]
- 35. Wang PY, Xi SY, Zhang GW, Hao Q (2009) [Prospective nested case - control study on relation between N-acetyltransferase-2 acetylator phenotype and hepatotoxicity in China] Zhongguo Gong Gong Wei Sheng. 25: 300–302. [Google Scholar]
- 36. Leiro V, Fernandez-Villar A, Valverde D, Constenla L, Vazquez R, et al. (2008) Influence of glutathione S-transferase M1 and T1 homozygous null mutations on the risk of antituberculosis drug-induced hepatotoxicity in a Caucasian population. Liver Int 28: 835–839. [DOI] [PubMed] [Google Scholar]
- 37. Bozok Cetintas V, Erer OF, Kosova B, Ozdemir I, Topcuoglu N, et al. (2008) Determining the relation between N-acetyltransferase-2 acetylator phenotype and antituberculosis drug induced hepatitis by molecular biologic tests. Tuberk Toraks 56: 81–86. [PubMed] [Google Scholar]
- 38. Possuelo LG, Castelan JA, de Brito TC, Ribeiro AW, Cafrune PI, et al. (2008) Association of slow N-acetyltransferase 2 profile and anti-TB drug-induced hepatotoxicity in patients from Southern Brazil. Eur J Clin Pharmacol 64: 673–681. [DOI] [PubMed] [Google Scholar]
- 39. Huang YS, Su WJ, Huang YH, Chen CY, Chang FY, et al. (2007) Genetic polymorphisms of manganese superoxide dismutase, NAD(P)H: quinone oxidoreductase, glutathione S-transferase M1 and T1, and the susceptibility to drug-induced liver injury. J Hepatol 47: 128–134. [DOI] [PubMed] [Google Scholar]
- 40. Cho HJ, Koh WJ, Ryu YJ, Ki CS, Nam MH, et al. (2007) Genetic polymorphisms of NAT2 and CYP2E1 associated with antituberculosis drug-induced hepatotoxicity in Korean patients with pulmonary tuberculosis. Tuberculosis (Edinb) 87: 551–556. [DOI] [PubMed] [Google Scholar]
- 41. Higuchi N, Tahara N, Yanagihara K, Fukushima K, Suyama N, et al. (2007) NAT2 6A, a haplotype of the N-acetyltransferase 2 gene, is an important biomarker for risk of anti-tuberculosis drug-induced hepatotoxicity in Japanese patients with tuberculosis. World J Gastroenterol 13: 6003–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, et al. (2006) CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol 62: 423–429. [DOI] [PubMed] [Google Scholar]
- 43. Shimizu Y, Dobashi K, Mita Y, Endou K, Moriya S, et al. (2006) DNA microarray genotyping of N-acetyltransferase 2 polymorphism using carbodiimide as the linker for assessment of isoniazid hepatotoxicity. Tuberculosis (Edinb) 86: 374–381. [DOI] [PubMed] [Google Scholar]
- 44. Roy B, Ghosh SK, Sutradhar D, Sikdar N, Mazumder S, et al. (2006) Predisposition of antituberculosis drug induced hepatotoxicity by cytochrome P450 2E1 genotype and haplotype in pediatric patients. J Gastroenterol Hepatol 21: 784–786. [DOI] [PubMed] [Google Scholar]
- 45. Wang JH, Liu JW, Wu XQ, Wang XJ, Zhang CY, et al. (2004) [The study on the susceptible gene of isoniazid and rifampicin-induced hepatotoxicity of pulmonary tuberculosis patients]. Jun Yi Jin Xiu Xue Yuan Xue Bao 25: 239–240. [Google Scholar]
- 46. Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, et al. (2003) Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 37: 924–930. [DOI] [PubMed] [Google Scholar]
- 47. Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, et al. (2002) Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology 35: 883–889. [DOI] [PubMed] [Google Scholar]
- 48. Roy B, Chowdhury A, Kundu S, Santra A, Dey B, et al. (2001) Increased risk of antituberculosis drug-induced hepatotoxicity in individuals with glutathione S-transferase M1 ‘null’ mutation. J Gastroenterol Hepatol 16: 1033–1037. [DOI] [PubMed] [Google Scholar]
- 49. Ohno M, Yamaguchi I, Yamamoto I, Fukuda T, Yokota S, et al. (2000) Slow N-acetyltransferase 2 genotype affects the incidence of isoniazid and rifampicin-induced hepatotoxicity. Int J Tuberc Lung Dis 4: 256–261. [PubMed] [Google Scholar]
- 50. Tang K, Li X, Xing Q, Li W, Feng G, He L, Qin S (2010) Genetic polymorphism analysis of cytochrome P4502E1 (CYP2E1) in Chinese Han populations from four different geographic areas of Mainland China. Genomics 95: 224–229. [DOI] [PubMed] [Google Scholar]
- 51. Lauterburg BH, Smith CV, Todd EL, Mitchell JR: Pharmacokinetics of the toxic hydrazine metabolites formed from isoniazid in humans. J. Pharmacol. Exp Ther (1985) 235(3): 566–570. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The flow chart of the included studies.
(TIF)
Begg’s funnel plot of NAT2 polymorphism and ATLI risk.
(TIF)
Begg’s funnel plot of CYP2E1 polymorphism and ATLI risk.
(TIF)
Begg’s funnel plot of GST M1 polymorphism and ATLI risk.
(TIF)
Begg’s funnel plot of GST T1 polymorphism and ATLI risk.
(TIF)
(DOC)