Abstract
Circadian pacemakers drive many daily molecular, physiological, and behavioral rhythms. We investigated whether the main olfactory bulb is a functional circadian pacemaker in rats. Long-term, multielectrode recordings revealed that individual, cultured bulb neurons expressed near 24-h oscillations in firing rate. Real-time recordings of Period1 gene activity showed that a population of cells within the bulb express synchronized rhythmicity starting on embryonic day 19. This rhythmicity was intrinsic to the mitral, and not the granule, cell layer, entrainable to physiological temperature cycles, and temperature compensated in its period. However, removal of the olfactory bulbs had no effect on running wheel behavior. These results indicate that individual mitral/tufted cells are competent circadian pacemakers which normally synchronize to each other. The daily rhythms in gene expression and firing rate intrinsic to the olfactory bulb are not required for circadian patterns of locomotion, indicating that they are involved in rhythms outside the canonical circadian system.
Keywords: pacemaker, suprachiasmatic nucleus, mitral cell, temperature compensation, Period gene, mPer1, multielectrode
Introduction
The mammalian retina and suprachiasmatic nucleus (SCN) of the hypothalamus regulate a variety of physiological and behavioral processes with periods close to 24 h. These two tissues fulfill the standard criteria for a circadian pacemaker: their daily rhythms synchronize to (and persist in the absence of) environmental cycles and show little change in their period over the physiological range of temperatures (Klein et al, 1991; Lee et al, 2003; Tosini and Fukuhara, 2003; Herzog and Huckfeldt, 2003). We recently reported that the isolated olfactory bulb (OB) expresses a circadian rhythm in gene transcription (Abe et al, 2002). It is not known whether this intrinsic rhythmicity in gene transcription has a function at the levels of cellular physiology or behavior and whether the OB retains the properties common to other circadian pacemakers. This study asks whether the OB, like the retina and SCN, acts as a circadian pacemaker and participates in entrainment of the circadian system.
The laminar organization and postnatal maturation of the OB has facilitated extensive studies on its development and plasticity. This study correlates the development of intrinsic circadian function with differentiation in the OB. Using a bioluminescent reporter of Period1 (Per1) gene activity, we found that, by E19, a population of OB cells expresses coordinated circadian rhythmicity in the rat OB. Using long-term multielectrode recording, we found that individual mitral, but not granule, cells can act as independent circadian pacemakers driving rhythms in firing rate. Removal of the OB showed that rhythms intrinsic to the OB were not required for normal circadian patterns of locomotion.
Materials and Methods
Animals
Wild-type and homozygous Per1-luc transgenic Wistar rats (Yamazaki et al, 2000) were maintained in the Hilltop animal facility at Washington University and fed ad libitum (lights on at 7:00 a.m., off at 7:00 p.m., 22°C). Proestrous females were paired with a male overnight. Pregnancy was confirmed within three hours after lights on the following morning by the presence of sperm in the vaginal smear or a copulatory plug. Embryonic day 1 (E1) and postnatal day 1 (P1) were defined as the day after successful mating and the day of birth, respectively. Gestational age was confirmed based on published morphological guidelines (Altman and Dittmer, 1962).
On the desired gestational day, pregnant mothers were anesthetized using 0.2% Avertin 30–60 min before lights off. Pups were quickly removed and decapitated. Rats older than 10 days of age were anesthetized by CO2 inhalation prior to brain removal. Embryonic olfactory bulbs were dissected with scalpels and neonatal OB were sectioned with a vibratome (coronal, 300 μm) in cold Hanks Buffered Saline (Sigma #H6136 St. Louis, MO). OB sections were used from Bregma 5.7 to 6.7 mm (Paxinos & Watson, 1998). In some cases, the mitral- or granule cell layers were separated with scalpel cuts along the division of the mitral cell layer (including the external plexiform layer) and the granular cell layer. Experiments on animals less than 12 days of age used both sexes and experiments on older animals used males. All procedures were approved by the Animal Care and Use Committee and conformed to National Institutes of Health guidelines.
Per1 expression
Per1 gene activity was assessed by measuring bioluminescence from cultured tissues harvested from Per1-luc transgenic rats using methods similar to previous reports (Yamazaki et al, 2000; Abe et al, 2002). Tissue explants were placed on membrane inserts (Millicell-CM, Millipore, Bedford, MA) with 1 ml of Dulbecco’s modified eagles medium (Sigma #D5030 St. Louis, MO) supplemented with 10 mM Hepes (Sigma #H0887 St. Louis, MO), 2.2 mg/ml NaHCO3 and 0.1 mM beetle luciferin (Promega #E1601, Madison, WI). Each culture was sealed in a Petri dish, maintained at 36°C in darkness, and its bioluminescence was continuously monitored for 5 to 7 days with a photomultiplier tube (HC135-11MOD, Hamamatsu Corp., Shizouka, Japan).
For temperature entrainment experiments, temperature cycles were imposed by changing the incubator set point at 12 h intervals. Culture temperature changed at a rate of 2.5°C per hour so that a 1.5°C shift was complete within 40 min. At each set point, temperature ranged by less than 0.3°C without circadian variation.
Multielectrode recordings
Long-term firing rate patterns were recorded from cells dispersed onto multielectrode arrays (60 electrodes, 200 μm spacing, 10 μm tips, Multichannel Systems, Reutlingen, Germany). The OB of two pups or the SCN from 8 pups (P1-P7) were pooled, dispersed and grown according to published methods (Herzog et al, 1998). OB (~90,000 cells/array) or SCN (~4,000 cells/array) cells were cultured at 37°C in 5% CO2/95% air for two to four weeks. Culture chambers were then covered with a fluorinated ethylene–propylene membrane and transferred to a recording incubator (Potter & DeMarse, 2001). Extracellular voltage signals from 60 electrodes were chosen from among four cultures and simultaneously recorded for at least 5 days. Action potentials larger than a user-defined voltage were digitized and a time-stamped, 2 ms cut-out of each spike was stored to the hard drive (MC Rack software; Multichannel Systems). Spikes from individual neurons were discriminated offline using principal component-based cluster analysis (Offline Sorter, Plexon Inc., Dallas, TX) and the discharge rate of each neuron was determined in 10-min bins using Neuroexplorer software (Plexon Inc.).
Temperature inside the recording chamber was recorded with six thermocouples (PT-6 with Thermes-16, Physitemp Instruments, Inc., Clifton, NJ). All temperature sensors were calibrated against a NIST-calibrated thermometer (Fisher Scientific #15043A). Long-term recordings showed that the temperature of the culture medium did not fluctuate with time of day and ranged by less than 0.2°C from the set point.
Morphological measurements
The diameter of OB cells cultured on electrode arrays (n= 2 cultures harvested from 2 animals) were made from linearly-calibrated, digitized images of living cultures (QImaging, Retiga 1350EX, Burna, Canada) using Northern Eclipse software (Empix, North Tonawanda, NY). Cells growing near the electrode array were randomly selected, the arborization of their dendrites was characterized and the diameter of their soma was measured at the widest point.
Locomotor activity measurements and olfactory bulbectomy
Male rats (3 weeks of age) were individually housed in cages (Nalgene, 32 × 20 × 17 cm high), outfitted with a 17.8-cm diameter, running wheel. Cages were placed in ventilated chambers illuminated by fluorescent bulbs for 3 h per day (F30T12-SP41-RS, General Electric, USA; 3.9 × 1017 to 6.9 × 1018 photons/s/cm2 at the bottom of the cages). Wheel running activity was recorded in 6 min bins (Clocklab, Actimetrics, Evanston, IL). After 7 days, rats were anaesthetized (75 mg/kg ketamine and 0.5 mg/kg medetomidine intraperitoneal) and mounted in a stereotaxic frame (David Kopf, Tujunga CA). Rats were either bulbectomized through a 3 mm hole above the OB using suction (OBX, n = 10) or sham operated (sham, n= 6). Wheel revolutions were recorded for 13 days under the same light schedule (lights on at 7:00 a.m. and off at 10:00 a.m.) and then for 21 days after delaying the light schedule by 6 h (lights on at 1:00 p.m.) and, finally, for another 19 days after advancing the light schedule by 6 h (lights on at 7:00 a.m.). The onsets and duration of daily activity were determined using a 6 h activity template in Clocklab. The average onset of activity relative to the time of lights on (termed the phase angle of entrainment) was determined from the 3 days prior to surgery and during the last 3 days in each of the three light schedules. The time to reentrain following the shift in the light schedule, calculated to the nearest day, started on the day of the shift and ended on the day when stable entrainment was observed. At the end of the locomotor activity recordings, rats were sacrificed to confirm the bulbectomy.
Data analysis
The phase, period and amplitude of the bioluminescence rhythms were determined as previously reported (Abe et al, 2002). Briefly, a 24-h running average was subtracted from the raw data to reduce trends in the baseline that occur over subsequent days. A 3-h and a 24-h running average were calculated from the detrended dataset. The phase of peak bioluminescence between the crossings of these two smoothed lines was used as the phase marker for each cycle of the recorded rhythms. All times were reported in “Zeitgeber Time” (ZT) relative to animal’s prior light cycle such that ZT0 was the time of light onset in the animal colony (7:00 a.m.) and ZT12 was the time of light offset (7:00 p.m.). Period and amplitude of Per1-luc, electrical and behavioral rhythms were also determined using Chi-square periodogram analysis (Clocklab). The temperature coefficient, a measure of the rate of change of a reaction as a function of temperature, was calculated using the equation where τ is the culture’s period and T is temperature. Statistical comparisons of phase, period and amplitude were analyzed by a one-way ANOVA followed by the LSD post-hoc test.
Results
Ontogeny of Per1 expression in the rat olfactory bulb
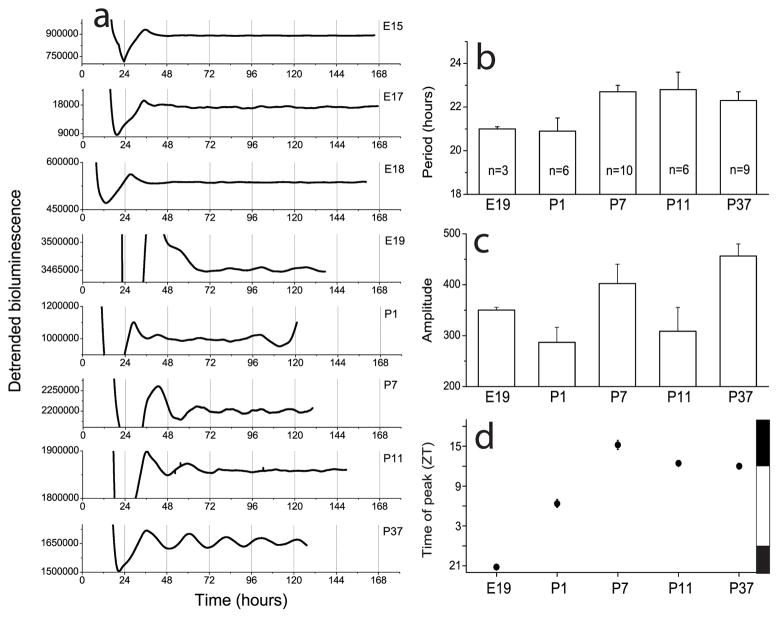
Per1-luc measurements showed the coordinated expression of the Period1 gene in a population of OB cells. Circadian rhythms in Per1-luc activity were apparent in the OB of rats as young as E19 (Fig. 1a; Table 1). Neither period (One way ANOVA, F(4,29) =2.3, p>0.05; Fig. 1b) nor amplitude (One way ANOVA, F(4,29) =3, p>0.05; Fig. 1c) of rhythmic OB cultures depended on age. The time of peak bioluminescence relative to the prior light schedule, however, was age dependent (One way ANOVA, F(4,29) =34.5, p<0.0001; Fig. 1d). While E19 OB peaked in the late subjective night (around ZT21), P1 OB peaked midday (around ZT6) and, by one week postnatal, the OB reached its adult phase peaking near dusk (around ZT12). This phase agrees with published results on the time of peak Per1 expression in the adult OB (Abe et al, 2002). These data suggest that a developmental program regulates circadian phase in a group of OB cells over the first week of life until it assumes its adult phase.
Figure 1.
Circadian patterns of bioluminescence recorded from olfactory bulb explants taken from rats carrying a Period1-luciferase (Per1-luc) transgene. a, Daily oscillations of Per1 gene activity were present in the OB by E19. b–c, The average period and amplitude of rhythmic OB cultures did not depend upon developmental age. d, From E19 to P7, the Per1-luc expression in the OB peaked progressively later until it reached its mature phase around one week postnatal. Phase is given in Zeitgeber Time where ZT0 is dawn in the animal colony (7:00 a.m.) and ZT12 is dusk (7:00 p.m.). The time of lights on and off are shown on the right as white and black bars. Error bars show S.E.M.
Table 1.
Percentage of rhythmic OB explants at different ages.
| Age | Percentage |
|---|---|
| E15 | 0 (n=6) |
| E17 | 0 (7) |
| E18 | 0 (3) |
| E19 | 100 (3) |
| P1 | 100 (5) |
| P7 | 100 (10) |
| P11 | 100 (6) |
| P37 | 100 (9) |
Circadian firing patterns of OB neurons
To address whether rhythms in Per1 transcription correlate with function in individual neurons, we recorded daily patterns of spontaneous firing rate from bulb neurons cultured on multielectrode arrays. OB neurons from P1-P7 grew readily on the arrays. After 3 weeks in vitro, two morphological cell types were apparent: small bipolar cells with a diameter of 11.1 ± 0.2 μm (n=50 cells; from 2 cultures; mean ± SEM) and pyramidal-shaped neurons with many processes and a diameter of 33.4 ± 1.0 μm (n=50; from 2 cultures; mean ± SEM). These small and large cell types are consistent with the descriptions of cultured granule and mitral/tufted cells, respectively (Trombley & Westbrook, 1990).
We were able to reliably discriminate the firing patterns of 35 OB and 30 SCN neurons for at least three days (average of 4.9 ± 0.5 days, range of 3–10 days for OB cells from 3 cultures; 12.7 ± 1.4 days, 3–20 days for SCN cells from 3 cultures). Approximately half of the recorded neurons (16 OB and 14 SCN) expressed circadian firing patterns. The peak-to-peak amplitudes of their biphasic action potentials were 101.6 ± 7.1 μV (mean ± SEM) for OB neurons and 67.0 ± 3.0 μV for SCN neurons.
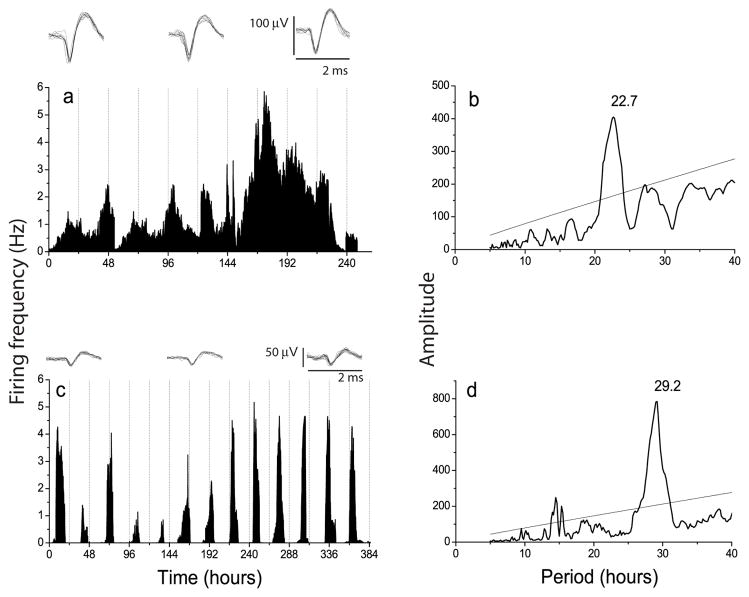
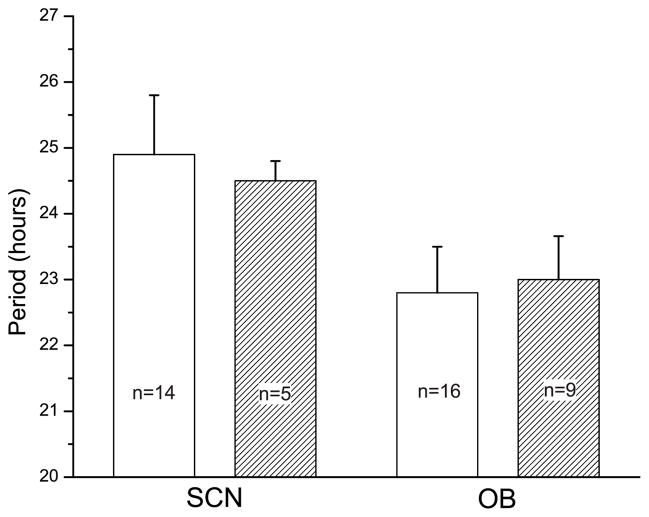
In general, rhythmic OB neurons showed similar peak firing rates to SCN neurons (0.4 to 4.2 Hz for OB neurons and 0.4 to 3.1 Hz for SCN neurons), but higher spontaneous activity during their daily trough and more variability in their amplitude and cycle-to-cycle onsets (compare Figs. 2a and 2c). The average periods of OB (22.8 ± 0.7 h) and SCN (24.9 ± 0.9 h) firing rate rhythms were similar to those seen in Per1-luc rhythms (23.0 ± 0.7 h for OB and 24.5 ± 0.3 h for SCN, mean ± SEM); the periods of the OB tended to be shorter than the SCN, but did not reach significance (Fig. 3, p = 0.06, Student’s t-test).
Figure 2.
a, Circadian rhythm in electrical activity of a representative OB neuron. Insets show 10 superimposed action potentials recorded at different times over 10 days to illustrate the reliable discrimination of the activity of a single OB cell. b, Chi-squared periodogram analysis of the firing pattern from the cell in a shows a dominant frequency at 22.7 h. c, Circadian firing pattern of an SCN neuron recorded for 16 days on a different multielectrode array. Insets show small action potentials, typical of SCN neurons. The period estimate for this cell, shown in d, was 29.2 h.
Figure 3.
The average periods of firing rate (white bars) and Per1-luc activity (hatched bars) for cultured SCN and OB were similar. Period estimates were made from firing patterns of cells dispersed on P4-P6 and cultured for 16 days and from Per1-driven bioluminescence of tissues explanted and recorded beginning on postnatal day 37. While there was a tendency for the SCN to have a longer period than the OB, period differences were not significant (p=0.06, Student’s t-test). The periods of Per1-luc and electrical rhythms did not differ for either the SCN or OB (p>0.1, Student’s t-test). Error bars show S.E.M.
Individual OB neurons showed different circadian periods in the same culture. In one culture, the firing patterns of 7 OB neurons were discriminated for 4 days. Periods ranged from 18.9 to 25.3 h and the times of peak firing on the first day of recording ranged from 13.9 to 22.1 h. Two cells recorded on two different electrodes 100 μm apart showed a period difference of 2.3 h and peaked 6 h apart on the first day of recording (Fig. 4). These results indicate that the OB is a multioscillator structure and that each OB neuron may function as an independent pacemaker.
Figure 4.
OB neurons in the same culture can express different circadian periods. a and c show actograms for the firing patterns shown in b and d, respectively. The two cells, recorded on electrodes 100 μm apart, had periods of 22.8 h (top) and 20.5 h (bottom).
Circadian oscillations localize to the mitral/tufted cell layer
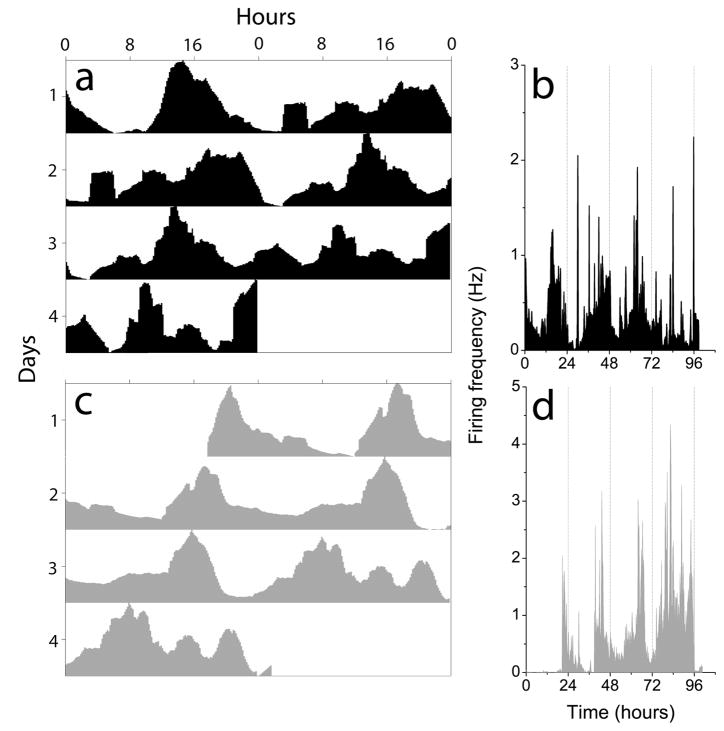
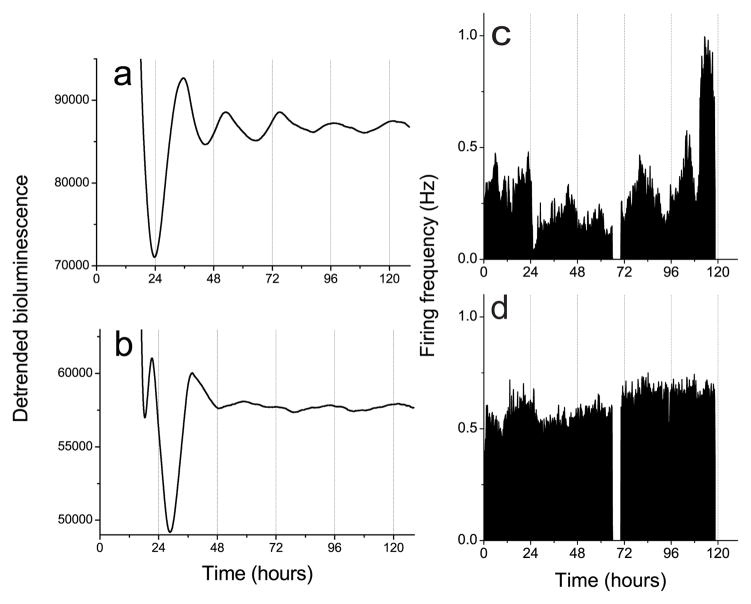
Within the OB, mitral/tufted and granule neurons are known to fire action potentials (Trombley & Westbrook, 1990; Chen & Shepherd, 1997; Cang and Issacson, 2003). To determine which cell types express circadian rhythms, we recorded Per1-driven bioluminescence and electrical activity from isolated cell layers of P7 OB. All explants of the mitral cell layer expressed robust circadian rhythms in Per1-activity (n=6; Fig. 5a), with an initial peak at ZT 12.5 ± 0.3 h and an average period of 21.5 ± 0.7 h. In contrast, explants of the granule cell layer did not express circadian rhythmicity (n=6; Fig. 5b). Similarly, 5 neurons recorded from a dispersal of mitral/tufted cells showed circadian rhythms in firing rate for at least 3 days with a period of 20.1 ± 0.6 h (mean ± SEM; Fig. 5c), while no rhythmic neurons were found in cultures prepared from the granular cell layer (Fig. 5d). We did not determine the percentage of rhythmic mitral cells. These results indicate that circadian oscillations likely arise within the mitral/tufted cells of the OB.
Figure 5.
Explant cultures of the mitral cell layer showed Per1-luc circadian rhythms (a) while the granule cell layer (b) did not. Similarly, cells dispersed from the mitral cell layer showed firing rate circadian rhythms (c) while cells from the granule cell layer did not (d). Data collection was briefly interrupted around 72 h for a medium change in c and d.
Temperature entrainment of circadian rhythmicity in the OB
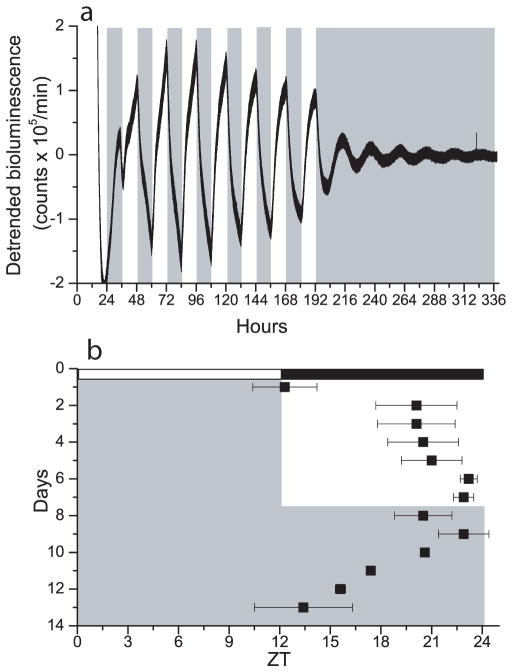
Circadian pacemakers are only useful to the organism if they can synchronize to local time. To determine whether the OB can entrain to daily cues, we exposed OB explants to a 1.5°C temperature cycle for 8 days (12 h: 12 h, 36.8°C: 35.3°C, warm at ZT 0). OB cultures synchronized their rhythmicity to these cycles (Fig. 6a). Cultures, which peaked at approximately ZT 12.3 in the first day of the temperature cycle, rapidly shifted to peak at approximately ZT 20.1 in the second day of the temperature cycle (n= 4, Fig. 6b). All cultures continued to peak just prior to the cool-to-warm transition for the next seven days. Following the temperature cycle, OB cultures free ran from their entrained phase with an average period of 21.9 ± 0.6 h (mean ± SEM). Results were similar in cultures taken from P1 and P19 animals (n= 2 at each age). These data indicate that the OB can entrain to physiological temperature oscillations and that this intrinsic entrainability develops early in the ontogeny of the OB.
Figure 6.
Temperature cycles entrain the OB in vitro. a, Bioluminescence recording from an OB explant in a temperature cycle of 35.3°C (white fill) to 36.8°C (gray fill). Per1 gene activity peaked near the cool-to-warm transitions during the 8 days of cyclic temperature. The rhythm free-ran from the last cool-to-warm transition in constant temperature. b, The peak of OB rhythmicity (black squares, mean ± SEM, n= 4) was tightly regulated by the temperature cycle (gray shows warm phases) so that after 8 days, the OB had delayed to peak approximately 1 h prior to the daily warm phase. Relative to the animals’ prior light cycle, the temperature cycle advanced the Per1-luc rhythm to around subjective dawn (white and black bars show the times of lights on and off in the animal colony, defined as ZT 0 and 12, respectively).
Temperature compensation of circadian rhythms in the OB
Circadian pacemakers also show relatively little change in their period at different constant temperatures. To determine if the OB is temperature compensated, we compared Per1-luc rhythms at 30°C (n=10) and 36°C (n=9). The circadian period of OB slices was slightly longer at 30°C than at 36°C (23.7 ± 0.5 h and 21.6 ± 0.4 h, respectively; mean ± SEM). This equates to a Q10 of 1.16 which is within the range seen for other circadian systems. In contrast, the amplitude of rhythmicity had a Q10 of 0.67 over the same temperature range (274.8 ± 15.9 at 30°C and 348.6 ± 15.5 at 36 °C). These results suggest that circadian period, but not amplitude, is temperature compensated.
Removal of the olfactory bulbs has no effect on locomotor behavior
Indirect evidence has implicated the olfactory bulb in the regulation of daily rhythms in locomotor activity. Removal of the bulbs lengthens the free running period in mice and hamsters (Possidente et al, 1990; Lumia et al, 1992) and delays photic reentrainment in male Octogon degus (Goel et al, 1998). We assessed the necessity of the OB for normal locomotor patterns in bulbectomized rats. Although rats without their olfactory bulbs tended to synchronize to a delayed light schedule approximately 1.1 days sooner than intact rats (p = 0.08), we found no significant effect of bulbectomy on the phase angle of entrainment, the rate of reentrainment to advances or delays in the light schedule, or the amount of daily activity (Table 2 and Supplementary Fig. 1). These data suggest that, in rats, the OB is not required for photic entrainment or period regulation of locomotor rhythmicity.
Table 2.
Bulbectomy (OBX) had no significant effect on entrainment or activity in rats. The delay from the daily onset of running wheel activity to the onset of light (phase angle of entrainment), the number of days required to synchronize locomotor activity following a shift in the light schedule (rate of reentrainment) and the number of wheel revolutions during the daily bouts of activity (Nighttime) and inactivity (Daytime) are expressed as mean ± SEM.
| Phase angle of entrainment (h) | Rate of reentrainment (days) | Activity (average counts/day) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lights on at | 6 h delay | 6 h advance | Daytime | Nighttime | |||
| 7am | 1pm | 7am | |||||
| OBX (n=10) | 11.6±0.5 | 11.5±0.5 | 11.5±0.2 | 11.6±0.4 | 14.0±0.4 | 14.6±4.8 | 3300±1138.4 |
| Sham (n=6) | 11.7±0.4 | 11.5±0.2 | 11.3±0.2 | 12.7±0.3 | 14.7±0.3 | 5.2±1.8 | 1411±174.5 |
| Student t-test | p=0.9 | p=0.5 | p=0.6 | p=0.08 | p=0.3 | p=0.2 | p=0.2 |
Discussion
The OB is an independent, functional circadian pacemaker
Three features conserved among circadian oscillators are their abilities to: express near 24-h rhythms in the absence of environmental timing cues, synchronize physiological rhythms to daily cycles, and accurately mark time over the range of physiological temperatures. The mammalian OB expresses intrinsic circadian rhythms in gene expression and neuronal firing rate, can be entrained by daily temperature cycles of 1.5°C and is temperature compensated in its period. These properties are also found in the mammalian SCN and retina (Tosini & Menaker, 1996; Ruby et al, 1999; Herzog & Huckfeldt, 2003). It is not yet known if the multitude of other tissues and cell types which exhibit circadian rhythms in gene expression also fulfill the criteria for a functional circadian pacemaker (Balsalobre et al, 1998; Yamazaki et al, 2000; Abe et al, 2002). It may be that multiple tissues function as semi-autonomous timekeepers as in other vertebrates (Underwood, 2001), invertebrates (Page, 2001) and plants (Thain et al, 2000). We recently found that, for example, the OB can continue to oscillate for at least a month in vivo in the absence of circadian rhythmicity in the SCN (Granados-Fuentes et al, 2003, in press). However, it is also possible that some rhythms in gene expression may have no functional consequence; conversely, some tissues which are rhythmic in vivo may lack their own pacemaking ability and be driven by other pacemaking tissues. Regardless, the identification of multiple circadian pacemakers leads to a number of basic questions: Do these pacemakers use a common molecular machinery to generate oscillations and drive physiology? Do they interact in vivo to regulate common or distinct behaviors?
Limited evidence indicates that circadian rhythms of the retina, SCN and OB share some of the same molecular bases. Recordings from individual SCN and OB neurons show that the clock regulates neural firing rates and is likely cell autonomous. The periodicities of the SCN and retina are also similarly shortened by the tau mutation (Tosini & Menaker, 1996; Liu et al, 1997). Importantly, however, the delayed phase and reduced robustness of OB rhythms, compared to SCN, suggests a functional difference between these oscillators. These differences are likely intracellular, as has been found for the pacemakers in the antennae and lateral neurons of Drosophila (Krishnan et al, 2001), but may also reflect differences in intercellular communication.
The similar periods of firing rate and Per1-luc rhythms in the SCN and in the OB suggest a functional relationship between Per1 expression and electrical excitability. This is consistent with the recent report which correlated levels of Per1 expression and firing rate of individual SCN neurons (Kuhlman et al, 2003). How the molecular timekeeping mechanism controls diurnal membrane excitability and how membrane potential controls clock gene expression are unknown (McMahon & Block, 1987; Nitabach et al, 2002; Pennartz et al, 2002; Panda et al, 2002).
Rhythmicity and cellular differentiation mature postnatally in the OB
The mechanisms that initiate circadian oscillations in any cell type are unknown. Circadian rhythms in the rat OB were first detectable on E19. At this age, many mitral cells have differentiated, grown axons and begun to receive dendritic input from olfactory sensory neurons (Lopez-Mascaraque & De Castro, 2002). The phase of circadian rhythmicity gradually matures over the first postnatal week. During this time, neuronal precursor cells migrate into the bulb, differentiate into granule and peri-glomerular cells and form synapses with relay neurons (Hinds, 1968; Hinds & Hinds, 1976; Bayer, 1983). This ontogeny is consistent with our localization of circadian rhythmicity to mitral/tufted, and not granule, cells. However, mitral cell differentiation and Per1 expression in the OB clearly precede the onset of circadian rhythmicity in the OB. Notably, initiation of rhythmicity in the rat SCN has been reported around E19 (Fuchs & Moore, 1980) and its synaptic organization matures over the first three weeks postnatal (Lenn et al, 1977). Thus, circadian periodicity develops concurrently in the SCN and OB.
What function does the OB circadian oscillator play in behavior?
The circadian rhythm in firing rate seen in OB neurons indicates that the circadian clock in these cells functions to modulate membrane excitability. This is the first demonstration that firing rate fluctuates with time of day in mitral/tufted cells. The firing rates seen in vitro from dispersed OB neurons are similar to the spontaneous rates reported for mitral/tufted cells in anesthetized rats (c.f. Cang & Isaacson, 2003). Although it is not known if in vivo OB neurons express daily rhythms in discharge rate, circadian modulation of olfaction has been reported in mammals (Amir et al, 1999; Funk & Amir, 2000) and shown to be intrinsic to the Drosophila antennae (Krishnan et al, 1999). The presence of a circadian pacemaker in the OB may, therefore, simply reflect the need for local, temporal regulation of olfactory processing. How the circadian rhythms in transcriptional and electrical activity in the bulb relate to olfaction is unknown.
In addition, the OB may also regulate other circadian pacemakers or co-regulate some behaviors with other pacemakers. We found bulbectomized rats showed no changes in the period or entrainment to light of their locomotor behavior. This is in contrast to results found in mice, hamsters and degus implicating the OB in regulation of period and entrainment of circadian rhythms in locomotion (Possidente et al, 1990; Lumia et al, 1992; Goel et al, 1998). This may reflect species differences. Alternatively, the role of the OB may develop later in adulthood since we found no effects of bulbectomy on rats from 4 to 11 weeks of age, where the other studies found effects on mice at ~ 50 days, hamsters at ~ 22 days old and degus at 1 to 2 years of age. This would likely reflect a delay in the maturation of the output of the OB circadian pacemaker since we found that Per1 rhythmicity doesn’t change in phase, period or amplitude after one week of age.
It may be that the OB plays a role in olfactory entrainment of the pacemaker in the SCN. For example, daily exposure to the scent of a conspecific suffices to entrain degus (Governale & Lee, 2001). Others have noted the robust multisynaptic connections from the OB to the rat SCN (Krout et al, 2002). In flies, social entrainment of the rest-activity cycle has been shown to depend upon olfaction and the antennae (Levine et al, 2002). It will therefore be important to determine whether the clocks in the OB (and antennae) play a role in entraining the rhythms in multiple circadian pacemakers.
Finally, the rhythmicity in the OB is likely to be influenced by rhythmicity in the SCN. For example, the daily rhythm in brain temperature is regulated by the SCN (Eastman et al, 1984). This daily temperature rhythm influences circadian timing in the SCN and in peripheral tissues (Brown et al, 2002; Herzog & Huckfeldt, 2003) and, based on the in vitro results presented here, is sufficient to entrain rhythmicity in the OB.
Taken together, these results indicate the circadian system must be modeled as multiple pacemaking tissues which can function semi-autonomously from each other. Cells within the mitral cell layer of the OB are likely competent circadian pacemakers regulating their own gene expression and membrane excitability. Their daily rhythms are, like those in the SCN, entrainable to 24-h schedules and temperature compensated, but unlike the SCN in that they do not play an important role in locomotor behaviors. Future work should reveal the role of circadian pacemaking in the OB on behavior.
Supplementary Material
Supplementary Figure 1. Removal of the olfactory bulbs had no significant effect on the circadian locomotor patterns. Actograms show wheel running activity (black tick marks) of representative sham-operated (a) and bulbectomized (b) rats in double-plotted format. Each horizontal line represents a 48 h period; the second 24 h period is plotted to the right and also below the first. Grey bars indicate the 3 h each day when the lights were on and the arrow marks the day of surgery.
Acknowledgments
We are especially grateful to Dr. Hajime Tei for generating the Per1-luc rats. This work was supported by NIH grant MH63104 and the McDonnell Center for Higher Brain Function.
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman PL, Dittmer DS. Biological Handbooks. FASEB; Washington D.C: 1962. Growth, including reproduction and morphological development; pp. 304–313. [Google Scholar]
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J. In rats, odor-induced Fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci Lett. 1999;272:175–178. doi: 10.1016/s0304-3940(99)00609-6. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- Brown S, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of Mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- Cang J, Issacson JS. In vivo whole-cell recording of odor-evoked synaptic transmission in the olfactory bulb. J Neurosci. 2003;23:4108–4116. doi: 10.1523/JNEUROSCI.23-10-04108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Shepherd GM. Membrane and synaptic properties of mitral cells in slices of rat olfactory bulb. Brain Res. 1997;745:189–196. doi: 10.1016/s0006-8993(96)01150-x. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav. 1984;32:357–368. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Moore RY. Development of circadian rhythmicity and light responsiveness in the rat suprachiasmatic nucleus: a study using the 2-deoxy[1–14C]glucose method. Proc Natl Acad Sci USA. 1980;77:1204–1208. doi: 10.1073/pnas.77.2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Amir S. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- Goel N, Lee TM, Pieper DR. Removal of the olfactory bulbs delays photic reentrainment of circadian activity rhythms and modifies the reproductive axis in male Octodon degus. Brain Res. 1998;792:229–236. doi: 10.1016/s0006-8993(98)00134-6. [DOI] [PubMed] [Google Scholar]
- Governale MM, Lee TM. Olfactory cues accelerate reentrainment following phase shifts and entrain free-running rhythms in female Octodon degus (Rodentia) J Biol Rhythms. 2001;16:489–501. doi: 10.1177/074873001129002169. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. doi: 10.1523/JNEUROSCI.4002-03.2004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Huckfeldt RM. Circadian entrainment to temperature, but not light, in the isolated suprachiasmatic nucleus. J Neurophysiol. 2003;90:763–770. doi: 10.1152/jn.00129.2003. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. I Time of origin of neurons and neuroglia. J Comp Neurol. 1968;134:287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Synapse formation in the mouse olfactory bulb. I Quantitative studies. J Comp Neurol. 1976;169:15–40. doi: 10.1002/cne.901690103. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: The mind’s clock. Oxford University Press; New York: 1991. [Google Scholar]
- Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Levine JD, Lynch MK, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. A new role for cryptochrome in a Drosophila circadian oscillator. Nature. 2001;411:313–317. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience. 2002;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat Neurosci. 2003;6:111–112. doi: 10.1038/nn1006. [DOI] [PubMed] [Google Scholar]
- Lenn NJ, Beebe B, Moore RY. Postnatal development of the suprachiasmatic hypothalamic nucleus of the rat. Cell & Tissue Research. 1977;178:463–475. doi: 10.1007/BF00219568. [DOI] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Mascaraque L, De Castro F. The olfactory bulb as an independent developmental domain. Cell Death Differ. 2002;9:1279–1286. doi: 10.1038/sj.cdd.4401076. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B. Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res. 1992;587:181–185. doi: 10.1016/0006-8993(92)90995-l. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Block GD. The Bulla ocular circadian pacemaker. II Chronic changes in membrane potential lengthen free running period. J Comp Physiol [A] 1987;161:347–354. doi: 10.1007/BF00603960. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Page TL. Circadian systems of invertebrates. In: Takahashi JS, Turek FW, Moore RY, editors. Handbook of behavioral neurobiology. Kluwer Academic; New York: 2001. pp. 79–110. [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, De Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Possidente B, Lumia AR, McGinnis MY, Teicher MH, deLemos E, Sterner L, Deros L. Olfactory bulb control of circadian activity rhythm in mice. Brain Res. 1990;513:325–328. doi: 10.1016/0006-8993(90)90475-q. [DOI] [PubMed] [Google Scholar]
- Potter SM, DeMarse TB. A new approach to neural cell culture for long-term studies. J Neurosci Methods. 2001;110:17–24. doi: 10.1016/s0165-0270(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Burns DE, Heller HC. Circadian rhythms in the suprachiasmatic nucleus are temperature-compensated and phase-shifted by heat pulses in vitro. J Neurosci. 1999;19:8630–8636. doi: 10.1523/JNEUROSCI.19-19-08630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thain SC, Hall A, Millar AJ. Functional independence of circadian clocks that regulate plant gene expression. Curr Biol. 2000;10:951–956. doi: 10.1016/s0960-9822(00)00630-8. [DOI] [PubMed] [Google Scholar]
- Tosini G, Fukuhara C. Photic and circadian regulation of retinal melatonin in mammals. J Neuroendocrinol. 2003;15:364–369. doi: 10.1046/j.1365-2826.2003.00973.x. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Westbrook GL. Excitatory synaptic transmission in cultures of rat olfactory bulb. J Neurophysiol. 1990;64:598–606. doi: 10.1152/jn.1990.64.2.598. [DOI] [PubMed] [Google Scholar]
- Underwood H. Circadian organization in nonmammalian vertebrates. In: Takahashi JS, Turek FW, Moore RY, editors. Handbook of behavioral neurobiology. Kluwer Academic; New York: 2001. pp. 111–140. [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Removal of the olfactory bulbs had no significant effect on the circadian locomotor patterns. Actograms show wheel running activity (black tick marks) of representative sham-operated (a) and bulbectomized (b) rats in double-plotted format. Each horizontal line represents a 48 h period; the second 24 h period is plotted to the right and also below the first. Grey bars indicate the 3 h each day when the lights were on and the arrow marks the day of surgery.