Abstract
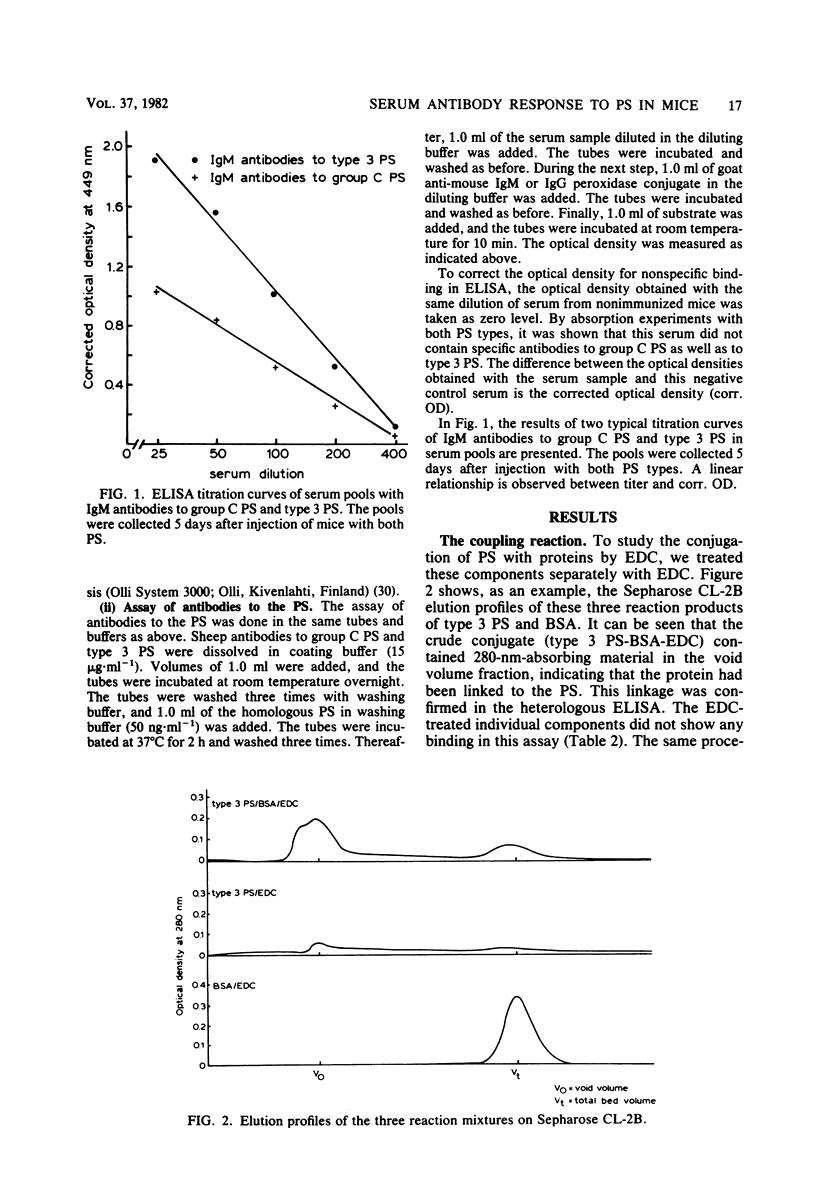
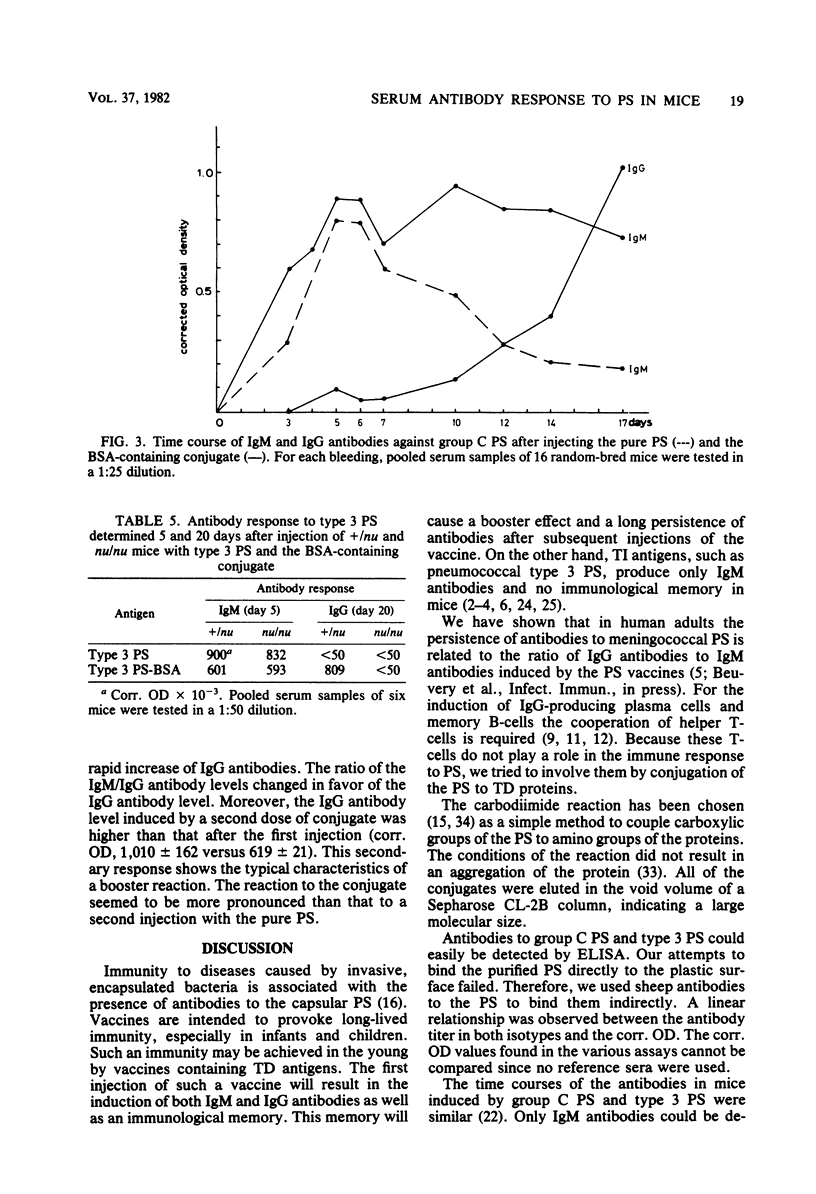
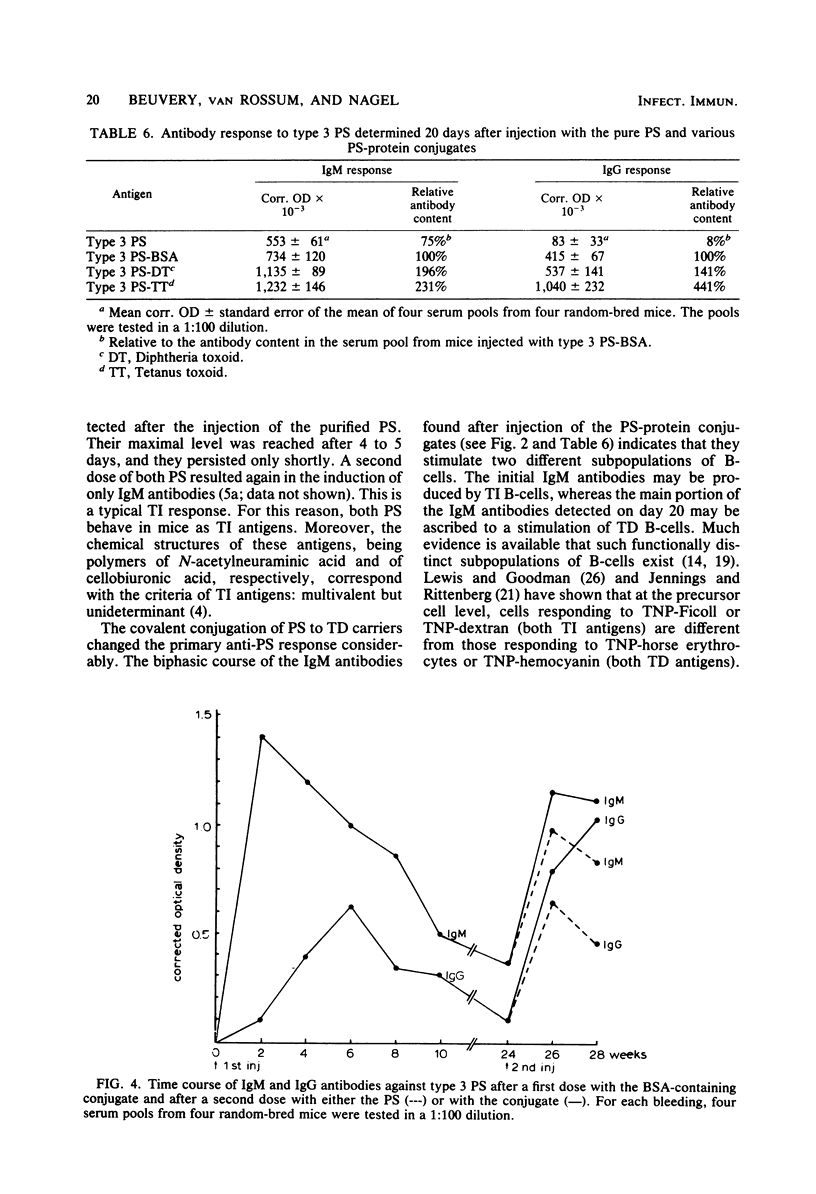
The nature and kinetics of the serum antibody response to pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates were studied in mice. Bovine serum albumin and diphtheria and tetanus toxoids were used as carrier proteins. The purified polysaccharides induced only immunoglobulin M (IgM) antibodies in thymus-bearing as well as congenic athymic (nude) mice. The polysaccharides covalently conjugated to proteins produced IgM and IgG antibodies in normal mice, but only IgM antibodies in nude mice. A second dose of the polysaccharide-protein conjugates resulted in a booster effect in the IgG response to the polysaccharides. Moreover, memory B-cells, generated after a primary injection with the polysaccharide-protein conjugates, could be triggered to the production of IgG antibodies after a second injection with the pure polysaccharides alone. These data indicate that the antibody response to the pure polysaccharides is thymus independent and that this response can be changed into a thymus-dependent response by covalent conjugation of the polysaccharide to a thymus-dependent protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C. Immunisation against bacterial meningitis. J Infect. 1981 Mar;3(1 Suppl):71–79. doi: 10.1016/s0163-4453(81)80011-4. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Antigen requirements for priming of IgG producing B memory cells specific for Type III pneumococcal polysaccharide. Immunology. 1980 Aug;40(4):521–527. [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Regulatory role of T cells in IgG antibody formation and immune memory to type III Pneumococcal polysaccharide. J Immunol. 1974 Dec;113(6):1909–1920. [PubMed] [Google Scholar]
- Braley-Mullen H. Secondary IgG responses to type 3 pneumococcal polysaccharide. III. T cell requirement for development of B memory cells. Eur J Immunol. 1977 Nov;7(11):775–781. doi: 10.1002/eji.1830071106. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Secondary IgG responses to type III pneumococcal polysaccharide. I. Kinetics and antigen requirements. J Immunol. 1975 Nov;115(5):1194–1198. [PubMed] [Google Scholar]
- Braley-Mullen H. Secondary IgG responses to type III pneumococcal polysaccharide. II. Different cellular requirements for induction and elicitation. J Immunol. 1976 Apr;116(4):904–910. [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Role of T lymphocytes in the humoral immune response. I. Proliferation of B lymphocytes in thymus-deprived mice. J Immunol. 1974 Nov;113(5):1438–1445. [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. The influence of T cells on the initiation and expression of immunological memory. Immunology. 1979 Oct;38(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- Frantz I. D. Growth Requirements of the Meningococcus. J Bacteriol. 1942 Jun;43(6):757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- GREGORY J. D. The effect of borate on the carbazole reaction. Arch Biochem Biophys. 1960 Aug;89:157–159. doi: 10.1016/0003-9861(60)90036-9. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Austrian R., Cvjetanović B., Robbins J. B. Prospects for the prevention of bacterial meningitis with polysaccharide vaccines. Bull World Health Organ. 1978;56(4):509–518. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. Functional analysis of murine and human B lymphocyte subsets. Transplant Rev. 1975;24:177–236. doi: 10.1111/j.1600-065x.1975.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981 Sep;127(3):1011–1018. [PubMed] [Google Scholar]
- Jennings J. J., Rittenberg M. B. Evidence for separate subpopulations of B cells responding to T-independent and T-dependent immunogens. J Immunol. 1976 Nov;117(5 PT2):1749–1752. [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Prescott B. Kinetics of the antibody response to type III pneumococcal polysaccharide (SSS-III). I. Use of 125I-labeled SSS-III to study serum antibody levels, as well as the distribution and excretion of antigen after immunization. J Immunol. 1976 Jan;116(1):41–51. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lerman S. P., Romano T. J., Mond J. J., Heidelberger M., Thorbecke G. J. Induction of primary and inhibition of secondary antibody response to hapten by hapten conjugates of type III pneumococcal polysaccharide. Cell Immunol. 1975 Feb;15(2):321–335. doi: 10.1016/0008-8749(75)90011-8. [DOI] [PubMed] [Google Scholar]
- Lewis G. K., Goodman J. W. Carrier-directed anti-hapten responses by B-cell subsets. J Exp Med. 1977 Jul 1;146(1):1–10. doi: 10.1084/jem.146.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A., Brosi B. J., Buys J. Reliability of the enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Trichinella spiralis infections in conventionally raised pigs. J Immunol Methods. 1976;10(1):67–83. doi: 10.1016/0022-1759(76)90008-9. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Immunochemistry of Salmonella O-antigens: preparation of an octasaccharide-bovine serum albumin immunogen representative of Salmonella serogroup B O-antigen and characterization of the antibody response. J Immunol. 1978 May;120(5):1750–1757. [PubMed] [Google Scholar]
- Timkovich R. Detection of the stable addition of carbodiimide to proteins. Anal Biochem. 1977 May 1;79(1-2):135–143. doi: 10.1016/0003-2697(77)90387-6. [DOI] [PubMed] [Google Scholar]


