Abstract
Avian scavengers, such as American crows (Corvus brachyrhynchos), have potential to translocate infectious agents (prions) of transmissible spongiform encephalopathy (TSE) diseases including chronic wasting disease, scrapie, and bovine spongiform encephalopathy. We inoculated mice with fecal extracts obtained from 20 American crows that were force-fed material infected with RML-strain scrapie prions. These mice all evinced severe neurological dysfunction 196–231 d postinoculation ( = 198; 95% CI: 210–216) and tested positive for prion disease. Our results suggest a large proportion of crows that consume prion-positive tissue are capable of passing infectious prions in their feces (
= 198; 95% CI: 210–216) and tested positive for prion disease. Our results suggest a large proportion of crows that consume prion-positive tissue are capable of passing infectious prions in their feces (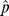 = 1.0; 95% CI: 0.8–1.0). Therefore, this common, migratory North American scavenger could play a role in the geographic spread of TSE diseases.
= 1.0; 95% CI: 0.8–1.0). Therefore, this common, migratory North American scavenger could play a role in the geographic spread of TSE diseases.
Introduction
Transmissible spongiform encephalopathies (TSE) are most likely caused by pathogenic isoforms (PrPRes) of prion proteins [1] that naturally occur across many classes of animals, including mammals and birds [2]. A number of livestock and wildlife species in North America are susceptible to TSE diseases. Mule deer (Odocoileus hemionus), white-tailed deer (O. virginianus), elk (Cervus elaphus), and moose (Alces alces) are susceptible to chronic wasting disease (CWD); domestic sheep and goats are susceptible to scrapie; and domestic cattle are susceptible to bovine spongiform encephalopathy (BSE) (although this disease is rare in North America [3]). These TSE diseases are always fatal to infected animals, and upon death, carcasses allowed to remain in the environment can be scavenged by an array of avian and mammalian scavengers [4].
Mechanisms for the spread of TSE in wild and domestic ungulates are incompletely understood. We hypothesized that avian scavengers have potential to translocate PrPRes in their feces. American crows (Corvus brachyrhynchos) are significant avian scavengers of deer carcasses [4], they are migratory, and their overall range [5] includes most areas where TSE diseases occur in North America [6]. Crows forage in groups, traveling up to 80 km/d from communal roosts [5]. Thus, crows have opportunity to encounter PrPRes-infected carcasses, consume infected tissue, and move long distances before depositing feces. Once in the soil, PrPRes may persist >2 years [7], [8], potentially enabling increased site contamination over time. For example, residual contamination of soil with PrPRes caused recurrence of CWD in confined mule deer in Colorado [7] and lateral transmission via environmental contamination is likely an important route of infection [9].
Insects [10], [11], poultry [12], and scavengers, including crows [4], have been suggested as passive carriers or dispersers of infectious prions. We found no studies that evaluated passage of PrPRes through avian digestive systems, though several studies have evaluated resistance of PrPRes to mammalian digestive fluids. Ruminant digestive fluids used during in-vitro trials have shown substantial [13], [14] to no reduction [15] in Western blot signal after incubation periods of approximately 13–24 h. Shorter incubation times (15–210 min) resulted in intermediate levels of Western blot signal loss [16]. Studies that investigated effects on PrPRes from full passage through rodent digestive systems found scrapie and BSE PrPRes present in mouse feces [17] and scrapie PrPRes in hamster feces (ca. 5% of original dose excreted 24 h postinoculation) [18]. Thus, it appears that mammalian digestive fluids and processes can reduce PrPRes concentration but are unlikely to eliminate it.
Proteolysis occurs in the avian digestive system due to the presence of hydrochloric acid (HCl) and the proteolytic enzymes pepsin, trypsin, chymotrypsin and various peptidases [19], [20]. Although experimentally induced hypoacidity was associated with reduced scrapie infection rates in mice [21], it is unlikely that gastric HCl would fully degrade PrPRes in the crow digestive system given extreme temperature and concentration required [22] and mild conditions present in the avian gut [19], [23]. Although early investigations suggested that trypsin reduced scrapie titer under certain circumstances [1], [24], subsequent studies found pepsin and trypsin were not effective for reducing infectivity of scrapie and BSE PrPRes [25] or variant Creutzfeldt-Jakob disease PrPRes [26]. Thus, there is little evidence to suggest that the crow digestive system would eliminate PrPRes infectivity prior to excretion of feces. Similar arguments can be made for nonruminant mammals because of similarities in endogenous enzymes in vertebrate digestive systems [27], yet PrPRes was substantially reduced by passage through hamster digestive systems [18].
Little is known about effects of avian digestive systems on infectivity of PrPRes. As a first step in understanding the potential role of avian scavengers in TSE transmission, we tested the hypothesis that readily available mouse-adapted scrapie PrPRes can remain infectious after passage through the digestive tract of crows. Results of our study support this hypothesis.
Materials and Methods
We evaluated infectivity of the RML Chandler strain (RML) of mouse-adapted scrapie [28] (obtained from Rocky Mountain Laboratories, Hamilton, MT) after passage through digestive systems of crows. Crows were captured during winter in central Oklahoma, USA. We used mouse-brain source material from uninfected (normal) and terminally ill RML-infected C57BL/6 mice (Hilltop Lab Animals, Scottsdale, PA; this strain used throughout study). We separately pooled and homogenized infected and normal mouse brains and diluted portions of each homogenate 1∶10 w/v in sterile phosphate-buffered saline (SPBS). We estimated passage time through the alimentary canal by gavaging 1 crow (not part of the experimental group) with 5 ml of whole egg mixed with blue dye; by 4 h postgavage all stained feces had been excreted. We withdrew feed (but not water) from study crows approximately 17 h pregavage. We randomly allocated 25 crows to treatment groups and gavaged each crow with 5 ml of either PrPRes-infected (n = 20) or normal (n = 5) mouse-brain homogenate diluted 1∶10 w/v in SPBS (Table 1). We then transferred each crow to an individual single-use cage. At 4 h postgavage, we collected and pooled all feces within each cage. We homogenized crow-specific pooled feces and gamma irradiated them at 24,000 Gy to destroy viruses and microbes. For each crow, we then diluted a 500 µl sample of fecal homogenate in SPBS to a total volume of 10 ml, centrifuged it for 15 min at 13,730 m/s2, and extracted the supernatant for use as inoculum for mice. We removed solids to minimize risk of toxicity to mice from uric acid contained in bird feces. Crows were not held or examined after collection of fecal samples.
Table 1. Experimental design used to estimate proportion of crows able to pass infectious RML scrapie prion (PrPRes) in feces (numbers of animals).
Mice intraperitoneally inoculated with gamma-irradiated crow fecal (CF) extract from crows gavaged with PrPRes (+) or control (−) mouse brain homogenate; additional control mice were inoculated with mouse-brain homogenate with (MB+) or without (MB−) PrPRes.
Five mice were randomly allocated to each crow and housed together in 1 cage postinoculation. Additional control mice were allocated randomly to MB treatment groups and 5 mice/treatment group were housed together in 1 cage postinoculation.
We randomly allocated 5 mice/crow to treatment groups (Table 1). Mice received crow-specific fecal supernatant from PrPRes or control crows (CF+ and CF− groups, respectively), or PrPRes-infected or normal mouse brain homogenate diluted to 1∶100 w/v in SPBS (MB+ and MB− groups, respectively). We intraperitoneally inoculated each mouse with 1 ml of either crow fecal supernatant or diluted mouse brain homogenate.
All 5 mice/crow, or 5 mice/MB treatment group, were caged together under biosafety level 2 conditions. We monitored mice daily until all those in PrPRes treatment groups expressed clinical symptoms of mouse scrapie and were thereafter euthanized. Remaining mice were monitored every 2 d until study termination at 365 d postinoculation (dpi). We scored mice for each of 6 clinical symptoms of mouse scrapie (kyphosis, ataxia, stiff tail, lack of grooming, emaciation, and lethargy), where 0 = none visible, 1 = moderate, and 2 = severe. We euthanized mice when total daily scores reached ≥8 for 1 d, ≥6 continuously for 3 d, or at 365 dpi. Brains were immediately harvested and stored at −70°C for analysis. Samples from harvested brains (1∶10 w/v homogenate) were tested at Colorado State University's Veterinary Diagnostic Laboratory for PrPRes using the ELISA-based Bio-Rad TeSeE BSE rapid assay (Bio-Rad Laboratories, Hercules, CA, USA) to confirm scrapie diagnosis.
We used exact methods [29] to estimate a 95% confidence interval (CI) on the proportion of crows able to excrete infectious prions in feces (SAS PROC FREQ [30]). We used Fisher's exact test, due to low count (i.e., 2) in 1 cell of the 2×2 contingency table, to evaluate whether early death (≤3 dpi) was associated with source of CF inoculum (PrPRes or control). We estimated means and 95% CI for incubation time or time-to-death (contingent on surviving >3 dpi) for CF+ and MB+ mice using general linear mixed modeling [31], where cage was a random effect to account for clustering of mice within cages (SAS PROC GLIMMIX [30]). Traditional time-to-event (or survival) analyses were not required for CF+ and MB+ mice because none were censored >3 dpi. As most CF− mice were censored at study termination, we tested for equality of survival functions between CF+ and CF− using the log-rank test (SAS PROC LIFETEST [30]).
Ethics Statement
The Institutional Animal Care and Use Committee of the United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center approved all procedures used in this study (QA-1406).
Results
All 20 crows gavaged with scrapie-infected mouse brain transmitted PrPRes to mice via fecal inoculum (estimated proportion: 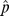 = 1.00, CI: 0.83–1.00). Sixteen mice from CF+ and 2 from CF− groups died ≤3 d postinoculation (likely from residual uric acid toxicity; Table 2). No early deaths occurred in MB groups and estimated probabilities of early death were not statistically different between CF+ (
= 1.00, CI: 0.83–1.00). Sixteen mice from CF+ and 2 from CF− groups died ≤3 d postinoculation (likely from residual uric acid toxicity; Table 2). No early deaths occurred in MB groups and estimated probabilities of early death were not statistically different between CF+ (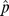 = 0.16) and CF− (
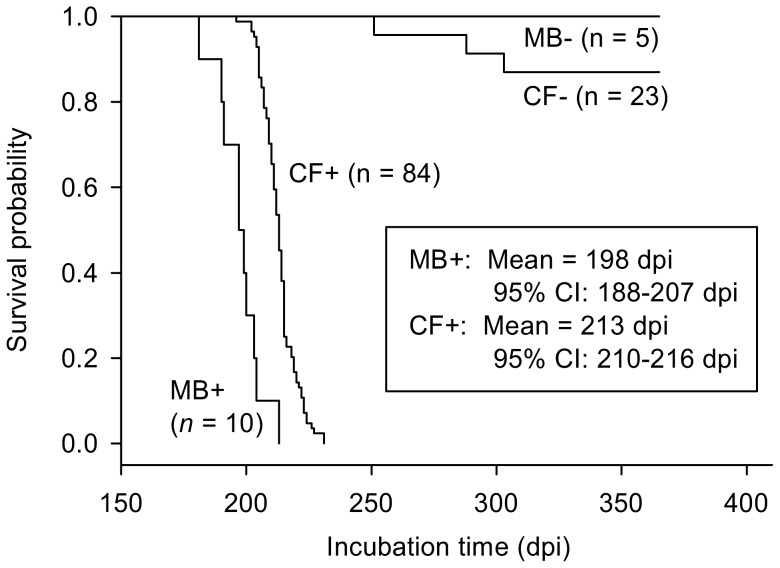
= 0.16) and CF− (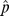 = 0.08) mice (Fisher's exact P = 0.524). After these early deaths, 2 crows were represented by only 1 mouse/crow and all other crows were represented by 3–5 mice/crow. Surviving mice appeared healthy until onset of clinical symptoms of mouse scrapie. Based on scoring for multiple clinical symptoms, we euthanized mice in MB+ and CF+ groups 181–231 dpi (Fig. 1). These mice subsequently tested positive for PrPRes (Table 2). On average, MB+ mice had shorter incubation times (by 15 d) than CF+ mice (Fig. 1). We observed no clinical symptoms in MB− or CF− control mice. All MB− mice lived to study termination at 365 dpi, though 3 CF− mice died at 251–303 dpi. Time to death was significantly longer for CF− than for CF+ mice (
= 0.08) mice (Fisher's exact P = 0.524). After these early deaths, 2 crows were represented by only 1 mouse/crow and all other crows were represented by 3–5 mice/crow. Surviving mice appeared healthy until onset of clinical symptoms of mouse scrapie. Based on scoring for multiple clinical symptoms, we euthanized mice in MB+ and CF+ groups 181–231 dpi (Fig. 1). These mice subsequently tested positive for PrPRes (Table 2). On average, MB+ mice had shorter incubation times (by 15 d) than CF+ mice (Fig. 1). We observed no clinical symptoms in MB− or CF− control mice. All MB− mice lived to study termination at 365 dpi, though 3 CF− mice died at 251–303 dpi. Time to death was significantly longer for CF− than for CF+ mice (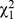 = 71.0, p<0.0001). One of these CF− mice (251 dpi) tested positive for PrPRes. This unexpectedly positive mouse was inoculated directly after 5 MB+ mice and may have been inadvertently exposed to PrPRes-positive material.
= 71.0, p<0.0001). One of these CF− mice (251 dpi) tested positive for PrPRes. This unexpectedly positive mouse was inoculated directly after 5 MB+ mice and may have been inadvertently exposed to PrPRes-positive material.
Table 2. Numbers of mice by treatment group that suffered early inoculation-related death, exhibited clinical symptoms of prion disease, and tested positive for scrapie prion (PrPRes) by ELISAA.
| Treatment groupB | Early deathC | Clinical diseaseD | PrPRes detected |
| CF+ | 16 (100) | 84 (84) | 84 (84) |
| CF− | 2 (25) | 0 (23) | 1 (23) |
| MB+ | 0 (10) | 10 (10) | 9 (9) |
| MB− | 0 (5) | 0 (5) | 0 (4) |
Numbers in parentheses indicate sample size.
Mice intraperitoneally inoculated with gamma-irradiated crow fecal (CF) extract from crows gavaged with PrPRes (+) or control (−) mouse brain homogenate; additional control mice were inoculated with mouse-brain homogenate with (MB+) or without (MB−) PrPRes.
Mice that died ≤3 d postinoculation, presumably from fecal uric acid toxicity. These mice were removed from the data set.
Mice that achieved a minimum threshold score, based on multiple symptoms such as kyphosis, ataxia, stiff tail, lack of grooming, emaciation, and lethargy, demonstrating strong clinical evidence of prion disease.
Figure 1. Survival functions for treatment groups of mice.
Twenty-five crows were fed infected (PrPRes) or normal (control) mouse brain homogenate. Five mice/crow were subsequently inoculated with crow fecal extract from PrPRes (CF+) or control (CF−) crows. Additional control mice were inoculated with mouse-brain homogenate with or without PrPRes (MB+ and MB−, respectively). Sample sizes reflect early deaths of 16 mice ≤3 d postinoculation (dpi). Mean and interval estimates of survival time for MB+ and CF+ groups showed these groups were significantly different, indicating different dose levels of PrPRes in crow fecal extracts compared to mouse brain homogenate. Time to death was significantly longer for CF− than for CF+ mice (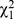 = 71.0, p<0.0001). Because all mice exposed to CF+ extracts died of transmissible spongiform encephalopathy (given survival >3 dpi), all 20 crows gavaged with PrPRes-infected mouse brain homogenate passed infectious doses of PrPRes to mice via fecal extracts.
= 71.0, p<0.0001). Because all mice exposed to CF+ extracts died of transmissible spongiform encephalopathy (given survival >3 dpi), all 20 crows gavaged with PrPRes-infected mouse brain homogenate passed infectious doses of PrPRes to mice via fecal extracts.
Discussion
We tested the hypothesis that PrPRes would remain infectious after passage through the digestive tract of crows. After inoculation with fecal supernatant from crows gavaged with PrPRes-infected material, we observed clinical disease and obtained positive results from ELISA in all 84 CF+ mice that survived >3 dpi. Thus, we confirmed passage of infectious PrPRes through all 20 crows gavaged with infected material. We conclude that 83–100% of crows from the population we sampled can excrete infectious RML PrPRes in feces under conditions similar to those in our study.
The MB+ mice developed clinical scrapie 15 d earlier than CF+ mice indicating inoculated dose of PrPRes infectivity was likely lower for CF+ mice. We inoculated MB+ and MB− mice to demonstrate that brain source materials were infectious or not infectious, respectively, not to serve as standards for titer assessment. However, comparison with unpublished titration results from intraperitoneal inoculation of RML mouse scrapie into C57BL10 mice (Ann Ward and Sue Priola, Rocky Mountain Laboratories, personal communication) suggest MB+ mice received approximately 10-times more infectivity than CF+ mice. Dilutions of brain and fecal material with SPBS (see Methods) indicate that the amount of infectivity inoculated into MB+ mice would have been about double that of CF+ mice, assuming no influence on concentration of infectivity due to passage or centrifuge processing. It is reasonable to expect some loss of infectivity after removing solids from diluted crow feces by centrifugation. It is also possible that some degradation or absorption of infectivity occurred during passage through crow alimentary tracts.
Our study clearly shows that RML PrPRes can persist after passage through the crow alimentary tract. As there is variability in resistance of different strains of PrPRes to degradation [32]–[36], we cannot definitively state that passage of strains of concern would occur. However, RML PrPRes has been shown more sensitive to degradation than TSE field isolates after 4 h exposure to enzymatic digestion [36]. Therefore, results of our study likely understate potential for prion passage through the alimentary canal of crows. Further experimental trials involving TSE prions obtained from ovine, bovine, and cervine carcasses would be required to definitively evaluate passage of natural TSEs through digestive systems of scavengers and predators. Other additional research topics could include in-vitro evaluation of PrPRes degradation in crow digestive fluids; effects of solid, semisolid, and liquid delivery of infective materials on passage rate and residual infectivity in feces; postexcretion continued enzymatic and bacterial degradation of infectivity in feces; infectivity of feces excreted >4 h postgavage; susceptibility of crows to TSE disease and potential for postinfection shedding of PrPRes in feces.
Acknowledgments
K. Kellett, M. Smith, S. Tupper, and S. Werner provided crows used in our study. H. VanRoekel, T. Camenisch-Ruby, and USDA, National Wildlife Research Center animal care staff assisted in laboratory procedures and animal monitoring. Colorado State University Veterinary Diagnostic Laboratory conducted ELISA tests. T. Nichols reviewed an early draft of the manuscript. Mention or use of a product does not imply USDA endorsement.
Funding Statement
Funding was provided by U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services (VS). A representative of VS served on a panel that provided input to the 5-year plan for the Chronic Wasting Disease Project of the National Wildlife Research Center, within which this project was conducted. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. [DOI] [PubMed] [Google Scholar]
- 2.Collinge J, Wadsworth JDF (2006) Molecular basis of prion diseases. In: Siegel G, Albers RW, Brady S, Price D, editors. Basic neurochemistry: Molecular, cellular, and medical aspects. Seventh edition. Burlington: Elsevier Academic Press. pp. 791–803.
- 3.Centers for Disease Control and Prevention (2011) BSE (Bovine Spongiform Encephalopathy, or Mad Cow Disease). Available: http://www.cdc.gov/ncidod/dvrd/bse/. Accessed 8 Mar 2011.
- 4. Jennelle CS, Samuel MD, Nolden CA, Berkley EA (2009) Deer carcass decomposition and potential scavenger exposure to chronic wasting disease. J Wildlife Manage 73: 655–662. [Google Scholar]
- 5. Verbeek NAM, Caffrey C (2002) American crow. Birds of N Am 647. [Google Scholar]
- 6.Chronic Wasting Disease Alliance (2011) Chronic wasting disease in North America. Available: http://www.cwd-info.org/images/CWDmaphi.jpg. Accessed 30 Mar 2011.
- 7. Miller MW, Williams ES, Hobbs NT, Wolfe LL (2004) Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10: 1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, et al. (2007) Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS ONE 2: e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller MW, Hobbs NT, Tavener SJ (2006) Dynamics of prion disease transmission in mule deer. Ecol Appl 16: 2208–2214. [DOI] [PubMed] [Google Scholar]
- 10. Wisniewski HM, Sigurdarson S, Rubenstein R, Kascsak RJ, Carp RI (1996) Mites as vectors for scrapie. Lancet 347: 1114. [DOI] [PubMed] [Google Scholar]
- 11. Post K, Riesner D, Walldorf V, Mehlhorn H (1999) Fly larvae and pupae as vectors for scrapie. Lancet 354: 1969–1970. [DOI] [PubMed] [Google Scholar]
- 12. Matthews D, Cooke BC (2003) The potential for transmissible spongiform encephalopathies in non-ruminant livestock and fish. Rev Sci Tech OIE 22: 283–296. [DOI] [PubMed] [Google Scholar]
- 13. Scherbel C, Pichner R, Groschup MH, Mueller-Hellwig S, Scherer S, et al. (2006) Degradation of scrapie associated prion protein (PrPSc) by the gastrointestinal microbiota of cattle. Vet Res 37: 695–703. [DOI] [PubMed] [Google Scholar]
- 14. Jeffrey M, González L, Espenes A, Press C, Martin S, et al. (2006) Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol 209: 4–14. [DOI] [PubMed] [Google Scholar]
- 15. Nicholson EM, Richt JA, Rasmussen MA, Hamir AN, Lebepe-Mazur S, et al. (2007) Exposure of sheep scrapie brain homogenate to rumen-simulating conditions does not result in a reduction of PrPSc levels. Lett Appl Microbiol 44: 631–636. [DOI] [PubMed] [Google Scholar]
- 16. Dagleish MP, Hamilton S, González L, Eaton SL, Steele P, et al. (2010) Digestion and transportation of bovine spongiform encephalopathy-derived prion protein in the sheep intestine. J Gen Virol 91: 3116–3123. [DOI] [PubMed] [Google Scholar]
- 17. Maluquer de Motes C, Grassi J, Simon S, Herva ME, Torres JM, et al. (2008) Excretion of BSE and scrapie prions in stools from murine models. Vet Microbiol 131: 205–211. [DOI] [PubMed] [Google Scholar]
- 18. Krüger D, Thomzig A, Lenz G, Kampf K, McBride P, et al. (2009) Faecal shedding, alimentary clearance and intestinal spread of prions in hamsters fed with scrapie. Vet Res 40: 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziswiler V, Farner DS (1972) Digestion and the digestive system. In: Farner DS, King JR, Parkes KC, editors. Avian biology: Volume II. New York: Academic Press. pp. 343–430.
- 20.Duke GE (1986) Alimentary canal: Secretion and digestion, special digestive functions, and absorption. In: Sturkie PD, editor. Avian physiology. Fourth edition. New York: Springer-Verlag. pp. 289–302.
- 21. Martinsen TC, Taylor DM, Johnsen R, Waldum HL (2002) Gastric acidity protects mice against prion infection? Scand J Gastroenterol 37: 497–500. [DOI] [PubMed] [Google Scholar]
- 22. Appel TR, Lucassen R, Groschup MH, Joncic M, Beekes M, et al. (2006) Acid inactivation of prions: Efficient at elevated temperature or high acid concentration. J Gen Virol 87: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 23.Evans HE, Heiser JB (2004) What's inside: Anatomy and physiology. In: Podulka S, Rohrbaugh Jr RW, Bonney R, editors. Handbook of bird biology. Second edition. Ithaca: Cornell Lab of Ornithology. pp. 4.1–4.162.
- 24. Hunter GD, Millson GC (1967) Attempts to release the scrapie agent from tissue debris. J Comp Pathol 77: 301–307. [DOI] [PubMed] [Google Scholar]
- 25. Langeveld JPM, Wang J-J, Van de Wiel DFM, Shih GC, Garssen GJ, et al. (2003) Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis 188: 1782–1789. [DOI] [PubMed] [Google Scholar]
- 26. Jackson GS, McKintosh E, Flechsig E, Prodromidou K, Hirsch P, et al. (2005) An enzyme-detergent method for effective prion decontamination of surgical steel. J Gen Virol 86: 869–878. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Nielsen K (1997) Animal physiology: Adaptation and environment. Fifth edition. Cambridge: Cambridge University Press. 607 p.
- 28. Chandler RL (1961) Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 277: 1378–1379. [DOI] [PubMed] [Google Scholar]
- 29.Agresti A (2002) Categorical data analysis. Second edition. Hoboken: John Wiley. 710 p.
- 30.SAS Institute Inc. (2008) SAS/STAT® 9.2 User's Guide. Cary: SAS Institute Inc.
- 31.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models. Second edition. Cary: SAS Institute Inc. 814 p.
- 32. Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, et al. (2006) Inactivation of prions by acidic sodium dodecyl sulfate. J Virol 80: 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, et al. (2008) Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathog 4: e1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kascsak RJ, Rubenstein R, Merz PA, Carp RI, Wisniewski HM, et al. (1985) Biochemical differences among scrapie-associated fibrils support the biological diversity of scrapie agents. J Gen Virol 66: 1715–1722. [DOI] [PubMed] [Google Scholar]
- 35. Bessen RA, Marsh RF (1992) Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol 66: 2096–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuczius T, Groschup MH (1999) Differences in proteinase K resistance and neuronal deposition of abnormal prion proteins characterize bovine spongiform encephalopathy (BSE) and scrapie strains. Mol Med 5: 406–418. [PMC free article] [PubMed] [Google Scholar]