Abstract
The fusion of NS-1 myeloma cells with spleen cells from mice chronically infected with Leishmania tropica resulted in nine clones of hybridomas producing monospecific antibodies to membrane antigens of L. tropica. One of the antibodies (L-5-85) bound specifically to the promastigote form of the parasite, and the remaining eight recognized antigens shared by the promastigote and amastigote of L. tropica. Four of the antibodies (L-5-16, L-5-34, L-5-44, and L-2-3F11) detected parasite antigens on the surface of L. tropica-infected macrophages. Common antigens shared by L. tropica, L. mexicana, and L. donovani were identified as well as one antigen apparent on most Leishmania spp. and present also in Crithidia fasciculata. Two monoclonal antibodies (L-5-27 and L-5-41) were found to bind only to strains of L. tropica from simple cutaneous leishmaniasis. A special property shared by these two antibodies was the inhibition of parasite growth in macrophages in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. D., Dwyer D. M. Expression of Leishmania antigen on the surface membrane of infected human macrophages in vitro. Clin Exp Immunol. 1981 May;44(2):342–348. [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M., Langreth S. G., Dwyer N. K. Evidence for a polysaccharide surface coat in the developmental stages of Leishmania donovani: a fine structure-cytochemical study. Z Parasitenkd. 1974 Jun 21;43(4):227–249. doi: 10.1007/BF00328879. [DOI] [PubMed] [Google Scholar]
- Farah F. S., Samra S. A., Nuwayri-Salti N. The role of the macrophage in cutaneous leishmaniasis. Immunology. 1975 Oct;29(4):755–764. [PMC free article] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
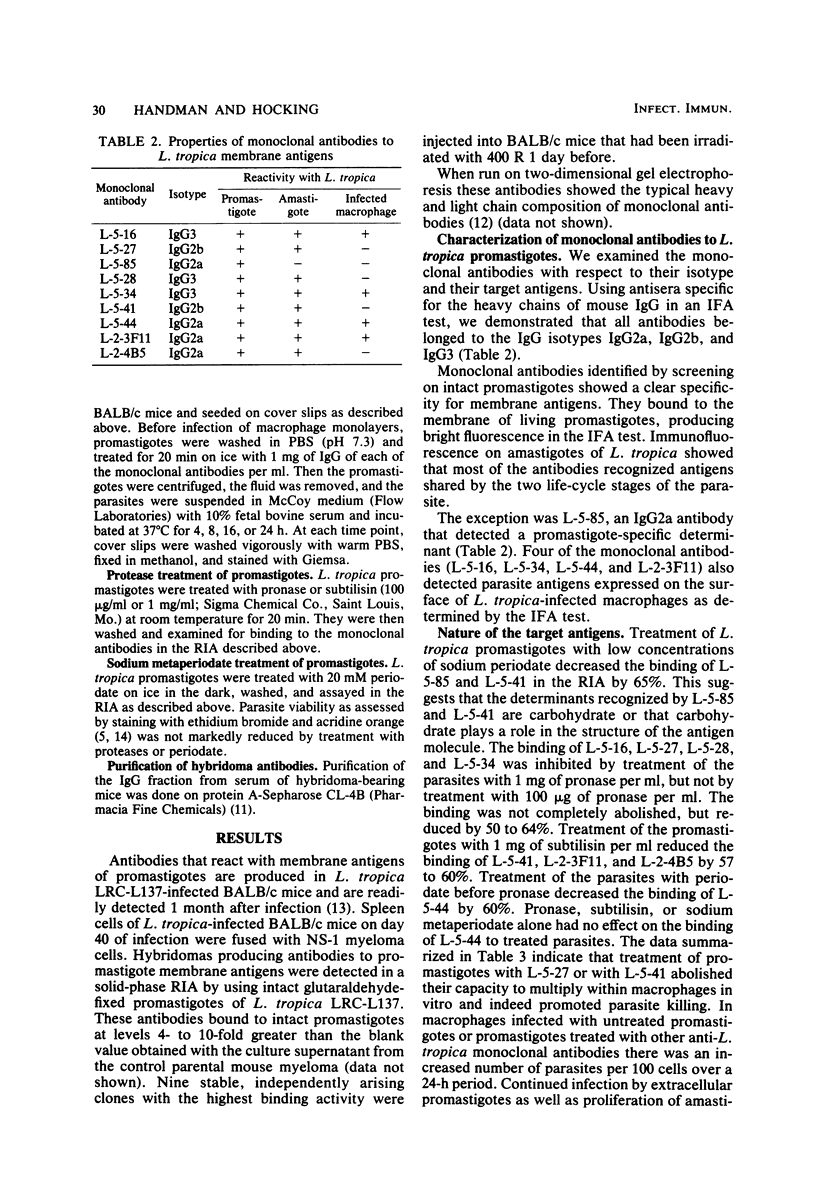
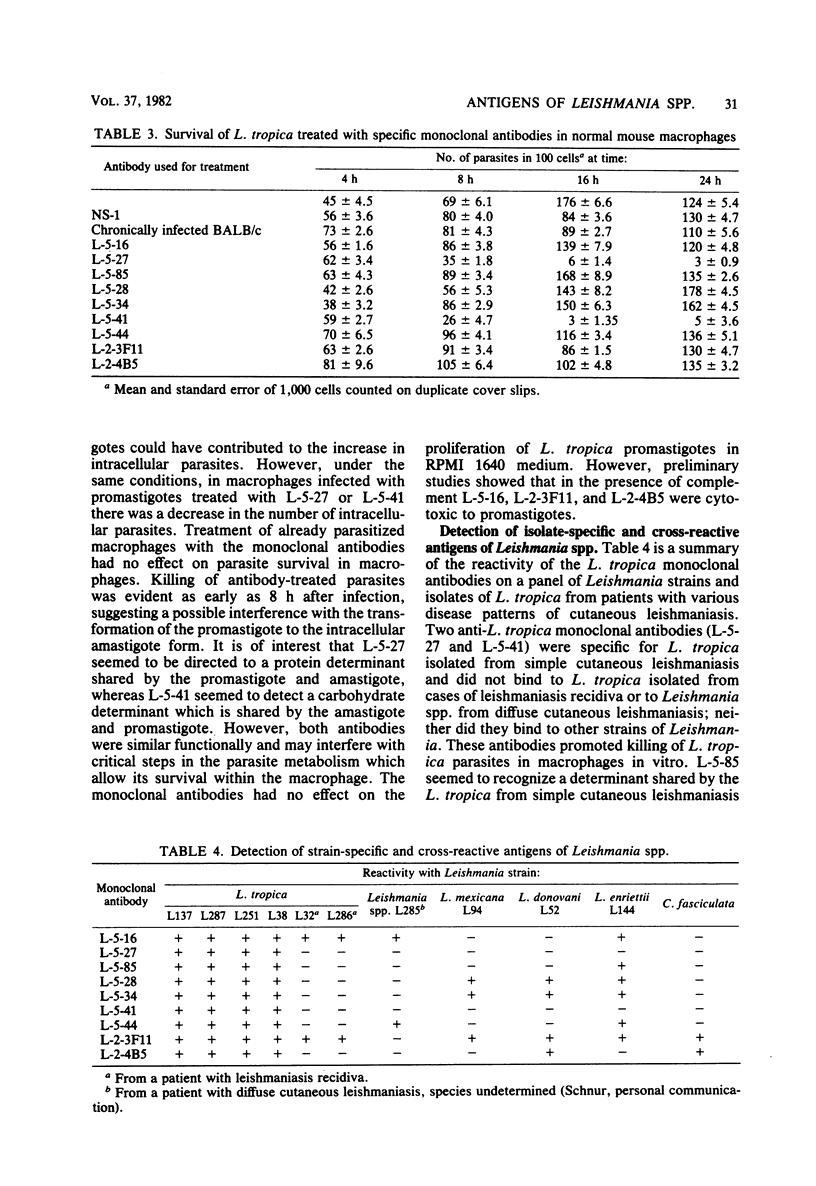
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Handman E., Remington J. S. Serological and immunochemical characterization of monoclonal antibodies to Toxoplasma gondii. Immunology. 1980 Aug;40(4):579–588. [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lewis D. H., Peters W. The resistance of intracellular Leishmania parasites to digestion by lysosomal enzymes. Ann Trop Med Parasitol. 1977 Sep;71(3):295–312. doi: 10.1080/00034983.1977.11687192. [DOI] [PubMed] [Google Scholar]
- Louis J. A., Zubler R. H., Coutinho S. G., Lima G., Behin R., Mauel J., Engers H. D. The in vitro generation and functional analysis of murine T cell populations and clones specific for a protozoan parasite, Leishmania tropica. Immunol Rev. 1982;61:215–243. doi: 10.1111/j.1600-065x.1982.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Olobo J. O., Handman E., Curtis J. M., Mitchell G. F. Antibodies to Leishmania tropica promastigotes during infection in mice of various genotypes. Aust J Exp Biol Med Sci. 1980 Dec;58(6):595–601. doi: 10.1038/icb.1980.61. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]



