Abstract
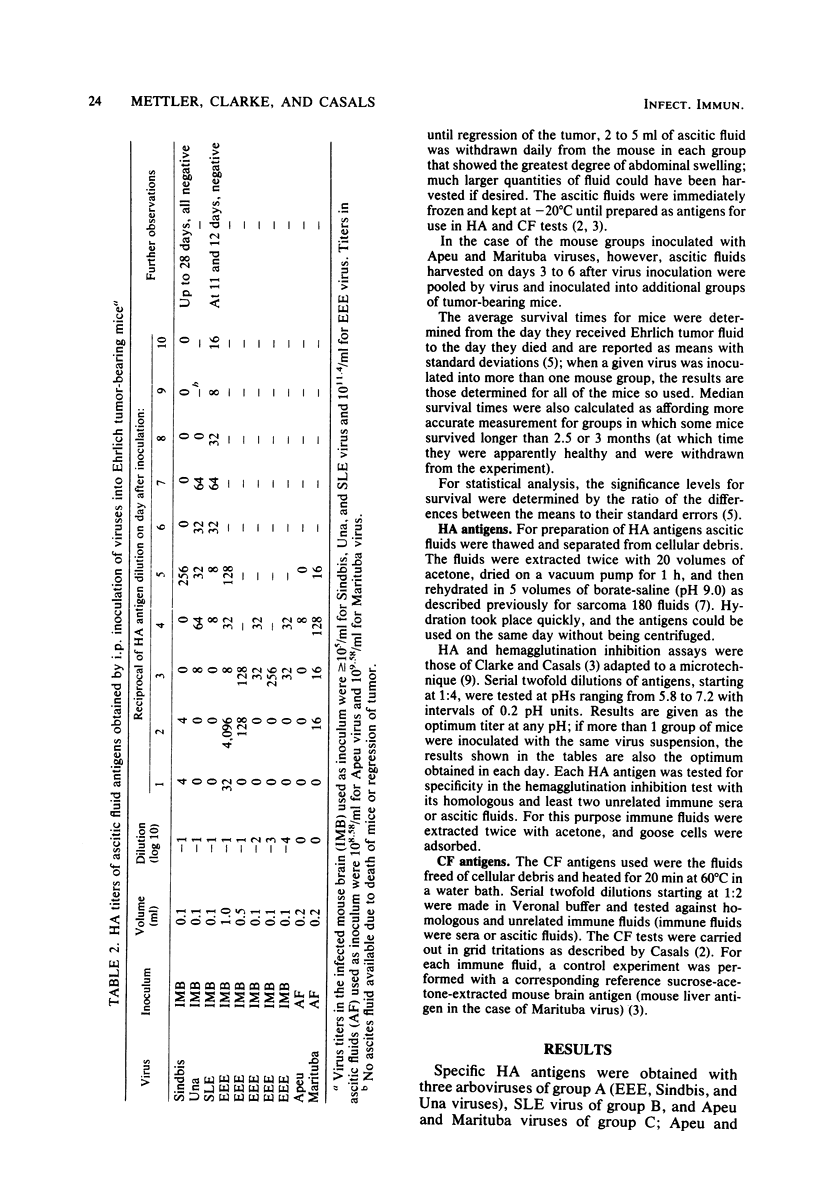
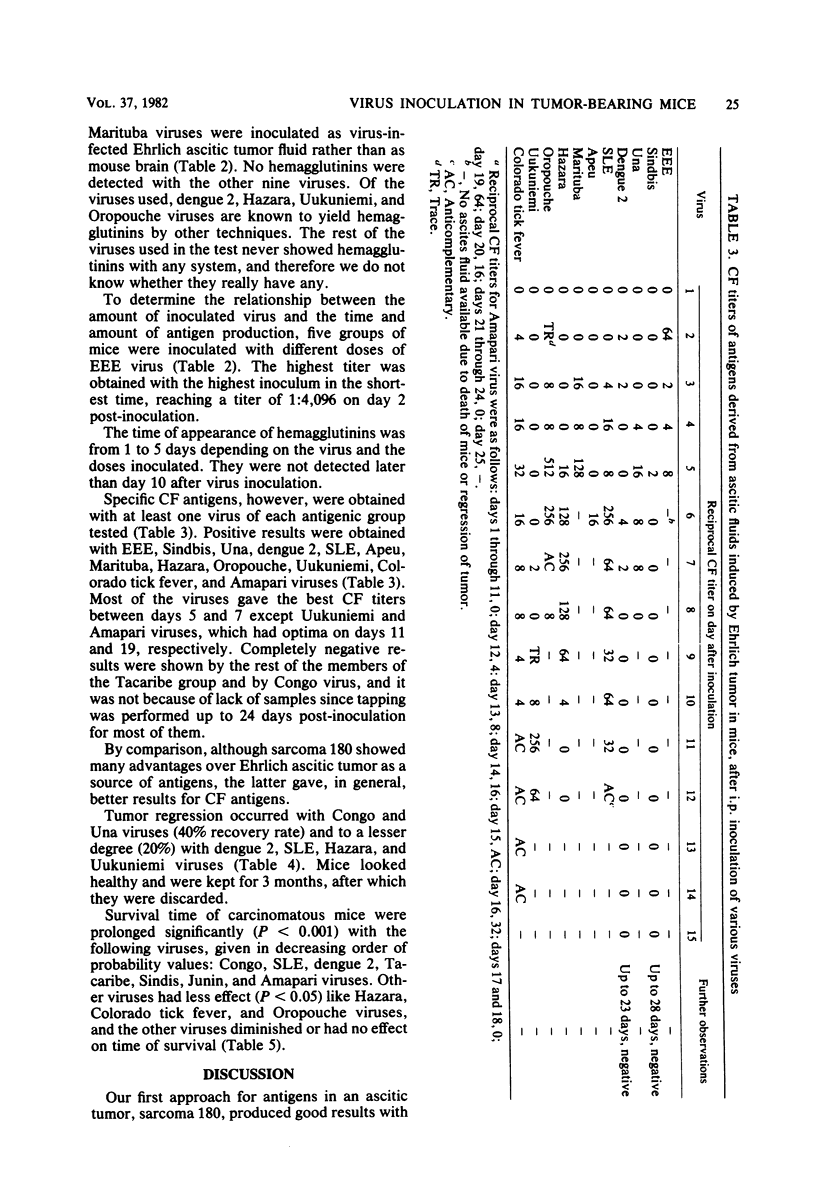
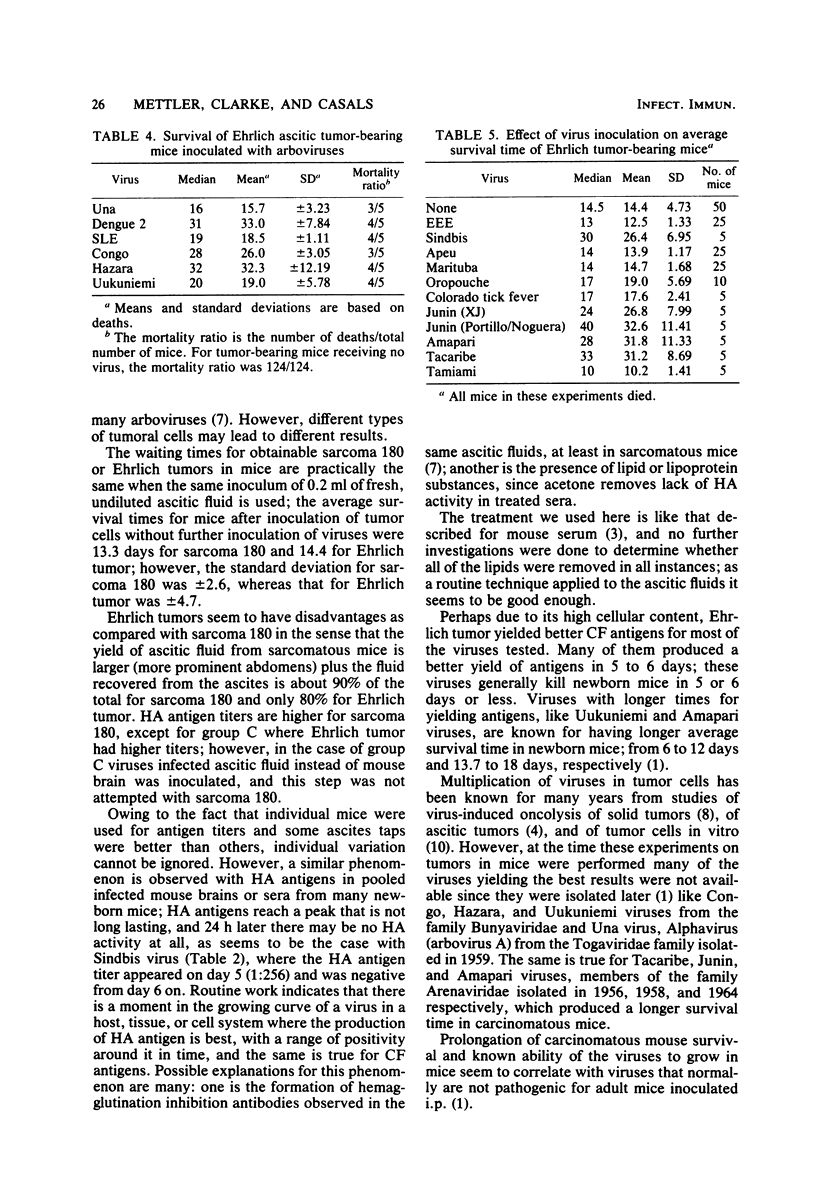
Ehrlich ascitic carcinoma, as developed in albino mice, has been used as a source of hemagglutinating and complement-fixing antigens, and it proved to be suitable for one type of antigen, or both, for at least 12 viruses of 16 tested. Hemagglutinins were obtained with members of arbovirus groups A, B, and C; complement-fixing antigens were obtained for at least one member of each antigenic group tested. Ehrlich ascitic tumor was compared with sarcoma 180 as a source of antigens; although sarcoma 180 showed many advantages over Ehrlich tumor, the latter gave, in general, better results for complement-fixing antigens. Oncolytic effect with complete recovery of the mice was observed in some instances. The highest recovery rate resulted with Congo and UNA viruses (40%), and the second highest rate resulted with dengue 2, St. Louis, Hazara, and Uukuniemi viruses (20%). The best survival was observed, in decreasing order, with Congo, St. Louis, dengue 2, Tacaribe, Sindbis, Junin, and Amapari viruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- KOPROWSKI H. Ascites tumors as culture media in quantitative growth studies of viral agents. Ann N Y Acad Sci. 1956 Mar 14;63(5):895–914. doi: 10.1111/j.1749-6632.1956.tb50899.x. [DOI] [PubMed] [Google Scholar]
- Mettler N. E., Casals J., Clarke D. H., Downs W. G., Shope R. E. Use of sarcoma 180 to prepare hemagglutinating and complement-fixing antigens for viruses in adult mice. Proc Soc Exp Biol Med. 1971 Apr;136(4):1355–1359. doi: 10.3181/00379727-136-35491. [DOI] [PubMed] [Google Scholar]
- Mettler N. E. Isolation of a microtatobiote from patients with hemolytic-uremic syndrome and thrombotic thrombocytopenic purpura and from mites in the United States. N Engl J Med. 1969 Nov 6;281(19):1023–1027. doi: 10.1056/NEJM196911062811901. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Stim T. B., Fischer G. A., Downs W. G., Chu M. Y. Cytolytic activity of arboviruses in murine leukemic lymphoblasts. Cancer Chemother Rep 2. 1971 Apr;2(1):57–64. [PubMed] [Google Scholar]



