Abstract
Most G protein-coupled receptors (GPCRs) started as orphan GPCRs. Matching them to known neuromodulators led to the elucidation of the broad diversity of the neuroreceptor families. Moreover, orphan GPCRs have also been used as targets to discover novel neuromodulators. These discoveries have had profound impact on our understanding of brain function. Here, I present an overview of how some of the novel neuropeptides have enlarged our comprehension of responses that direct sleep/wakefulness, the onset of obesity and the feeding response. I also discuss other advances gained from orphan GPCR studies such as the concept of specificity in neuromodulation or of receptors acting as sensors instead of synaptic transmitters. Finally, I suggest that the recently-discovered neuromodulators may hold the keys to our understanding of higher brain functions and psychiatric disorders.
Keywords: G protein-coupled receptors, neuropeptides, neuromodulators, orphanin FQ, orexins, ghrelin, kisspeptin, sensor receptors, pharmacological specificity, schizophrenia
Introduction
Synaptic transmission is viewed as depending primarily on the actions of glutamate and y-aminobutyric acid (GABA), and the majority of CNS interneuronal interactions monitored electrophysiologically are driven by these two neurotransmitters. A few other neurotransmitters such as acetylcholine, serotonin or ATP also elicit electrophysiological responses. Together these responses constitute the trunk of synaptic transmission. The role of the branches is carried out by neuromodulators.
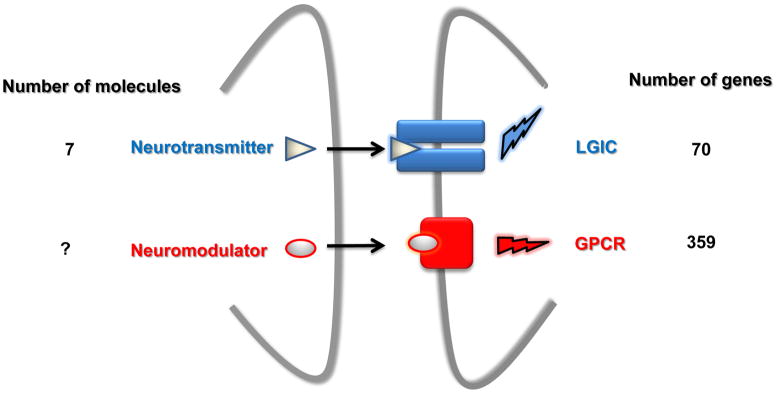
Neuromodulators can be classified as transmitters that, like glutamate and GABA, are released from a neuron and interact with specific receptors found on the membrane of a recipient neuron (Fig 1). Contrary to glutamate or GABA, they do not interact with ligand-gated ion channels but with G protein-coupled receptors (GPCRs). Activation of these GPCRs induces second messenger response(s) that changes the biochemical properties of the recipient cell and consequently can modulate its electrophysiological responsiveness but also its transcriptional activity or its metabolism. Note that glutamate, GABA and the neurotransmitters mentioned above activate their own GPCRs, they therefore can also act as neuromodulators. Because only a limited number of second messenger pathways exist, it is the neuromodulator-receptor interaction that needs to provide the complexity required for brain function. However, because this interaction is based on a binary mechanism, complexity must rely on the diversity of the neuromodulators and their receptors.
Figure 1. The synaptic transmission.
The numbers of molecules or genes are derived from the IUPHAR data base (www.iuphar-db.org/DATABASE) LGIC: Ligand-gated ion channel; GPCR: G protein-coupled receptors
The neuromodulators and their receptors
The search for neuromodulators began indeed with the discovery of acetylcholine, although it was discovered as a neurotransmitter according to the above definition. Neuromodulators were discovered through their abilities to regulate physiological responses in organ or tissue preparations (Langley and Magnus, 1905). This search gained another dimension when peptides were found to belong to this class of transmitters. Then lipid mediators and other small molecules were found to act as neuromodulators.
In parallel, neuromodulator receptors were being defined by pharmacological means. Synthetic ligands were being developed and used to differentiate these receptor identities. A few receptors were even purified and their sequences determined. This culminated with the discovery that the β2-adrenergic receptor and the opsins share a seven transmembrane domains topology (7TMs) and some sequence similarities (Dixon et al., 1986). Since the sole link between these two receptors is the fact that they induce G protein-mediated cellular responses, this discovery suggested that GPCRs may belong to a supergene family. This suggestion was reinforced with the cloning of the first neuropeptide receptor (Substance K receptor) (Masu et al., 1987) and opened the door to the search for GPCRs by homology screening approaches such as low-stringency hybridization and degenerate polymerase chain reaction (PCR) (Bunzow et al., 1988; Libert et al., 1989).
The receptors cloned via these strategies are by definition unmatched to natural ligands. They are “orphan” receptors. But at that time, some 50 neuromodulators were known to exist but had no cognate cloned receptors. The cloning of the orphan GPCRs offered a solution to this problem. The approach was to express orphan GPCRs in cells in culture and to use these heterologous GPCRs as targets for matching to possible neuromodulators. Insights in the orphan GPCR tissue expression profile as well as random testing proved to be successful in matching the first orphan GPCRs to known neuromodulators. The first deorphanized GPCRs, the 5HT-1A and the D2 dopamine receptors were reported in 1988 (Bunzow et al., 1988; Fargin et al., 1988). This strategy was rapidly espoused worldwide and is now known as reverse pharmacology (Libert et al., 1991; Mills and Duggan, 1994).
During the first part of the 90’s, application of the reverse pharmacology strategy led to the molecular identification of many GPCRs (Civelli et al., 2006) (Table 1). These DNA sequences allowed for the determination of their definitive pharmacological profiles as well as for in depth analyses of their sites of expression. In turn, these receptors DNA probes led to the discovery of sequentially-related related GPCRs and the blossoming of the GPCR subfamilies. Most often, the cloning of the GPCR genes have greatly extended the diversity of the subfamilies (Civelli et al., 2006). These discoveries have had lasting impact in the fields of pharmacological and pharmaceutical research.
TABLE 1.
Orphan GPCRs and neuromodulators
| 1988 Two orphan GPCRs as neuromodulator receptors: the 5-HT1A and the dopamine D2 receptors. |
| 1995 The first novel neuromodulator discovered as target of an orphan GPCR: orphanin FQ/nociceptin |
| 1988 – present Orphan GPCRs linked to neuromodulators |
| Adenosine A1, A2a, A2b, A3; Adenosine diphosphate; Adrenergic a1A, a1d, a2b, a2c, b1, b3; Adrenomedulin; Anaphylatoxin C3a, C5a; b-alanine; Angiotensin AT1b; Apelin, Basic L-α amino acids; N-arachidonylglycine; Bile acids; Bradykinin; Bombesin BB1, BB3; Bovine adrenal medulla peptide 22; Cannabinoid CB1, CB2; Calcitonin gene related peptide; Chemokine CCR1, CCR2, CCR3, CCR4, CCR5, CXCR2, CXCR3, CXCR4; Cholecystokinin CCKa; Cortistatin; Dopamine D1, D2, D3, D4, D5; ; Eskine; Estrogen; 5-oxo-ETE; Farnesyl pyrophosphate and N-arachidonylglycine; Follicle-stimulating hormone; Formyl-peptide FPR2; Galanin type 2; Ghrelin; Gonadotropin-releasing hormone; Histamine H2, 3; a-ketoglutarate; Kynurenic acid; Kisspeptin/metastin; Lactate; Latrotoxin; L-DOPA; Leukotriene B4,C4,D4; Lysophosphatidic acid; ; Lysophosphatidylcholine; Lysophosphatidylinositol ; Medium and long fatty acids; Melanin-concentrating hormone; Melanocortin MC1, MC2, MC3, MC4, MC5; Melatonin ML1a, ML1B; 3- and 4-methyl-valeric acid; Motilin; Muscarinic acetylcholine M3, M4, M5; Neurokinin NK1, NK3; Neuromedin S,U; Neuropeptides B/W; Neuropeptide FF, AF; Neuropeptide S; Neuropeptide YY1, YY1-like, YY4; Neurotensin NTR2; Nicotinic acid; Oleoylethanolamide; Opioid k, m; Orexins/Hypocretins; OrphaninFQ/nociceptin; D-phenylalanine; Prokineticins1/2; Prolactin-releasing peptide, Prostaglandin D2; Prostanoid EP1, EP2, EP3, EP4, DP, FP, IP; Protease-activated 2,3; Proton; Purinoceptor P2Y1, P2Y3, P2Y4, P2Y6, P2Y8; Psychosine; RFamide-related protein 1,3; Pyroglutamylated arginine-phenylalanine-amide peptide; R-spondins; Relaxin; Relaxin-3; Serotonin 5-HT1a, 5-HT1b, 5-HT1d, 5-HT1e, 5-HT1f, 5-HT2a, 5-HT2b, 5-HT4, 5-HT5a, 5-HT5b, 5-HT6, 5-HT7; Sphingosine 1-phosphate; Sphingosylphosphorylcholine; Somatostatin SST1, SST2, SST3, SST4, SST5; Trace amines; Thyrotropin-stimulating hormone; UDP-glucose; Unsaturated long chain FFAs; Uracil nucleotide/ cysteinyl leukotrienes; Urotensin II; Vasopressin V1b, V2; Zn++ |
Derived from Civelli et al Ann. Rev. Pharm. 2012, in press
Concomitantly, the overall number of orphan GPCRs was steadily increasing due to the mining of the database of expressed sequence tagged cDNAs (Marchese et al., 1994; O’Dowd et al., 1998). It became clear that the numbers of GPCRs outnumbers the number of known neuromodulators (Later the completion of the human genome revealed the full extent of the GPCR diversity). The conclusion of this recognition was (and still is) that many orphan GPCRs must be activated by undiscovered neuromodulators, since inactive GPCRs would have been evolutionarily discarded. The corollary of this conclusion was that orphan receptors may be used as baits to identify novel neuromodulators (Civelli et al., 2001).
The search for novel neuromodulators
The approach used to isolate novel neuromodulators consists of expressing an orphan GPCR in heterologous cells and test these against brain tissue extracts. Receptor reactivity is monitored by quantifying changes in second messenger levels. The extract displaying reactivity is fractionated and its purification is pursued to homogeneity. The first orphan GPCR used in this approach was ORL-1, an orphan GPCR sequentially-related to the opioid receptors (Henderson and McKnight, 1997). Its activation was monitored by monitoring intracellular decreases in cAMP levels. Its neuromodulator was extracted from brain tissues and shown to be a neuropeptide, named orphanin FQ or nociceptin (OFQ/N)(Meunier et al., 1995; Reinscheid et al., 1995). This neuropeptide shares sequence similarities to the opioid peptides but also precise differences that render it inactive at opioid receptors (Reinscheid et al., 1998).
This strategy has since been used to discover the following neuropeptides (Fig 2): the two orexins (Oxs) (Sakurai et al., 1998), also identified through an RNA subtraction approach as hypocretins (Hcrts) (de Lecea et al., 1998), prolactin-releasing peptide (PrRP) (Hinuma et al., 1998), apelin (Tatemoto et al., 1998), ghrelin (Kojima et al., 1999), kisspeptin/metastin (Ohtaki et al., 2001), the two prokineticins (Lin et al., 2002; Masuda et al., 2002), neuropeptide B and neuropeptide W (NPB/W) (Brezillon et al., 2003; Fujii et al., 2002; Shimomura et al., 2002; Tanaka et al., 2003), neuropeptide S (NPS) (Sato, 2002; Xu et al., 2004), neuromedin S (Mori et al., 2005) and finally relaxin-3 (Liu et al., 2003). Each of these neuropeptides has been the subject of intense research, which has helped better understand a number of physiological processes. I will not cover all the advances that they have brought to neuroscience here, but in the next section, I review, as examples, three of the brain-directed responses for which our understanding has been drastically impacted by orphan GPCR research.
Figure 2.
The discovery of the novel neuropeptide and the structures of several of them. In blue are residues that are conserved in the neuropeptide families
Neuromodulatory responses directed by orphan GPCRs
Sleep/wakefulness
Studies on one orphan GPCR system, the orexin/hypocretin (Oxs/Hcrts) system, has had a great impact on our understanding sleep/wakefulness states. Soon after the discovery of the Oxs/Hcrts it was shown that mice devoid of Oxs/Hcrts exhibit a pronounced narcoleptic behavior (Chemelli et al., 1999). This conclusion was paralleled by genetic studies of an autosomal recessive mutation responsible for narcolepsy in dogs which showed that the responsible gene is a defective Oxs/Hcrts receptor 2 (Lin et al., 1999), further confirmed by the lack of Ox’s/Hcrt’s in several individuals afflicted with narcolepsy (Nishino et al., 2000).
The mode of action of Ox/Hcrt system on sleep an arousal has been investigated (Fig 3A). From the afferent side, it is known that the preoptic area, especially the ventrolateral preoptic nucleus (VLPO), plays a critical role in the initiation of non-rapid eye movement (NREM) sleep and maintenance of both NREM and rapid eye movement (REM) sleep (Sherin et al., 1998). Neurons in the VLPO fire at a rapid rate during sleep and slow down during the waking period. These neurons contain GABA and/or galanin and promote sleep. GABAergic neurons originating in the preoptic area densely innervate Ox/Hcrt neurons (Sakurai et al., 2005; Yoshida et al., 2006). The orexin neurons are inhibited by activation of the GABA system (Xie et al., 2006; Yamanaka et al., 2003). These observations therefore suggest that VLPO neurons send GABAergic projections to orexin neurons to turn off orexin neurons during sleep.
Figure 3. The role of the orexins/ hypocretins in the sleep/wake response.
In blue: GABA and its inhibitory effect on monoaminergic centers. In red: the stimulatory effect of the Oxs/Hcrts on the same centers which activation results in arousal and wake. BF: basal forebrain; VLPO: ventrolateral preoptic nucleus; TMN: tuberomammilary nucleus; LDT/PPT: laterodorsal tegmental nucleus/pedunculopontine nucleus: LC: locus coeluleus: DR: dorsal raphe
From the efferent side, it has been shown that Ox/Hcrt neurons innervate wake promoting centers such as the noradrenergic neurons of the locus coerulues (LC), the serotonergic neurons of the dorsal raphe (DR) and the histyaminergic neurons of the tuberomammilary nucleus of the hypothalamus (TMN) (Saper et al., 2005) (Fig 3). These monoaminergic neurons are synchronized and modulate sleep/wake states. They fire tonically during the awake state, less during NREM sleep, and not at all during REM sleep (Lee et al., 2005; Vanni-Mercier et al., 1984). Ox/Hcrt neurons discharge during active waking and virtually cease firing during sleep, including the NREM and REM periods (Lee et al., 2005) and thus should exert an excitatory influence on the wake-active neurons and help them sustain their activity. In addition, Ox/Hcrt neurons project to laterodorsal tegmental nucleus/pedunculopontine nucleus (LDT/PPT) cholinergic neurons and affect the activity of these neurons in wakefulness and REM sleep. Finally the OX/Hcrt neurons project and excite the cholinergic neurons of the basal forebrain (BF) which also regulate arousal. All together these data point at the Ox/Hcrt system as a central modulator for the maintenance of wakefulness. When dysfunctional it is a primary cause of the narcolepsy-catalepsy syndrome.
Onset of puberty
At the onset of puberty, neurons in the medial preoptic area of the hypothalamus initiate the pulsatile secretion of gonadotropin releasing hormone (GnRH) which reaches the pituitary gland where it stimulates the release of the gonadotopic hormones luteinizing hormone (LH) and follicle stimulating hormone (FSH). These hormones in turn act on the gonads to stimulate synthesis of the sex steroids which are required for spermatogenesis and oogenesis. The mechanism that initiates the pulsatile secretion of GnRH at puberty was unknown.
Then studies on patients with idiopathic hypogonadotropic hypogonadism (IHH) (de Roux et al., 2003; Marchese et al., 1994; Seminara et al., 2003) found that mutations in a particular orphan GPCR, GPR54, were responsible for the phenotype. These mutations resulted in loss of function or putative reductions in the GPR54 signaling. IHH is a clinical condition characterized by absence of pubertal sexual development and low gonadotropin levels and sex steroids. The role that GPR54 plays in initiating fertility has been confirmed in mice by the generation of lines with disruptions of the GPR54 gene (Funes et al., 2003; Kauffman et al., 2007; Lapatto et al., 2007; Messager et al., 2005; Seminara et al., 2003).
The discovery of kisspeptin/metastin as the neuropeptide that activates GPR54 allowed in-depth studies of all aspects of the system (Fig 4). First, mice with a disrupted kisspeptin gene display the similar reproductive defects found in the GPR54 mutants (d’Anglemont de Tassigny et al., 2007) (Lapatto et al., 2007). These mice exhibit IHH, have abnormal pubertal maturation and low sex steroid levels but retain the ability to secrete gonadotrophic hormones after kisspeptin injection. Then, the expression patterns of GPR54 and kisspeptin in the hypothalamus are also consistent with the function of these genes in the control of reproduction. Kisspeptin and GPR54 are expressed in discrete nuclei of the hypothalamus. Kisspeptin is expressed in the arcuate nucleus (ARC), anteroventral periventricular nucleus (AVPV) and the periventricular nucleus, (Gottsch et al., 2004; Irwig et al., 2004). GPR54 is localized in the preoptic areas and anterior and lateral hypothalamus, the diagonal band of Broca and the medial septum (Han et al., 2005; Irwig et al., 2004). More importantly, practically all the GnRH neurons express GPR54 (Chemelli et al., 1999; Han et al., 2005; Irwig et al., 2004). Kisspeptin and GPR54 expression increase at puberty in many species. These increases are confined to the AVPV and the periventricular nucleus and are not seen in the ARC.
Figure 4. Kisspeptin and the onset of puberty.
GnRH pulses produced at the onset of puberty by neurons of the preoptic area of the hypothalamus (POA) are under the regulation of kisspeptin (Kiss) secreted by the arcuate nucleus (ARC, negative feedback by sex steroids) and the anteroventral periventricular nucleus (AVPV, positive feedback by estrogen). GnRH acts on the pituitary to rpoduce LH/FSH which act on the gonads to produce sex hormones which in turn regulate the pituitary and the POA in feedbacks loops. In red stimulatory, in blue inhibitory pathways.
The hypothalamus-pituitary-gonadal axis implies that the sex steroids are part of feedback loops with the pituitary and the hypothalamus to regulate gonadotropin production. Yet direct action of estrogen on GnRH neurons is unlikely because they do not express estrogen receptor alpha. Studies on the kisspeptin system indicate that the kisspeptin-expressing neurons are the intermediaries that receive the signals from the sex steroids. They have therefore a very important modulatory role in the HPG axis and in particular direct the onset of puberty.
Food intake and energy homeostasis
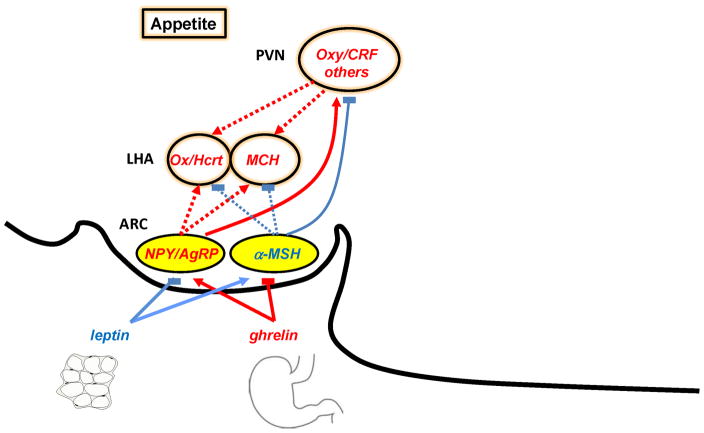
Orphan GPCRs have had an important impact on our understanding of appetite regulation and energy homeostasis. Ghrelin, the natural ligand of the GH-S receptor is produced in the stomach and is the most potent known circulating orexigen (Wiedmer et al., 2007). Intravenous injections of ghrelin into human volunteers increased their food intake by 28% (Wren et al., 2001) Elevated ghrelin has been linked to the hyperphagia and obesity of individuals with the Prader-Willi syndrome (Cummings et al., 2002) Ghrelin is a functional antagonist of leptin, produced in adipose tissues, which acts as a satiety signal (Friedman and Halaas, 1998). Humans genetically lacking leptin are hyperphagic and severely obese and respond dramatically to leptin administration (Friedman and Halaas, 1998). Ghrelin and leptin are released in the circulation and converge on one brain center, the hypothalamic arcuate nucleus (ARC), which is more readily accessed by blood-borne peptides, and there act on two populations of neurons. The first population expresses two orexigenic peptides, the melanocortin antagonist Agouti-related peptide (AgRP) and Neuropeptide Y (NPY). The second population expresses the pro-opiomelanocortin (POMC) precursor and the peptide cocaine and amphetamine-related transcript (CART) (Fig 5).
Figure 5. The role of ghrelin in the control of food intake.
Ghrelin produced by the stomach activates NPY/Agrp neurons and inhibit POMC (α-MSH) neurons in the arcuate nucleus. Leptin produced by the fat cells does the opposite. NPY/Agrp and POMC neurons project to several brain nuclei in particular the lateral hypothalamus (LHA) and the paraventricular nucleus where they convey orexigenic (in red) and anorexigenic (in blue) signals. The orexins/hypocretins and MCH in the LHA have been shown to stimulate appetite.
Ghrelin stimulates NPY/AgRP neurons and thus promote the production and secretion of NPY and AgRP peptides (Kojima and Kangawa, 2008; Mondal et al., 2005). Studies with NPY- or AgRP-knockout mice confirm these results (Chen et al., 2004). Note that there are also ghrelin receptors on vagal sensory nerves which play a role in the feeding response(Date et al., 2002). Leptin, on the other hand, stimulates POMC/CART neurons and inhibits NPY/AgRP neurons. Leptin effects on POMC are of importance since fasting decreases POMC expression (Coll et al., 2004). POMC is cleaved to α-melanocyte-stimulating hormone (αMSH) which is considered the predominant POMC-derived product controlling energy. αMSH acts at melanocortin 3 and melanocortin 4 receptors (MC3R and MC4R, first identified as orphan GPCRs) (Cone, 2005). The NPY/AgRP neurons project to many of the same brain areas as POMC neurons. They secrete AgRP which acts as an antagonist at MC3R and MC4R (Cone, 2005) such that one action of the NPY/AgRP neurons is to counter the activity of POMC neurons. They also secrete NPY which acts at NPY receptors (first identified as orphan GPCRs) to stimulate food intake (Clark et al., 1984; Stanley and Leibowitz, 1984). When either AgRP or NPY is administered chronically into the brain, body weight increases (Morton et al., 2006; Ollmann et al., 1997; Zarjevski et al., 1993).
Although the NPY and POMC neurons project throughout the brain, two target areas are of particular importance to food intake regulation. The first is the paraventricular nucleus (PVN) which expresses both MC3R/MC4R and NPY receptors and synthesize and secrete neuropeptides that have a net catabolic action, such as CRH and oxytocin (Atasoy et al., 2012). The second is the one is the lateral hypothalamic area (LHA) the brain center that lesion studies have identified as the center for feeding initiation. The LHA receives direct inputs from the ARC, and contains neurons that synthesize and secrete prominently two anabolic peptides, ligands of orphan GPCRs, melanin-concentrating hormone (MCH) and the orexins (Oxs/hcrts). Administrations of MCH (Qu et al., 1996) or orexins (Sakurai et al., 1998) increase food intake and body weight gain. The interplay between these opposing hypothalamic circuits is therefore seen as a major regulator of food intake and consequently of energy homeostasis.
Other advances gained from orphan GPCR studies
New modifications in neuropeptide structure
The natural ligands of two orphan GPCRs, two neuropeptides, stand out with regard to their structures (Fig 2). One is neuropeptide B (NPB), which is brominated at its N-terminus (Trp1) (Tanaka et al., 2003). This represents the first evidence of bromination in mammals. NPB is similar to NPW which is not brominated. Both act at two related GPCRs. Since des-Bromo-NPB is equipotent to brominated NPB at activating its receptors yet, the biological significance of the bromination event is unclear (Hondo et al., 2008; Tanaka et al., 2003).
The other neuropeptide is ghrelin, the only neuropeptide thus far that is modified by a fatty acid. It is n-octanoylated at serine 3 (Ser3) (Kojima et al., 1999). In contrary to NPB, this modification is essential for its activity. In view of the role of ghrelin as an orexigenic factor, as discussed above, its acylation has become a subject of research in its own right. It was found that there exist one enzyme, ghrelin O-acyltransferase (GOAT) that attaches octanoate to proghrelin (Gutierrez et al., 2008; Yang et al., 2008), which is then processed to ghrelin. GOAT’s activity is specific to ghrelin since no other acyltransferases do it and is only expressed in tissues that express ghrelin (Yang et al., 2008). This indicates that nature evolved a specific posttranslational system for regulating the activity of a single neuropeptide, which putatively adds to the physiological importance of ghrelin and opens a new way for developing ghrelin-related therapies.
The concept of specificity in neuromodulation
Studies on orphan GPCRs have also impacted our understanding of specificity in neuromodulation. Receptors are expected to bind one or several closely-related neuromodulators. This is expected to result from evolutionary constrain that aim at specifying neuromodulation. For example, the three opioid receptors bind all the opioid peptides and have evolved structures that ensure that they do not bind the related neuromodulator OFQ/N (Meng et al., 1996; Reinscheid et al., 1998). The catecholaminergic receptors are closely related phylogenetically and are activated by structurally-related neuromodulators. Yet, nordrenaline and dopamine direct different neuromodulatory responses, although it has been shown that adrenaline and noradrenaline can efficiently activate the dopamine D4 receptor in vitro (Lanau et al., 1997) and as does dopamine at adrenergic receptors in brain slices (Cornil et al., 2002).
The studies on the ligands of the Mas-related GPCRs (Mrgprs or Sensory Neuron-Specific Receptors, SNSRs) may revise our understanding of specificity. This receptor family comprises more than 50 orphan GPCRs in mice, many of which are expressed in specific subsets of nociceptive sensory neurons (Dong et al., 2001; Lembo et al., 2002). This highly restricted expression suggests that Mrgprs are likely to be involved in somatosensation, including pain or itch. Variable numbers of Mrgprs exist in human, rat and mouse making any attempt at orthologous classification difficult (Zylka et al., 2003). Being part of a subfamily, one could have expected that the Mrgprs would bind similar transmitters. Ligands for a number of these receptors have been identified (Bender et al., 2002; Lembo et al., 2002; Robas et al., 2003; Shinohara et al., 2004). Many ligands have been peptides which contain the C-terminal RF/Y-G or RF/Y-amide motif. Peptides bearing this motif, referred to as RFamides, are known to possess anti-nociceptive properties (Han et al., 2002; Tang et al., 1984; Yang et al., 1985). But other Mrgprs have been found to be activated by adenine or cortistatin-14 or β-alanine, highlighting the lack of ligand specificity of this receptor family (Bender et al., 2002; Lembo et al., 2002; Robas et al., 2003; Shinohara et al., 2004).
The majority of the Mrgprs are constitutively active in vitro. Constitutive activity may reflect a role for these receptors as sensors more than as carriers of a specific neuromodulator message. This could mean that Mrgprs evolved to recognize more than one structural motif. This would be expected for receptors signaling pleiotropic responses as is the case for the human MrgprX1. This Mrgpr can respond to both the endogenous peptide BAM8-22 at nanomolar concentrations and exogenous chloroquine at micromolar concentrations (Liu et al., 2009). It has been shown that this Mrgpr is responsible for the itching reaction associated with chloroquine administration. Mice lacking a cluster of Mrg genes, including the Mrgprs orthologous to MrgprX1, display significant deficits in chloroquine-induced itch, suggesting that one role for some of the Mrgprs maybe to act as itch receptors (Liu et al., 2009).
The neuromodulator receptors that act as sensors
The concept that some GPCRs are not confined to transmitting signals at the synapse but instead act as sensors has already been shown for the calcium sensing receptor (Brown, 1999). Indeed the largest family of orphan GPCRs, the olfactory receptors, act as sensors. More recently, the orphan GPRC6A has been first shown to be activated by not one but a series of basic L-α-amino acids with a preference for basic amino acids (Wellendorph et al., 2005) (Pi et al., 2005) but also for androgen and for osteocalcin (Pi et al., 2005). GPRC6A is therefore a cation-, calcimimetic-, and osteocalcin-sensing receptor (Pi and Quarles, 2012).
Other GPCRs may be sensing cell damage. For example the orphan GPCRs, GPR91 and GPR99, have been shown to be activated by succinate and α-ketoglutarate, respectively (He et al., 2004). Succinate participates in the reabsorption of phosphate and glucose in the proximal tubules and stimulates gluconeogenesis (He et al., 2004). Succinate is however unable to induce hypertension in GPR91-deficient mice thus demonstrating that its activity relies on GPR91 activation. Succinate belongs to the tricarboxylic acid cycle intermediates, which are not expected to be secreted as neuromodulators, but which will be released upon mechanical stress or cell death. Indeed, it has been shown that succinate, acting through GPR91, governs retinal angiogenesis and show the propensity of retinal ganglion neurons to act as sensors of ischemic stress (Sapieha et al., 2008). It is therefore possible that several GPCRs are monitoring cell death.
Perspective
Studies on orphan GPCRs have had a profound impact on our understanding of neuromodulation. First the majority of the neuromodulator receptors, the GPCRs, started as orphan GPCRs, and novel neuromodulators have been discovered as ligands of orphan GPCRs. Novel neuromodulators have proven to be the missing link in our understanding of biological function and disorders, for example, the Oxs/hcrts in narcolepsy and kisspeptin in the initiation of puberty.
The neuromodulator GPCR family has undergone large expansion during vertebrate evolution. A characteristic of many of the neuromodulator receptor genes is their lack of introns. RNA-based mechanisms, instead of classical gene duplications, may have driven evolutionary events that are responsible for the lack of introns (Fridmanis et al., 2007). This expansion coincides with development of the so-called “higher” brain functions. It is therefore not surprising that neuromodulation has taken a prominent place in our understanding of behavior, cognition and affect. In this respect, neuromodulation offers probably the highest hopes for our understanding the pathophysiology of psychiatric disorders and indeed recent studies emphasize this point (Table 2).
Table 2.
| Therapeutic indication | Neuromodulator system | |
|---|---|---|
| Schizophrenia | MCH | activation increases apomorphine-induced PPI inhibition reverses PPI deficits in APO-SUS rats higher pMCH mRNA levels in APO-SUS rats |
| NPS | activation decreases MK-801-induced PPI activation decreases MK-801-induced vacuolization of the retrosplenial cortex |
|
| Kisspeptin | activation reverses PPI deficits in a model of schizophrenia (maternal polyriboinosinic-polyribocytidylic acid (poly I:C) injection), | |
| Anxiety | OFQ/N | activation reduces anxiety responses OFQ/N precursor KO mice exhibit anxiogenic behavior |
| NPS | activation reduces anxiety responses facilitates extinction of conditioned fear responses |
|
| NPB/W | NPB/W Receptor 1 KO mice exhibit impaired contextual fear conditioning impaired safety conditioning increased social interaction |
Schizophrenia
Schizophrenia has, and still is, viewed as being in significant part the result of one neuromodulator imbalance, dopamine (Carlsson, 1988; Toda and Abi-Dargham, 2007). Other newer neuromodulator systems such as the metabotropic glutamate system are taking stand (Coyle, 2006; Stone et al., 2007) ((Moghaddam and Javitt, 2012; Raedler et al., 2007) but orphan GPCR systems may also play a role. The pathophysiology of schizophrenia is difficult to study in animals. Possibly our best bet is to record sensorimotor gating, the ability to selectively allocate attention to a significant event by silencing the background. Sensorimotor gating can be monitored by prepulse inhibition (PPI), the phenomenon where a startle response produced by an intense stimulus (pulse) is suppressed when a weak prestimulus (prepulse) immediately precedes it. Significant PPI deficits have been observed in patients with schizophrenia and other psychopathological disorders. Three orphan GPCR systems have recently been shown to play a role in regulating PPI (Cardon et al., 2010; Chung et al., 2011; Okamura et al., 2010)
Neuropeptide S (NPS) is expressed in only a few brainstem nuclei where it is co-expressed with glutamate. Dysfunctions in glutamatergic neurotransmission via N-methyl-D-aspartate (NMDA) receptors might be involved in the pathophysiology of schizophrenia since a NMDA receptor antagonists, MK-801, has been shown to induce psychotic-like behavior in humans and animal models. MK-801 is further known to produce histological changes such as cytoplasmic vacuoles in retrosplenial cortex neurons where NPS receptors are highly expressed. It was shown that NPS treatment attenuates MK-801-induced vacuolization in a dose-dependent manner. Furthermore, animals pretreated with NPS recover significantly from MK-801-induced disruption of PPI. (Okamura et al., 2010)
The role of kisspeptin system has been investigated in a neurodevelopmental animal model for schizophrenia (maternal poly I:C treatment) in which abnormal PPI develops only at adulthood. In this system it was shown that kisspeptin expression is related to the late onset of the schizophrenia-like behavior. Furthermore, administrations of kisspeptin overcome the behavioral deficits measured by PPI (Cardon et al., 2010)
Finally the MCH system was also shown to affect schizophrenia-like responses (Chung et al., 2011) MCH had been shown to potentiate dopamine-induced cellular firing in the shell of the nucleus accumbens (NAcSh), center of many dopamine-directed responses and in particular of its role in sensorimotor gating. As expected, administration of MCH to the NAcSh potentiates apomorphine-induced PPI deficits without affecting startle reactivity. This observation was extended by using the APO-SUS and APO-UNSUS outbred rat model. These animals have been selected and bred to exhibit differences in their susceptibility to apomorphine. The APO-SUS rats have been described as an animal model displaying aspects of schizophrenia. MCH was shown to disrupt PPI in APO-UNSUS rats, but not in APO-SUS rats, in line with their hyper-dopaminergic activity of their mesolimbic dopamine pathway which may not be increased further upon exogenous MCH injection. Moreover, blockade of the MCH system in APO-SUS rats restores PPI deficits to levels similar to those found in APO-SUS rats. Furthermore, this correlates with pMCH mRNA levels which were found increased in APO-SUS versus APO-UNSUS rats. That there may be a link between schizophrenia and the activity of the MCH system is further suggested by a genomic linkage study which revealed significant associations between schizophrenia and a number of SNPs and haplotypes located in the MCH receptor gene locus (Chung et al., 2011)
Anxiety disorders
Central administrations of OFQ/N exert anxiolytic effects comparable to those resulting from classic anxiolytic drugs treatment, such as diazepam (Civelli, 2008). A synthetic OFQ/N agonist induces anxiolytic-like effects in a variety of paradigms such as the elevated plus maze, pup isolation-induced ultrasonic vocalization, fear-potentiated startle, Geller-Seifter conflict and conditioned lick suppression. Mice devoid of OFQ/N precursor display signs of impaired stress adaptation and increased anxiety-like behavior. OFQ/N knockout mice exhibit increased stress-induced analgesia when housed in groups, an environmental condition that may be a source of chronic mild stress.
Central administrations of NPS also produce anxiolytic-like effects independent of its effects on locomotion (Xu et al., 2004). NPS furthermore facilitates extinction of conditioned fear responses when administered into the amygdala, a response that can be reversed by an NPS receptor antagonist (Jungling et al., 2008). Consequently these data indicate that the NPS system is involved not only in anxiety behavior and but also in extinction. These results are in line with the observation that specific NPSR alleles appear to be associated in human with panic disorder, a specific form of anxiety disorder (Okamura et al., 2007).
The role of the NPB/W system in fear conditioning has been revealed by behavioral studies of the NPB/W receptor 1 KO mice (Nagata-Kuroiwa et al., 2011). These mice exhibit an intriguing pattern of behavioral abnormalities in the resident-intruder paradigm. When presented with an intruder mouse, these mice display impulsive contact with the strange mice, produce more intense approaches and longer contact toward them. They also sustain a higher elevation of heart rate and blood pressure as compared to wild type mice. Histological and electrophysiological studies show that NPB/W receptor 1 acts as an inhibitory regulator on a subpopulation of GABAergic neurons in the lateral division of the central amygdala and terminates stress responses. Together these data suggest that impairment of the NPB/W system leads to stress vulnerability (Nagata-Kuroiwa et al., 2011).
Concluding remarks
The discussion of these five orphan GPCR systems provides only a few examples of the data implicating novel neuromodulators in the pathophysiology of neuropsychiatric disorders. While these studies are still preliminary, they set new bases to investigate brain function. Since there exist some 70 GPCRs that are still orphan and that classify, on the basis of their sequences, as potential neuromodulator receptors, many neuromodulators remain to be found which could drastically enrich our understanding of mental health. In this respect, because GPCRs are excellent targets for drug design, the newest neuromodulator receptors carry our best hope for devising therapies that aim at managing psychiatric disorders in a radically new way.
Acknowledgments
The author is thankful to his colleagues, Zhiwei Wang, Rainer Reinscheid, Yan Zhang Nayna Sanathara and Shinjae Chung for their help during the preparation of the manuscript. The work done in the author’s laboratory was supported by National Institute of Health Grants MH60231, DA024746, an Established Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD) and a Tourette Syndrome Association award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012 doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender E, Buist A, Jurzak M, Langlois X, Baggerman G, Verhasselt P, Ercken M, Guo HQ, Wintmolders C, Van den Wyngaert I, et al. Characterization of an orphan G protein-coupled receptor localized in the dorsal root ganglia reveals adenine as a signaling molecule. Proc Natl Acad Sci U S A. 2002;99:8573–8578. doi: 10.1073/pnas.122016499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezillon S, Lannoy V, Franssen JD, Le Poul E, Dupriez V, Lucchetti J, Detheux M, Parmentier M. Identification of natural ligands for the orphan G protein-coupled receptors GPR7 and GPR8. Journal of Biological Chemistry. 2003;278:776–783. doi: 10.1074/jbc.M206396200. [DOI] [PubMed] [Google Scholar]
- Brown EM. Physiology and pathophysiology of the extracellular calcium-sensing receptor. Am J Med. 1999;106:238–253. [PubMed] [Google Scholar]
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Cardon M, Ron-Harel N, Cohen H, Lewitus GM, Schwartz M. Dysregulation of kisspeptin and neurogenesis at adolescence link inborn immune deficits to the late onset of abnormal sensorimotor gating in congenital psychological disorders. Mol Psychiatry. 2010;15:415–425. doi: 10.1038/mp.2009.66. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Chung S, Verheij MM, Hesseling P, van Vugt RW, Buell M, Belluzzi JD, Geyer MA, Martens GJ, Civelli O. The melanin-concentrating hormone (MCH) system modulates behaviors associated with psychiatric disorders. PLoS One. 2011;6:e19286. doi: 10.1371/journal.pone.0019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O. The orphanin FQ/nociceptin (OFQ/N) system. Results Probl Cell Differ. 2008;46:1–25. doi: 10.1007/400_2007_057. [DOI] [PubMed] [Google Scholar]
- Civelli O, Nothacker HP, Saito Y, Wang Z, Lin SH, Reinscheid RK. Novel neurotransmitters as natural ligands of orphan G-protein-coupled receptors. Trends Neurosci. 2001;24:230–237. doi: 10.1016/s0166-2236(00)01763-x. [DOI] [PubMed] [Google Scholar]
- Civelli O, Saito Y, Wang Z, Nothacker HP, Reinscheid RK. Orphan GPCRs and their ligands. Pharmacol Ther. 2006;110:525–532. doi: 10.1016/j.pharmthera.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Fargin A, Raymond JR, Lohse MJ, Kobilka BK, Caron MG, Lefkowitz RJ. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988;335:358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Fridmanis D, Fredriksson R, Kapa I, Schioth HB, Klovins J. Formation of new genes explains lower intron density in mammalian Rhodopsin G protein-coupled receptors. Molecular Phylogenetics and Evolution. 2007;43:864–880. doi: 10.1016/j.ympev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fujii R, Yoshida H, Fukusumi S, Habata Y, Hosoya M, Kawamata Y, Yano T, Hinuma S, Kitada C, Asami T, et al. Identification of a neuropeptide modified with bromine as an endogenous ligand for GPR7. J Biol Chem. 2002;277:34010–34016. doi: 10.1074/jbc.M205883200. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci U S A. 2002;99:14740–14745. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- Henderson G, McKnight AT. The orphan opioid receptor and its endogenous ligand--nociceptin/orphanin FQ. Trends Pharmacol Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, et al. A prolactin-releasing peptide in the brain. Nature. 1998;393:272–276. doi: 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- Hondo M, Ishii M, Sakurai T. The NPB/NPW neuropeptide system and its role in regulating energy homeostasis, pain, and emotion. Results Probl Cell Differ. 2008;46:239–256. doi: 10.1007/400_2007_056. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Structure and function of ghrelin. Results Probl Cell Differ. 2008;46:89–115. doi: 10.1007/400_2007_049. [DOI] [PubMed] [Google Scholar]
- Lanau F, Zenner MT, Civelli O, Hartman DS. Epinephrine and norepinephrine act as potent agonists at the recombinant human dopamine D4 receptor. J Neurochem. 1997;68:804–812. doi: 10.1046/j.1471-4159.1997.68020804.x. [DOI] [PubMed] [Google Scholar]
- Langley JN, Magnus R. Some observations of the movements of the intestine before and after degenerative section of the mesenteric nerves. J Physiol. 1905;33:34–51. doi: 10.1113/jphysiol.1905.sp001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, et al. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, Simons MJ, Dumont JE, Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244:569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- Libert F, Schiffmann SN, Lefort A, Parmentier M, Gerard C, Dumont JE, Vanderhaeghen JJ, Vassart G. The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. Embo J. 1991;10:1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DCH, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. Journal of Biological Chemistry. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin XY, Qiu XH, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen J, Sutton S, Roland B, Kuei C, Farmer N, Sillard R, Lovenberg TW. Identification of relaxin-3/INSL7 as a ligand for GPCR142. J Biol Chem. 2003;278:50765–50770. doi: 10.1074/jbc.M308996200. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Docherty JM, Nguyen T, Heiber M, Cheng R, Heng HHQ, Tsui LC, Shi XM, George SR, Odowd BF. Cloning of human genes encoding novel g-protein-coupled receptors. Genomics. 1994;23:609–618. doi: 10.1006/geno.1994.1549. [DOI] [PubMed] [Google Scholar]
- Masu Y, Nakayama K, Tamaki H, Harada Y, Kuno M, Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature. 1987;329:836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- Meng F, Taylor LP, Hoversten MT, Ueda Y, Ardati A, Reinscheid RK, Monsma FJ, Watson SJ, Civelli O, Akil H. Moving from the orphanin FQ receptor to an opioid receptor using four point mutations. J Biol Chem. 1996;271:32016–32020. doi: 10.1074/jbc.271.50.32016. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mills A, Duggan MJ. Orphan 7 transmembrane domain receptors - reversing pharmacology. Trends in Biotechnology. 1994;12:47–49. doi: 10.1016/0167-7799(94)90099-X. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, Nakazato M. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept. 2005;126:55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Mori K, Miyazato M, Ida T, Murakami N, Serino R, Ueta Y, Kojima M, Kangawa K. Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. Embo Journal. 2005;24:325–335. doi: 10.1038/sj.emboj.7600526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Nagata-Kuroiwa R, Furutani N, Hara J, Hondo M, Ishii M, Abe T, Mieda M, Tsujino N, Motoike T, Yanagawa Y, et al. Critical role of neuropeptides B/W receptor 1 signaling in social behavior and fear memory. PLoS One. 2011;6:e16972. doi: 10.1371/journal.pone.0016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- O’Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski LF, Jr, George SR. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47:310–313. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Okamura N, Hashimoto K, Iyo M, Shimizu E, Dempfle A, Friedel S, Reinscheid RK. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1444–1448. doi: 10.1016/j.pnpbp.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Okamura N, Reinscheid RK, Ohgake S, Iyo M, Hashimoto K. Neuropeptide S attenuates neuropathological, neurochemical and behavioral changes induced by the NMDA receptor antagonist MK-801. Neuropharmacology. 2010;58:166–172. doi: 10.1016/j.neuropharm.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Quarles LD. Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology. 2012;153:2062–2069. doi: 10.1210/en.2011-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem. 1998;273:1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem. 2003;278:44400–44404. doi: 10.1074/jbc.M302456200. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1, 696. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal JS, Cho JH, Honore JC, Kermorvant-Duchemin E, Varma DR, Tremblay S, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- Sato S, Shintani Y, Miyajima N, Yoshimura K. Novel G protein-coupled receptor protein and DNA thereof. World patent application 2002
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Harada M, Goto M, Sugo T, Matsumoto Y, Abe M, Watanabe T, Asami T, Kitada C, Mori M, et al. Identification of neuropeptide W as the endogenous ligand for orphan G-protein-coupled receptors GPR7 and GPR8. J Biol Chem. 2002;277:35826–35832. doi: 10.1074/jbc.M205337200. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, et al. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–23564. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol. 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yoshida T, Miyamoto N, Motoike T, Kurosu H, Shibata K, Yamanaka A, Williams SC, Richardson JA, Tsujino N, et al. Characterization of a family of endogenous neuropeptide ligands for the G protein-coupled receptors GPR7 and GPR8. Proc Natl Acad Sci U S A. 2003;100:6251–6256. doi: 10.1073/pnas.0837789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Yang HY, Costa E. Inhibition of spontaneous and opiate-modified nociception by an endogenous neuropeptide with Phe-Met-Arg-Phe-NH2-like immunoreactivity. Proc Natl Acad Sci U S A. 1984;81:5002–5005. doi: 10.1073/pnas.81.15.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep. 2007;9:329–336. doi: 10.1007/s11920-007-0041-7. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Jouvet M. Specific neurons for wakefulness in the posterior hypothalamus in the cat. C R Acad Sci III. 1984;298:195–200. [PubMed] [Google Scholar]
- Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Brauner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- Wiedmer P, Nogueiras R, Broglio F, D’Alessio D, Tschop MH. Ghrelin, obesity and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:705–712. doi: 10.1038/ncpendmet0625. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Xie X, Crowder TL, Yamanaka A, Morairty SR, Lewinter RD, Sakurai T, Kilduff TS. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574:399–414. doi: 10.1113/jphysiol.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yang HY, Fratta W, Majane EA, Costa E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci U S A. 1985;82:7757–7761. doi: 10.1073/pnas.82.22.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133:1753–1758. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]