Abstract
Background
Deficiency of interleukin 1 receptor antagonist (DIRA) is a recently described autoinflammatory syndrome of skin and bone caused by recessive mutations in the gene encoding the interleukin 1 receptor antagonist. Few studies have been published about this debilitating condition. Early identification is critical for targeted lifesaving intervention.
Observations
A male infant, born to nonconsanguineous Puerto Rican parents, was referred for management of a pustular eruption diagnosed as pustular psoriasis. At 2 months of age, the infant developed a pustular eruption. After extensive evaluation, he was confirmed to be homozygous for a 175-kb genomic deletion on chromosome 2 that includes the IL1RN gene, commonly found in Puerto Ricans. Therapy with anakinra was initiated, with rapid clearance of skin lesions and resolution of systemic inflammation.
Conclusions
Recent identification of DIRA as a disease entity, compounded by the limited number of reported cases, makes early identification difficult. It is critical to consider this entity in the differential diagnosis of infantile pustulosis. Targeted therapy with the recombinant human interleukin 1 receptor antagonist anakinra can be lifesaving if initiated early. A high carrier frequency of the 175-kb DIRA-associated genomic deletion in the Puerto Rican population strongly supports testing infants presenting with unexplained pustulosis in patients from this geographic region.
Diffuse Infantile Pustulosis is a rare cutaneous presentation for myriad dermatologic conditions, such as bullous impetigo, tinea, bacterial folliculitis, scabies, miliaria, pustular psoriasis, IgA pemphigus, acrodermatitis enteropathica, eosinophilic pustular folliculitis, erythema toxicum neonatorum, and transient neonatal pustular melanosis.1 The least commonly reported and most recently recognized cause of infantile pustulosis is deficiency of interleukin 1 receptor antagonist (DIRA). DIRA is an early-onset autoinflammatory syndrome of skin and bone caused by recessively inherited loss-of-function mutations in IL1RN, the gene encoding the interleukin 1 (IL-1) receptor antagonist.2–4 Both IL-1α and IL-1β are proinflammatory cytokines that bind to the IL-1 type I receptor and are capable of inducing an inflammatory response. The IL-1 receptor antagonist is encoded by the IL1RN gene and has homology with IL-1α and IL-1β but functions as an antagonist of IL-1α and IL-1β by competitively binding to the IL-1 receptor and preventing the receptor from forming a signaling complex. It can, thus, function as a negative regulator that is important in controlling and stopping the response to IL-1. DIRA leads to unopposed action of IL-1 with resultant life-threatening systemic inflammation involving the skin and other organs. Treatment with the recombinant IL-1 receptor antagonist anakinra (Kineret; Swedish Orphan Biovitrum) can be lifesaving due to opposing IL-1 action and its inflammatory properties, as shown in the present patient.
REPORT OF A CASE
A 5-month-old boy was born prematurely at 31 weeks to healthy nonconsanguineous Puerto Rican parents after an uncomplicated labor. At hospital discharge, the neonate appeared to thrive until approximately 2 months of age, when he developed severe pustular eruption. His clinical presentation and biopsy findings were thought to be indicative of infantile pustular psoriasis; however, he did not respond to high-potency topical corticosteroid therapy. He was referred to the Weill Cornell dermatology clinic for systemic psoriasis therapy
In addition to pustulosis, the patient was found to have onychomadesis (Figure 1) and failure to thrive, weighing 7.5 lb at 5 months of age (<5th percentile). At the time of his presentation to the Weill Cornell dermatology clinic, we repeated the biopsy, which demonstrated an acantholytic subcorneal pustular dermatosis. Histologically, these findings were believed to be inconsistent with psoriasis, and the possibility of IgA pemphigus was raised. The results of his laboratory studies revealed persistent leukocytosis, monocytosis, iron deficiency anemia, and thrombocytosis. His erythrocyte sedimentation rate was persistently elevated at 56 mm/h, and his C-reactive protein level was 192.5 mg/L (to convert to millimoles per liter, multiply by 9.524) (Table 1). Total IgE level, eosinophil count, myeloperoxidase level, biotinidase level, lymphocyte subset levels, and immunoglobulin levels were normal. Given his diffuse cutaneous eruption and failure to thrive, the patient was admitted to the hospital for further workup and optimization of therapy.
Figure 1.
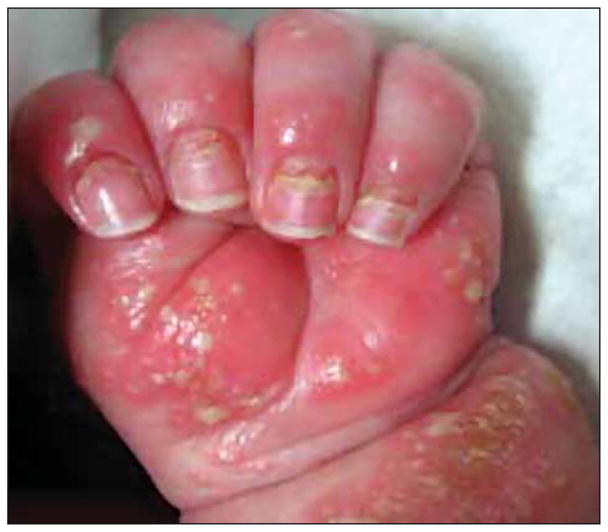
Infantile pustulosis and onychomadesis at initial presentation.
Table 1.
Laboratory Results Before and After Treatment With Anakinra
| Laboratory Test | During Hospital Admission | After Anakinra Treatment
|
Reference Range | ||
|---|---|---|---|---|---|
| Day 2 | Day 6 | Day 60 | |||
| CRP, mg/L | 192.5 | 9.5 | 9.3 | 10.0 | 0–9.9 |
| ESR, mm/h | 56 | 15.7 | 15 | 0.83 | 0–15 |
| Hemoglobin, g/dL | 7.6 | 9.2 | 9.7 | 13.4 | 10.5–13.5 |
| Hematocrit, % | 24.9 | 29.4 | 31.3 | 38.4 | 33–39 |
| Platelets, ×103/μL | 628 | 632 | 490 | 261 | 150–450 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
SI conversion factors: To convert CRP to millimoles per liter, multiply by 9.524; hemoglobin to grams per liter, multiply by 10.
On admission, the patient was tachycardic, with a heart rate of 166 beats/min. He had anemia, with a hemoglobin level of 7.6 g/dL (to convert to grams per liter, multiply by 10) and a hematocrit of 24.9%, and was transfused with 10 mL/kg of body weight of packed red blood cells. Chest radiography revealed a patchy right upper lobe opacity, widening of the lower ribs, and abnormally close-together vertebral attachments of ribs, suggestive of decreased spine height. The osseous skeletal survey revealed decreased height of the lower thoracic vertebrae with secondary rib crowding and angulated kyphosis centered at T9–T10; an irregular and widened proximal radius; and an irregular, bulbous appearance of the left proximal femur (Figure 2 and Figure 3). His left femur was shorter than his right. Skin scraping for fungal culture revealed few Mucor species, and the results of potassium hydroxide testing were negative. Results of skin bacterial cultures, blood cultures, and nasopharyngeal viral cultures were negative. Results of fecal examination for white blood cells, bacteria, ova, and parasites were all negative as well. The patient was treated with topical corticosteroids, an antifungal regimen, and oral antibiotic agents for pneumonia and later for possible secondary skin infection without improvement. He continued to feed poorly and to lose weight. He exhibited pain with movement and developed diffuse desquamating scaly skin, which covered his entire body, sparing only his palms and soles (Figure 4A); anemia; and persistent elevation of the erythrocyte sedimentation rate and C-reactive protein level (Table 1).
Figure 2.
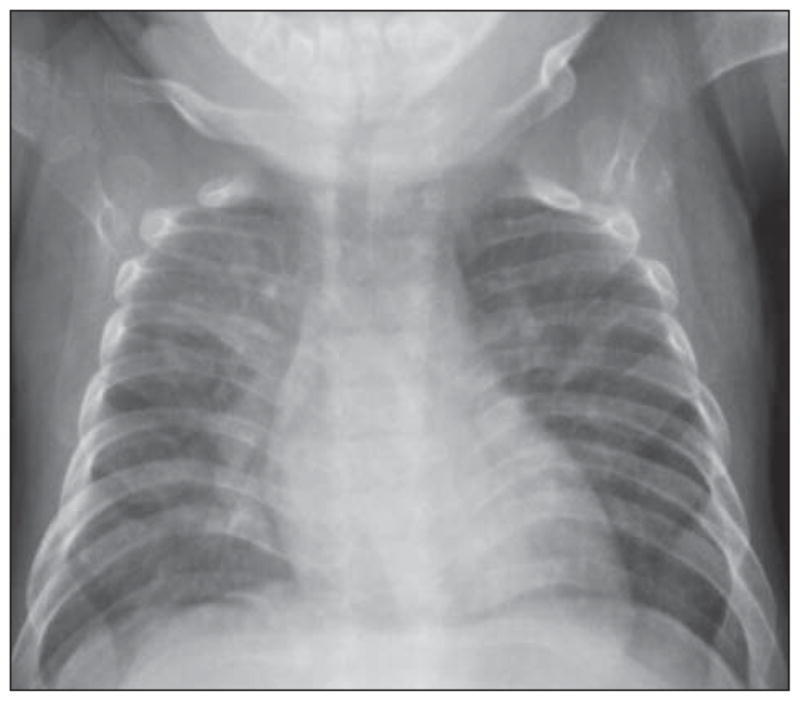
Chest radiograph showing crowding of the ribs due to decreased spine height.
Figure 3.
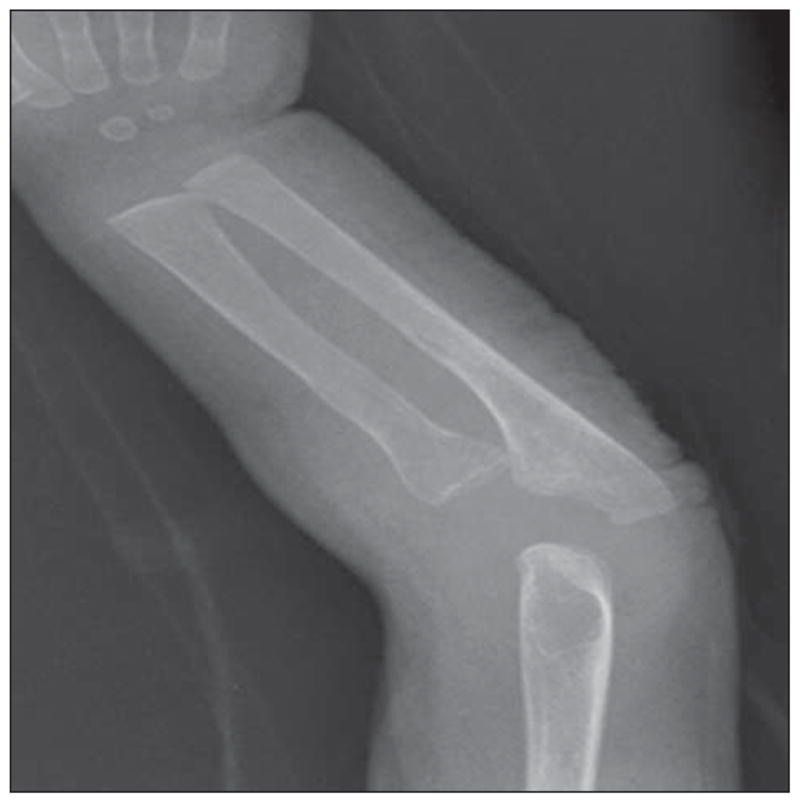
Irregular, widened left radius with increased lucency consistent with a lytic lesion.
Figure 4.
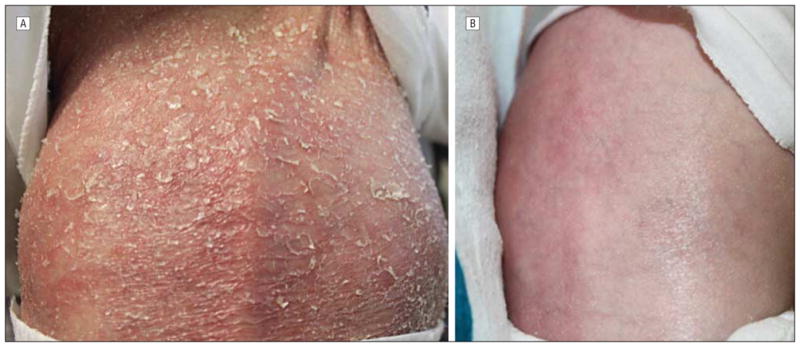
A, Diffuse desquamating scaly skin on the back before treatment. B, Clear back skin after anakinra therapy was initiated.
Another biopsy specimen was obtained and was submitted for direct immunofluorescent testing. Both biopsy specimens showed subcorneal and spongiform pustulation (Figure 5A) accompanied by a neutrophilic eccrine hidradenitis (Figure 5B). Other inflammatory cells were not seen. Foci of neutrophilimbued parakeratosis with attendant granular cell layer loss were noted (Figure 6). Results of the immunofluorescent studies were negative.
Figure 5.
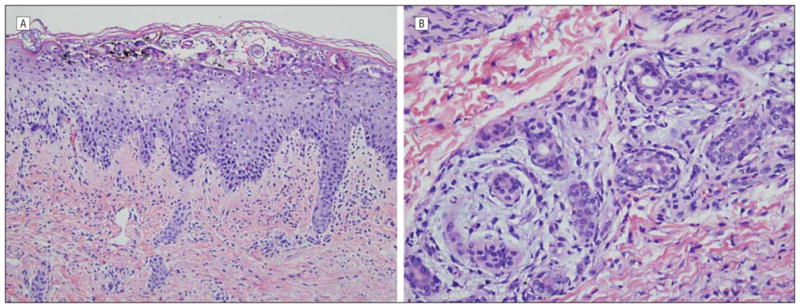
Skin biopsy specimens. At variance with psoriasis is the presence of subcorneal pustulosis (A) associated with superficial acantholysis along with a neutrophilic eccrine hidradenitis (B) (hematoxylineosin, original magnification ×20).
Figure 6.
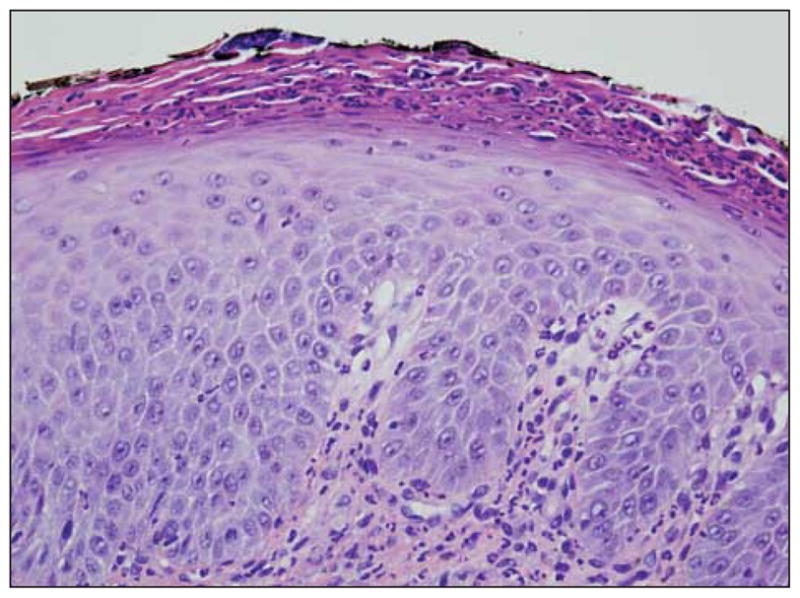
The skin biopsy specimen shows granular cell layer diminution with neutrophilimbued parakeratosis (hematoxylineosin, original magnification ×40).
The constellation and severity of the cutaneous and skeletal anomalies, and the persistence of the patient’s systemic inflammation, prompted initiation of treatment with subcutaneous anakinra at 3 mg/kg of body weight per day and genetic evaluation for DIRA. Several days after starting anakinra therapy, the patient was also administered prednisolone, 0.3 mg/kg of body weight per day, which was tapered and then discontinued after 3 months. Genetic analysis revealed a homozygous 175-kb genomic deletion that has previously been described in the Puerto Rican population, confirming the clinical diagnosis of DIRA. Within 48 hours of initiation of anakinra treatment, there was dramatic improvement in the skin condition, with cessation of exfoliation, appearance of fine superficial desquamation and erythema, decreased pustules, and, eventually, appearance of normal skin (Figure 4B). Inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein level, began to improve within 48 hours of initiation of treatment (Table 1), as did patient activity and weight gain. The anemia resolved as well within 2 months.
COMMENT
DIRA is a rare disorder recently described by Reddy et al3 and Aksentijevich et al.2,4 Thus far, only 2 other patients of Puerto Rican descent have been described in the literature with a genomic 175-kb deletion on chromosome 2q13 that encompasses 6 IL-1 family members, including the IL-1 receptor antagonist. As a result of these mutations, no functional IL-1 receptor antagonist protein is secreted, leading to the loss of an inhibitor to counteract the proinflammatory cytokines IL-1α and IL-1β. Aksentijevich et al2,4 determined that the allele frequency of the founder mutation in the Puerto Rican population around the area of Arecibo is approximately 1.3%. The present patient is the most recently identified patient who is homozygous for the Puerto Rican founder mutation. The biopsy findings in these earlier studies describe infiltration of the epidermis and dermis by neutrophils with a concomitant superficial folliculitis. The study by Reddy et al3 also emphasizes the subcorneal localization of the pustule. The present biopsy findings are similar to those described in the earlier studies. However, there was also significant accentuation of inflammation to involve the eccrine apparatus. This inherent tendency for the neutrophils to exhibit this pattern of syringotropism could also account for the prominent palmar-plantar involvement in this patient.
The recombinant IL-1 receptor antagonist anakinra, a drug approved by the Food and Drug Administration for the treatment of rheumatoid arthritis, is also used in other autoinflammatory conditions, including cryopyrin-associated periodic syndromes and adult- and juvenile-onset Still disease.5 A study6 of long-term use of the medication in children with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome showed that long-term treatment with anakinra is effective but needs to be initiated early, before the development of irreversible lesions. These facts underscore the importance of early diagnosis.
The present patient was initially diagnosed as having pustular psoriasis based on clinical presentation and biopsy results. The underlying illness was due to over-stimulation of native IL-1, resulting in a cytokine milieu that closely recapitulates that found in psoriasis. The mouse phenotype varies considerably depending on the genetic background onto which the IL1RN knockout is bred. The mouse ILRN-KO phenotype can vary from psoriasis-like skin lesions involving the pinna of the ear and arthritis on the BALB/cA background to aortitis and small vessel vasculitis on the BALBc/C57BL background.7–22 To date, 4 different mutations have been described to cause DIRA, and all of them are founder mutations. A fifth mutation is a novel 1-bp deletion introducing a frameshift in the protein sequence, followed by a premature termination codon, and the first described compound heterozygote with DIRA.4 In addition to the genomic deletion found in the Puerto Rican population, nonsense mutations leading to early truncation of the protein were found in Newfoundland, Dutch, and Lebanese populations,2 and the clinical presentation in patients from different populations is similar.
Osteolytic bone lesions and periostitis are hallmarks of the disease in humans that have not been described in mice. However, similar to what has been observed in mice, cells from patients with DIRA overproduce several chemokines and cytokines when stimulated with IL-1α, IL-1β, or lipopolysaccharide. Among cytokines or chemokines that are overproduced in humans are IL-1α, macrophage inflammatory protein-1α, IL-8, and IL-6. This cytokine milieu supports the expansion of IL-17–producing T cells (ie, TH17 cells)8,10 and is conducive to neutrophil accumulation since IL-17 elaborated by TH17 cells is a potent inducer of neutrophil accumulation.10,13 Also, IL-17 staining has been shown in the skin of patients with DIRA.2 Various mechanisms likely underlie neutrophil influx, including IL-1–induced IL-8 production. Although IL-17 seems to be an attractive cytokine that regulates granulocyte colony-stimulating factor and granulocyte colony-stimulating factor receptor activity and can also induce the gene that encodes IL-8, the most potent neutrophil chemoattractant, and CCL20 (macrophage inflammatory protein-3α), a chemokine that recruits dendritic cells and T cells, the contribution of IL-17 in this disease has not been sufficiently evaluated.8
Other murine studies7,14,17 have focused on the important role of tumor necrosis factor (TNF) in mediating the arthritis and dermatitis of mice with DIRA. Nakajima and coworkers7 found that TNF inhibitors improved the dermatitis, whereas blocking IL-17 production had no effect on the dermatitis, indicating a more important role for TNF in the pathogenesis of the cutaneous eruption. Presumably, unrepressed IL-1 enhances TNF production in keratinocytes. Indeed, keratinocyte-derived TNF is an important mediator of inflammation in psoriasis and works synergistically with IL-17. The role of TNF and IL-17 as possible mechanisms downstream of IL-1 in DIRA needs further evaluation. It remains interesting to speculate whether the similarity of the DIRA-associated cutaneous eruption to psoriasis in the murine setting and in humans could point to a role of IL-1 in psoriasis. However, the results of studies23–26 examining IL-1 expression in psoriasis and treatment of psoriasis with the IL-1 receptor antagonist anakinra have not been conclusive, therefore suggesting that the similarities of these diseases may lie in more downstream mechanisms. Since the initial description of DIRA, several new cases have been identified, including another patient born recently in the United States with the Puerto Rican mutation.27 Based on the high carrier frequency of 2.6% in the northern Puerto Rican population, the incidence of DIRA in the northern region of Puerto Rico might be as high as 1 in 6300 births.
Given the severity of the disease and the availability of specific therapy with anakinra, early identification of the illness is critical for targeted lifesaving intervention. In areas with known founder mutations, prenatal screening may help identify patients early.
In conclusion, it is important to include DIRA in the differential diagnosis of early-onset diffuse pustulosis, particularly in premature infants and those of Puerto Rican descent. In light of the existence of DIRA, a review of the literature for infantile pustular psoriasis leads one to establish distinct categories of reported children: those with pustular psoriasis with otherwise little chronic morbidity that responded to topical corticosteroid therapy and the reported cases of recalcitrant infantile pustular psoriasis associated with sterile lytic bone lesions.28–30 It is likely that the latter cases represent additional patients with DIRA (Table 2).
Table 2.
Deficiency of Interleukin 1 Receptor Antagonist Diagnosis
| Diagnostic Stage | Description |
|---|---|
| Clinical presentation | Neonatal to several months of age Pustular skin eruption Failure to gain weight Thrombosis Joint swelling Fever |
| Laboratory finding | Elevated inflammatory markers (white blood cell count, erythrocyte sedimentation rate, C-reactive protein level) |
| Skeletal survey | Multifocal osteolytic lesions, osteitis, widening of ribs |
| Skin biopsy | Neutrophil infiltration of the epidermis and dermis, concomitant superficial folliculitis, subcorneal localization of the pustule |
| Treatment | Anakinra subcutaneous |
| Confirmation of diagnosis | IL1RN gene sequencing |
Acknowledgments
Funding/Support: Drs Aksentijevich and Goldbach-Mansky are supported by the Intramural Research Program of the National Institute of Arthritis and Musculo-skeletal and Skin Diseases of the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
Author Contributions: All the authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Minkis and Davis. Acquisition of data: Aksentijevich, Goldbach-Mansky, Magro, Scott, Sardana, and Herzog. Analysis and interpretation of data: Aksentijevic, Goldbach-Mansky, and Magro. Drafting of the manuscript: Minkis and Aksentijevich. Critical revision of the manuscript for important intellectual content: Minkis, Aksentijevic, Goldbach-Mansky, Magro, Scott, Davis, Sardana, and Herzog. Administrative, technical, and material support: Minkis and Aksentijevich. Study supervision: Magro.
References
- 1.Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3(6):389–400. doi: 10.2165/00128071-200203060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360 (23):2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360(23):2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenerson M, Dufendach K, Aksentijevich I, Brady J, Austin J, Reed AM. The first case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1-receptor antagonist. Arthritis Rheum. 2011;63(12):4018–4022. doi: 10.1002/art.30565. [DOI] [PubMed] [Google Scholar]
- 5.Goldbach-Mansky R. Blocking interleukin-1 in rheumatic diseases. Ann N Y Acad Sci. 2009;1182:111–123. doi: 10.1111/j.1749-6632.2009.05159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neven B, Marvillet I, Terrada C, et al. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2010;62(1):258–267. doi: 10.1002/art.25057. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima A, Matsuki T, Komine M, et al. TNF, but not IL-6 and IL-17, is crucial for the development of T cell-independent psoriasis-like dermatitis in Il1rn−/ −mice. J Immunol. 2010;185(3):1887–1893. doi: 10.4049/jimmunol.1001227. [DOI] [PubMed] [Google Scholar]
- 8.Lamacchia C, Palmer G, Seemayer CA, Talabot-Ayer D, Gabay C. Enhanced Th1 and Th17 responses and arthritis severity in mice with a deficiency of myeloid cell-specific interleukin-1 receptor antagonist. Arthritis Rheum. 2010;62(2):452–462. doi: 10.1002/art.27235. [DOI] [PubMed] [Google Scholar]
- 9.Isoda K, Matsuki T, Kondo H, Iwakura Y, Ohsuzu F. Deficiency of interleukin-1 receptor antagonist induces aortic valve disease in BALB/c mice. Arterioscler Thromb Vasc Biol. 2010;30(4):708–715. doi: 10.1161/ATVBAHA.109.201749. [DOI] [PubMed] [Google Scholar]
- 10.Koenders MI, Devesa I, Marijnissen RJ, et al. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2008;58(11):3461–3470. doi: 10.1002/art.23957. [DOI] [PubMed] [Google Scholar]
- 11.Ganaiem M, AbuElhija M, Lunenfeld E, et al. Effect of interleukin-1 receptor antagonist gene deletion on male mouse fertility. Endocrinology. 2009;150(1):295–303. doi: 10.1210/en.2008-0848. [DOI] [PubMed] [Google Scholar]
- 12.Kagari T, Tanaka D, Doi H, Iwakura Y, Shimozato T. Anti-type II collagen antibody accelerates arthritis via CXCR2-expressing cells in IL-1 receptor antagonist-deficient mice. Eur J Immunol. 2007;37(10):2753–2763. doi: 10.1002/eji.200737313. [DOI] [PubMed] [Google Scholar]
- 13.Cho ML, Kang JW, Moon YM, et al. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176(9):5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 14.Ishida Y, Kondo T, Kimura A, Matsushima K, Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-β signal pathway. J Immunol. 2006;176(9):5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 15.Kotani M, Hirata K, Ogawa S, et al. CD28-dependent differentiation into the effector/ memory phenotype is essential for induction of arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2006;54(2):473–481. doi: 10.1002/art.21769. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, He X, Iwakura Y, Horai R, Stuart JM. Arthritis in mice that are deficient in interleukin-1 receptor antagonist is dependent on genetic background. Arthritis Rheum. 2005;52(12):3731–3738. doi: 10.1002/art.21481. [DOI] [PubMed] [Google Scholar]
- 17.Matsuki T, Isoda K, Horai R, et al. Involvement of tumor necrosis factor–α in the development of T cell–dependent aortitis in interleukin-1 receptor antagonist-deficient mice. Circulation. 2005;112(9):1323–1331. doi: 10.1161/CIRCULATIONAHA.105.564658. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd J, Nicklin MJ. Elastic-vessel arteritis in interleukin-1 receptor antagonist–deficient mice involves effector Th1 cells and requires interleukin-1 receptor. Circulation. 2005;111(23):3135–3140. doi: 10.1161/CIRCULATIONAHA.104.519132. [DOI] [PubMed] [Google Scholar]
- 19.Horai R, Nakajima A, Habiro K, et al. TNF-α is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist–deficient mice. J Clin Invest. 2004;114(11):1603–1611. doi: 10.1172/JCI20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepherd J, Little MC, Nicklin MJ. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J Invest Dermatol. 2004;122(3):665–669. doi: 10.1111/j.0022-202X.2004.22305.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100(10):5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horai R, Saijo S, Tanioka H, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist–deficient mice. J Exp Med. 2000;191(2):313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mee JB, Cork MJ, di Giovine FS, Duff GW, Groves RW. Interleukin-1: a key inflammatory mediator in psoriasis? Cytokine. 2006;33(2):72–78. doi: 10.1016/j.cyto.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Viguier M, Guigue P, Pagès C, Smahi A, Bachelez H. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153(1):66–67. doi: 10.7326/0003-4819-153-1-201007060-00030. [DOI] [PubMed] [Google Scholar]
- 25.Cooper KD, Hammerberg C, Baadsgaard O, et al. Interleukin-1 in human skin: dysregulation in psoriasis. J Invest Dermatol. 1990;95(5 suppl):24S–26S. doi: 10.1111/1523-1747.ep12505698. [DOI] [PubMed] [Google Scholar]
- 26.Debets R, Hegmans JP, Croughs P, et al. The IL-1 system in psoriatic skin: IL-1 antagonist sphere of influence in lesional psoriatic epidermis. J Immunol. 1997;158(6):2955–2963. [PubMed] [Google Scholar]
- 27.Levenson D. New inherited immune disorder revealed. Am J Med Genet A. 2009;149A(9):fm v–fm vi. doi: 10.1002/ajmg.a.33102. [DOI] [PubMed] [Google Scholar]
- 28.Zelickson BD, Muller SA. Generalized pustular psoriasis in childhood: report of thirteen cases. J Am Acad Dermatol. 1991;24(2 pt 1):186–194. doi: 10.1016/0190-9622(91)70025-w. [DOI] [PubMed] [Google Scholar]
- 29.Ivker RA, Grin-Jorgensen CM, Vega VK, Hoss DM, Grant-Kels JM. Infantile generalized pustular psoriasis associated with lytic lesions of the bone. Pediatr Dermatol. 1993;10(3):277–282. doi: 10.1111/j.1525-1470.1993.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 30.Prose NS, Fahrner LJ, Miller CR, Layfield L. Pustular psoriasis with chronic recurrent multifocal osteomyelitis and spontaneous fractures. J Am Acad Dermatol. 1994;31(2 pt 2):376–379. doi: 10.1016/s0190-9622(94)70176-8. [DOI] [PubMed] [Google Scholar]


