Abstract
Background
Alterations in circadian rhythms can have profound effects on mental health. High co-morbidity for psychiatric disorders has been observed in patients with circadian rhythm disorders, such as delayed sleep phase disorder (DSPD) and in those with an evening-type circadian preference. The aim of this study was to systematically determine the prevalence and type of DSM IV AXIS-I disorders in those with DSPD compared to evening-type controls.
Methods
Forty-eight DSPD and 25 evening-type participants took part in this study. Sleep and wake parameters were assessed with actigraphy, diary and questionnaires (Pittsburgh Sleep Quality Index (PSQI) and Functional Outcomes of Sleep Questionnaire (FOSQ)). Evening-type preference was defined by the Horne-Ostberg questionnaire. DSPD was determined by interview according to International Classification of Sleep Disorders criteria. Current and past diagnosis of psychiatric disorders were assessed with a Structured Clinical Interview for DSM-IV disorders.
Results
DSPD was associated with a later wake time, longer sleep time, higher PSQI score, lower Horne-Ostberg and FOSQ scores compared to evening-types. There were no significant differences in the prevalence or type of AXIS-I disorders between those with DSPD or evening type preference. Over 70% of participants met criteria for at least one past AXIS-I disorder. Approximately 40% of both the DSPD and evening-types met criteria for a past diagnosis of mood, anxiety (most frequently phobia) or substance use disorders. Evening types were more likely to have a past diagnosis of more that one AXIS-I disorder.
Conclusions
These results highlight the important link between circadian rhythms and mental disorders. Specifically, an evening circadian chronotype regardless of DSPD status is associated with a risk for anxiety, depressive or substance use disorders.
Keywords: circadian, sleep, depression, delayed sleep phase disorder
Introduction
Circadian (near 24 hour) rhythmicity is a fundamental property of all living organisms. Circadian rhythms are endogenously generated molecular, cellular, physiological and behavioral cycles that recur with a period of approximately 24 hours (1). A central pacemaker, located in the anterior hypothalamus regulates these circadian rhythms(2-3), via integration of clock controlled genes of central nervous system and peripheral tissues with external synchronizing agents, such as light and behavioral/social cycles (4-5). Evidence supports a role for the circadian clock system in the regulation of sleep–wake (6-7) and emotional behaviors (8-11). Indeed, alterations in circadian rhythms can have profound effects on sleep and mental health, and similarly, abnormalities in sleep and circadian rhythms are often observed in patients with depressive disorders, schizophrenia, bipolar disorder and anxiety disorders (11-13).
Circadian misalignment between the timing of the endogenous clock, the timing of sleep/wake and social behaviors is the basis for most circadian rhythm sleep disorders (CRSD). High co-morbidity for psychiatric disorders(14-15) has been observed in those with delayed sleep phase disorder (DSPD). DSPD is characterized by a delay in the sleep period relative to what is desired resulting in insomnia and impairment to daily functioning(16). The prevalence of DSPD in the general population is believed to be 0.17% (17) and to be more common (up to 10%) in those presenting at sleep clinics with insomnia complaints (18). There is also some evidence that individuals with an evening circadian preference (evening-type) without reported impairment of daytime function, may also be prone to psychiatric disorders (19-20). Because an evening circadian preference is what is common among these groups, an alteration in circadian timing at the molecular, cellular or physiological level, could potentially increase susceptibility to psychiatric disorders.
Systematic and detailed examination of the prevalence and type of psychiatric disorder in DSPD has been limited since many of the previous studies include questionnaire based assessment, and/or are primarily patient populations (recruited from sleep clinics or from inpatient psychiatric facility). Kamei and colleagues reported that 15.6% of their DSPD patients reported previously having a diagnosis of either depression or depressive symptoms (15). Reports using questionnaires such as the Minnesota Multiphasic Personality Inventory (MMPI) and the Yatabe-Guilford test (Y-G test), have indicated a high rate of depressive symptoms(21) and emotional features like nervousness, depression and lack of control of emotional expression in DSPD(22). Dagan and colleagues reported that in a group of hospitalized adolescents psychiatric patients approximately 15% had DSPD. Of the individuals determined to have DSPD, the most common disorders were borderline personality disorder (50%) and bipolar depression (30%)(23). Yamadera and colleagues (14) examined 72 cases of primary DSPD and 25 cases of secondary DSPD. A diagnosis of secondary DSPD was given due to a primary psychiatric disorder being present. The most prevalent Axis I DSM disorders reported in these patients were depression, social phobia and obsessive-compulsive disorders. More recently, in a study of 90 DSPD patients, evening-type preference, rather than sleep-wake phase was associated with self-reported depressive symptoms(24).
There is also indirect evidence (questionnaire) that having an evening-type preference is associated with depressive symptoms (19, 25-27). Furthermore, in patients with insomnia, depressive symptoms were greater in those with an evening-type preference (20, 28). It could also be the case that psychiatric disorders contribute to, or exacerbate circadian rhythm sleep disruption. For those with DSPD the inability to sleep at socially acceptable times can result in significant impairments of waking function and relationships, especially in severe cases when individuals are awake during most of the night, when others are sleeping. In addition to social isolation, individuals with delayed sleep phase may have difficulty maintaining regular employment.
Although DSPD patients nearly always have an evening-type circadian preference (prefer to be active during the late evening/early morning hours), not all evening-types have DSPD. It is also unclear whether the high prevalence of reported psychopathology in DSPD is related to the sleep disorder per se or because of their circadian chronotype (i.e. evening-type circadian preference). We hypothesized that the occurrence of psychiatric disorders would be higher in DSPD when compared to those with an evening-type circadian preference but without DSPD. Therefore, the aims of this study were 1) to characterize the types of psychiatric disorders and determine their occurrence in individuals with DSPD and in those who are evening-types using the Structured Clinical Interview for the DSM-IV (SCID) and 2) to determine whether there are differences in sleep and wake function in those with DSPD compared to evening-types.
Methods
Participants
Forty-eight DSPD and twenty-five evening-types participated in this study. The evening-type group (34.2 ± 11.8 yrs, 12 female) was defined as those having an evening-type diurnal preference on the Horne-Ostberg questionnaire without dissatisfaction with their sleep/wake times (i.e. they did not meet criteria for DSPD). Participants were considered to have DSPD (35 ± 11.4 yrs, 27 female) if they met the criteria for DSPD set forth in the International Classification of Sleep Disorders(29) and DSM-IV TR(30). DSPD status (yes/no) was determined by a board certified sleep physician during a clinical interview.
Procedures
Participants were recruited using printed and online advertisements between January 2004 and November 2007. Advertisements solicited participants who had an “extreme evening preference” or who were a “night owl”. Only those with an evening-type preference on the Horne-Ostberg Questionnaire and/or a diagnosis of DSPD were included in this analysis. There were no exclusion criteria for this study. All participants gave written informed consent and were compensated for their participation. All procedures were approved by the Internal Review Board at Northwestern University. After written consent was obtained, each subject completed the Horne-Ostberg Self-Assessment Questionnaire, Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale and the Functional Outcomes of Sleep Questionnaire. After completing the questionnaires, each subject wore an activity monitoring device and kept a sleep diary for 4 weeks. The participants then completed a Structural Clinical Interview for DSM-IV Disorders (SCID)(31) and had a clinical interview with a board certified sleep medicine physician to determine DSPD status.
Measures
Circadian Preference/Chronotype
The Horne-Ostberg Self Assessment Questionnaire (HO)(32) is an assessment of “morningness-eveningness”, consisting of 19 items that measure a subject's preference for the timing of daily activities. Each question has four answer choices that indicate a scale of “morningness” to “eveningness”. The lower scores indicate “eveningness” (16-30 Definitely Evening-Type, 31-41 Moderate Evening-Type), the higher scores indicate “morningness” (59-69 Moderately Morning-Type, 70-86 Definitely Morning-Type), and a score of 42-58 indicates a Intermediate- Type.
Characterization of Axis I Psychiatric Disorders
The Structured Clinical Interview for DSM IV (SCID) Axis I Disorders is a semi-structured interview used to diagnose DSM-IV Axis I disorders(31). It is administered in one session lasting an average 30-90 min, depending on the history of the participant. The SCID covers the diagnostic criteria for Mood Episodes, Psychotic Symptoms, Psychotic Disorders, Mood Disorders, Substance Use Disorders, Anxiety and Other Disorders. For this study, the clinician indicated whether the participant met diagnostic criteria currently (i.e. full criteria have been met during the criterion time period, such as the last 2 weeks) and or past (i.e. full criteria were met at some point over the course of the participant's lifetime) (31). The interview was conducted by a clinical psychology doctoral trainee and the diagnoses were confirmed by a supervising psychologist. The interviewer did not know the final sleep diagnosis or circadian chronotype of the participants at the time of the SCID interview. A detailed report of the interview was generated for each participant. Reports were reviewed to determine whether there was a seasonal pattern for the onset or worsening of psychiatric symptomatology.
Activity/Rest Cycle and Sleep
Sleep-wake parameters were measured by wrist activity monitoring on the non-dominant wrist (Actiwatch, Mini-Mitter Inc., Bend, OR) and sleep diary. Participants were instructed to wear the activity monitor at all times for 4 weeks. Wrist activity data was analyzed using Actiware-Sleep software (Mini-Mitter, Inc.). Actiwatch recorded sleep variables were calculated from the period between self reported bedtime to wake time (time in bed) from the sleep diary. The following variables were determined: sleep start (sleep onset), sleep end (actual wake time), actual sleep time (total sleep time), sleep latency (difference between bedtime and sleep start) and sleep efficiency (actual sleep time divided by total time in bed). Sleep start was defined as the first 10 minute period in which no more than one epoch was scored as mobile. Sleep end was defined as the last 10 minute period in which no more than one epoch was scored as immobile. Actual sleep time was defined as the amount of time between sleep start and sleep end, that was scored as sleep. Participants were also instructed to complete a daily sleep diary upon awakening and the following variables were determined: bed time, wake time, time in bed, and assumed sleep duration.
Self Reported Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) is a self-rated 21 item questionnaire to assess individual sleep habits (bedtime, morning rising time, sleep-onset latency, and night sleep duration), insomnia, and hypnotic use over a 1-month time interval (33).
Daytime Sleepiness
The Epworth Sleepiness Scale (ESS) provides a measurement of a subject's general level of daytime sleep propensity: it asks individuals to estimate the likelihood of dozing off or falling asleep in 8 different sedentary situations(34). The ESS correlates to some extent with mean sleep latencies on the objective Multiple Sleep Latency Test (MSLT) and with the degree to which people complain of sleepiness(35-36).
Sleep Related Quality of Life
The Functional Outcomes of Sleep Questionnaire (FOSQ) was developed to assess how sleepiness impacts activities of daily living(37). Participants rate the 30 items from “no difficulty” to “extreme difficulty.” The questions are grouped into five factors: activity level, vigilance, intimacy, sexual relationships, general productivity, and social outcomes. In addition to the total score obtained from the sum of factors, each factor is scored individually. A lower score indicates more sleepiness effects on a subject's daily activities.
Statistical Analysis
We conducted comparisons between DSPD and evening-types. Analyses included student's t-tests for continuous variables, or chi-square and Fisher exact tests for categorical variables. In order to quantify the magnitude of the effect, odds ratios (OR) were calculated for differences in occurrence of psychiatric disorders using R statistical software (38). Univariate OR models were conducted followed by multivariate models adjusting for age, race, gender, sleep duration (actigraphy), PSQI score and FOSQ score. Odds ratio of having Axis-I diagnoses were calculated with evening-type participants used as the reference group for each of the disorders. Missing questionnaire data were due to participants either failing to complete the questionnaires or not completing them correctly. Two DSPD participants had missing actigraphy data due to actigraph failure. Statistical significance was defined as p<.05 on two tailed tests.
Results
Comparison of DSPD and Evening-types
Sleep, Sleep related Quality of Life and Circadian Chronotype
Rest-activity data indicated that DSPD participants had a significantly later sleep end (p=0.02), and longer actual sleep time (p=0.04), than evening-types (Table 1). Questionnaire data indicated that DSPD participants also had significantly poorer sleep related quality of life on the FOSQ (p=0.04) and a trend for poorer sleep quality on the PSQI compared to evening-types (p=0.06). Furthermore the DSPD participants had significantly lower Horne-Ostberg questionnaire scores than the evening-type (p<0.01) group indicating a greater evening preference. There were no significant differences between the groups for the use of sleep medications (13% for both groups) or psychiatric medications (20% for DSPD and 13% for evening-types).
Table 1.
Mean (± standard deviation) of actigraphically recorded sleep and questionnaire data for participants with DSPD and Evening-type circadian preference.
| DSPD | Evening-types | ||||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | p | |
| Sleep | |||||
| Sleep Start | 46 | 3:03(42.6) | 25 | 2:22(38.8) | 0.11 |
| Sleep End | 46 | 10:26(1.9) | 25 | 09:26(1.6) | 0.02 |
| Actual Sleep Time (hours) | 46 | 6.4(1.9) | 25 | 5.9(0.76) | 0.04 |
| Sleep Efficiency (%) | 46 | 80.3(7.9) | 25 | 77.13(6.3) | 0.08 |
| Sleep Latency (minutes) | 46 | 24.26(0.38) | 25 | 24.27(0.26) | 0 .99 |
| Questionnaires | |||||
| Horne-Ostberg | 47 | 28.55(5.7) | 25 | 33.0(5.4) | <0.01 |
| PSQI | 47 | 7.38(3.1) | 23 | 5.82(3.6) | 0.06 |
| ESS | 46 | 7.39(4.4) | 25 | 7(3.8) | 0.71 |
| FOSQ | 45 | 16.76(2.4) | 24 | 17.91(1.9) | 0.04 |
Occurrence of Axis-I disorders in DSPD and Evening-types
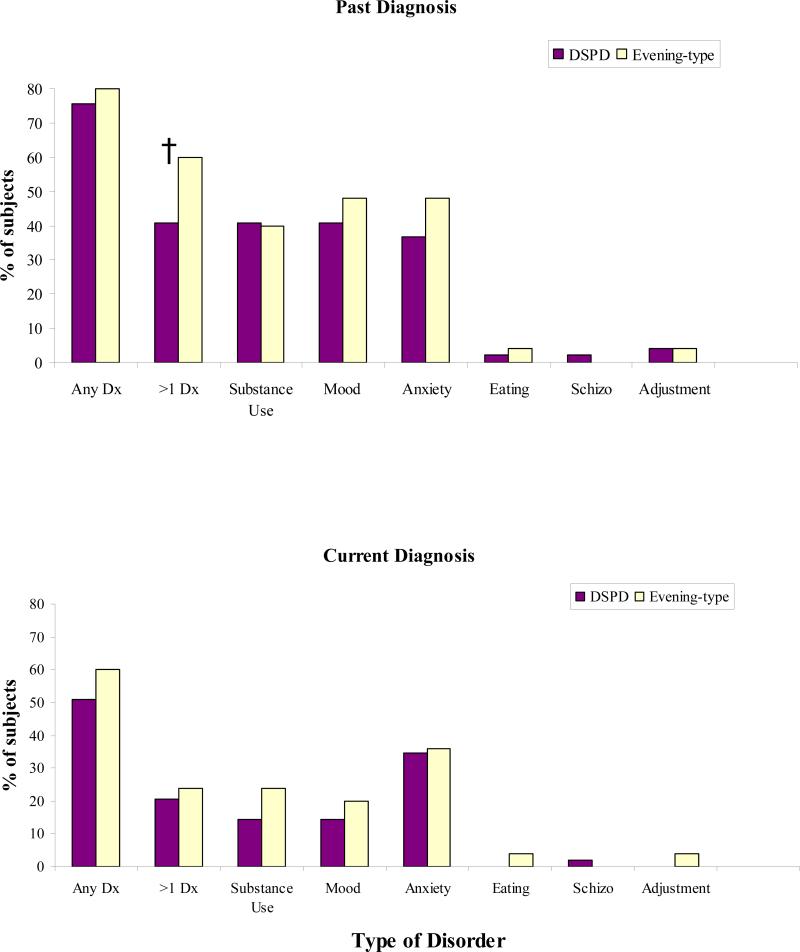
The percentage of participants that met criteria for an Axis-I disorder by major category is presented in Figure 1 and a detailed break down of the number of participants with a specific Axis-I disorder diagnosis is presented in table 2. For each Axis-I disorder there were no significant differences between the DSPD and evening-types (Table 2). However, there was a trend that evening-types were more likely to have met criteria for >1 past diagnosis of an Axis-I disorder than those with DSPD (p=0.07) (Figure 1).
Figure 1.
Graphic representation of the percent of participants that meet criteria for a particular category of disorder by history and currently. Any diagnosis (Any Dx), greater than one diagnosis (>1 DX). † indicates a trend (p=0.07) between DSPD and evening-types.
Table 2.
The number of cases of each type of Axis-I disorders by category according to the Structured Clinical Interview for DSM-IV recorded as past or current diagnoses in Delayed Sleep Phase Disorder (DSPD) and Evening-Types (ET).
| DSPD versus Evening-types (ET) |
||||
|---|---|---|---|---|
| Past | Current | |||
| DSPD (n=48) | ET (n=25) | DSPD (n=48) | ET (n=25) | |
| Substance Use Disorders | ||||
| Alcohol Use Disorders | ||||
| Alcohol Dependence | 6 | 5 | 5 | 2 |
| Alcohol Abuse | 9 | 4 | 2 | 4 |
| Other Substances Use Disorders# | ||||
| Substance Dependence | 6 | 4 | 1 | 0 |
| Substance Abuse | 8 | 1 | 0 | 1 |
| Mood Disorders | ||||
| Major Depressive Disorder | 17 | 10 | 4 | 4 |
| Other Depressive Disorders | ||||
| Dysthymic Disorder | 2 | 0 | 2 | 0 |
| Depressive Disorder NOS | 2 | 1 | 0 | 0 |
| Bipolar Disorders | ||||
| Bipolar I | 1 | 1 | 1 | 1 |
| Bipolar II | 0 | 0 | 0 | 0 |
| Anxiety Disorders | ||||
| Panic Disorder | 3 | 2 | 2 | 1 |
| Anxiety Disorder NOS | 2 | 1 | 2 | 1 |
| PTSD | 5 | 6 | 1 | 5 |
| OCD | 1 | 2 | 0 | 1 |
| Other Anxiety Disorders | ||||
| Social Phobia | 6 | 2 | 5 | 1 |
| Specific Phobia@ | 13 | 9 | 12 | 7 |
| Generalized Anxiety Disorder | 1 | 0 | 0 | 0 |
| Eating Disorders | ||||
| Bulimia | 1 | 0 | 0 | 0 |
| Anorexia | 0 | 1 | 0 | 1 |
| Schizophrenia and Psychotic Disorders | ||||
| Schizoaffective Disorder | 1 | 0 | 1 | 0 |
| Adjustment Disorders | ||||
| Adjustment Disorder | 2 | 2 | 0 | 1 |
| Other Disorders | ||||
| Impulse Control Disorder | 1 | 0 | 0 | 0 |
| Bereavement | 2 | 3 | 1 | 0 |
| Trichotillomania | 1 | 0 | 1 | 0 |
Example of commonly reported phobias to: Situational Type (planes, flying, cars, bridges, closed spaces), Blood-Injection-Injury Type, Natural Environment Type (heights, the dark, water), Animal Type (dogs, birds, sharks, insects, spiders)
Example of commonly abused substances: Cannabis, Cocaine, Amphetamines, Opioids, Hallucinogens, Crystal Meth, Unspecified.
Forty percent of the participants met criteria for past and 20% met criteria for current diagnosis of substance use disorder. Fifty percent of those who were diagnosed with alcohol abuse or dependence, were in college at the time of the abuse, and primarily reported drinking for social reasons. There were no significant differences between groups for alcohol or substance use disorders (Table 2.).
The most common mood disorder in this sample was major depressive disorder (MDD), a past diagnosis was reported in 35% of DSPD and 40% of evening-types. In addition, approximately 33% of those with a diagnosis of MDD reported recurrent episodes that did not appear to be seasonal since they occurred at different times of the year. Considerably fewer individuals in both groups met current criteria (symptoms in the past 2 weeks) for MDD.
The most common anxiety disorder was specific phobia, examples of the most frequently reported type of phobia are listed in Table 2. Eleven participants (n=5 DSPD and n=6 evening-types) met criteria for a past diagnosis of post traumatic stress disorder (PTSD). The trauma experienced by these participants was abuse in all but one case.
Evening-type participants were more likely to have greater than one past Axis-I diagnosis than DSPD (OR 0.2, 95% CI=0.05, 0.77, p=0.02). This was the only significant multivariate association between DSPD status and Axis-I disorders. There was, however, a trend for evening-types to be more likely to have a past diagnosis than DSPD (OR 0.3, 95% CI=0.09, 1.1, p=0.07).
Discussion
Although greater psychiatric symptoms have been reported previously in those with DSPD or evening-type preference, this study is the first to systematically examine differences in the occurrence of psychiatric diagnoses in those with DSPD compared to individuals with just an evening-type preference. We hypothesized that there would be a greater likelihood of an Axis-I disorder in those with DSPD compared to evening-types. However, in this study there were no significant differences in the occurrence or types of disorders between the groups. This would suggest that the occurrence of psychopathology in DSPD is primarily influenced by having an evening chronotype, rather than a sleep complaint (i.e. DSPD) per se, and that the underlying mechanism (s) is an involvement of the circadian timing system.
Although it should be pointed out that sleep is likely to play a role in the prevalence of psychiatric disorders, since both groups had poor sleep quality, but in different ways. While those in the evening-type group did not report insomnia, daytime sleepiness, or problems with daytime functioning due to their sleep, they did have a significantly shorter objective sleep duration, which was mostly likely due to the earlier wake time, and lower sleep efficiency compared to the DSPD group. This was an unexpected finding considering that those with DSPD are diagnosed with a sleep disorder and the evening-types are not. While the evening-types seem to have poorer objective sleep quality, the DSPD group had worse subjective sleep quality (PSQI score) and sleep related quality of life (FOSQ) and a lower Horne-Ostberg score (indicating greater eveningness),than the evening-types. Controlling for sleep duration, subjective sleep quality (PSQI) and sleep-related quality of life (FOSQ) did not change the associations between group and psychiatric diagnoses.
The only significant difference between the groups, after controlling for demographic and sleep variables, was for the evening-type group to have an increased likelihood for having met criteria for >1 disorder in the past. Since none of the sleep or demographic factors by themselves were associated with having more than one past diagnosis. Why then would the evening-types be more likely to have more than one past diagnosis? Perhaps for some evening-types this preference and the associated alterations in sleep manifests not in a sleep complaint as reported by those with DSPD but in psychiatric disorders.
The number of current axis-I diagnosis reported in this sample is considerably higher (~50% current) than that reported in the general population (26.2%, 12 months) (39). The most common psychiatric diagnoses in this study were mood, anxiety and substance use disorders, similar to those reported previously for DSPD and evening-types. These comparisons should be made with caution however, because our sample was not randomly selected and therefore not a true estimate of prevalence.
A past diagnosis of major depressive disorder (MDD) was the most common mood disorder reported (~40%) in both groups. Of those participants with MDD approximately a third had recurrent episodes. Although the presence of seasonal affective disorder was not specifically examined in this study, review of the time of the year of the reported depressive episodes did not indicate seasonality. This is an important distinction as seasonal affective disorder is thought in some cases to be associated with a circadian phase delay (40-41) and an evening-type preference (42). In the general population the prevalence for lifetime major depressive disorder is about 16.2% (43), considerably lower than the percentage reported in this sample of DSPD/evening-type individuals. Anxiety disorders were also common among the participants, with specific phobia being the most prevalent, a finding that has not been reported previously in those with DSPD or evening-type circadian preference.
Another interesting finding was the high occurrence of substance use disorder among both groups (current ≤15%). This is in contrast to data from the general population in the USA which reports substance use disorder in the past 12 months at 3.8% (39). The high occurrence in this sample was largely contributed by the high level of alcohol use disorder. For those who met the criteria for alcohol abuse, approximately 50% of episodes occurred when they were young adults or while in college, a time when alcohol abuse (44) and binge drinking are common (45). An alternate explanation is that those with an evening-type diurnal preference, particularly at the age of reported abuse, may be more likely to have poor health related behaviors. For example, previous studies have reported that evening-types may be more likely to smoke (46-47) or to use alcohol (48), sleep medications and caffeine (49). While there is no direct evidence of this from the current study self medication of insomnia symptoms with alcohol and drugs has been reported in DSPD (29) and may, in part, explain the number of individuals with a substance use diagnosis.
The underlying mechanisms that link psychiatric disorders with the evening circadian chronotype are unknown. We hypothesize a common link between circadian function and vulnerability for psychiatric disorders. However, alterations in homeostatic regulation of sleep or its interaction with circadian processes, sleep-wake behaviors and sleep disruption (such as the short sleep duration and poor sleep efficiency reported here in the evening-type group) may also play a role in the development of psychiatric disorders. Sleep-wake is believed to be controlled by two opposing processes; the circadian alerting signal and the homeostatic drive for sleep (6-7). The homeostatic drive for sleep increases with the time awake, and an alteration in the build up or dissipation of this homeostatic drive could influence sleep-wake timing and behavior. In the current study we did not directly assess the relative contribution of homeostatic versus circadian timing on the relationship between circadian chronotype and psychopathology. In the current study we only measured the timing of the rest/activity cycles. Therefore, a limitation of this study was the lack of direct markers of circadian phase (i.e. melatonin profiles) or homeostatic decay. Recent data indicate that “morningness” and “eveningness” may also be determined by homeostatic mechanisms. For example, there is evidence that evening-types with intermediate circadian phases have lower levels of slow wave activity and slower homeostatic decay of sleep pressure than morning-types with an intermediate circadian phase, interestingly this relationship is not evident in morning and evening-types with extreme circadian phases(50).
Furthermore, behavioral factors such as a reduction in light exposure across the day could contribute to depressive symptomatology. For example, low light levels in the fall and winter have been implicated as a mechanism in seasonal affective disorder. The limited data of light exposure in adult DSPD and evening-types are inconsistent (51-52). Further support for the role of exposure to circadian synchronizing agents and therefore the circadian hypothesis comes from the finding that stabilization or strengthening of social and behavioral circadian rhythms improves recovery from bipolar depression and could decrease vulnerability to the illness (53-55).
The finding in our study that the evening chronotype, rather than DSPD per se was associated with psychiatric disorders also supports a common physiological or molecular basis for this relationship. For example, both DSPD (56-57) and evening chronotype (58) have been associated with a longer than normal circadian period. A longer circadian period can make it more difficult to entrain to a 24 hour cycle and to maintain a regular social and professional schedule. Circadian period is likely genetically determined and linked to circadian clock genes. Several studies have found alterations in circadian clock genes in those with circadian rhythm sleep disorders and extreme circadian preference (59-65). Examination of molecular changes in evening-types with and without psychiatric diagnosis may be extremely useful in elucidating the role of the circadian clock in mental health.
Results of our study are limited by aspects of our methodology. The use of a convenience sample limits the generalizablility of our findings to the population as a whole. Furthermore, while the use of a DSM-IV based diagnostic classification provides information on whether individuals meet criteria for diagnoses it does not provide information on the severity of those illnesses. The sample size for our study was relatively small, yet larger than most studies of DSPD. However, a post-hoc power analysis demonstrated power >90% for more than one past diagnosis and phobia (66), which suggests that Type II error is an unlikely explanation for our results.
In summary, results of this study support the hypothesis of a common link or genetic susceptibility between evening circadian chronotype and psychiatric disorders, highlighting the important role of circadian regulation in mental illness. Thus, it is important for clinicians to recognize that mood, anxiety and substance use disorders are common in patients with circadian rhythm misalignment, such as seen in DSPD or extreme evening-types who attempt to conform to the “usual” work day. The likely bidirectional nature of the relationship between circadian rhythms and mood regulation suggest that a multimodal approach that incorporates both circadian measures to optimize synchronization of circadian rhythms, as well as treatment of the psychiatric disorder is likely to yield the best outcomes in the management of anxiety and mood disorders.
Acknowledgements
The authors would like to acknowledge the contribution of Gregory Kodesh for aid with data collection and Sridhar Jatla, Prasanth Manthena and Ramadevi Gourineni for diagnosis of DSPD status. Funding for this project was provided by a grant awarded to PC Zee (PI) from the National Institute of Health, grant R01 HL069988.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work was performed at the Department of Neurology, Northwestern University Feinberg School of Medicine
References
- 1.Mistlberger R, Rusak B. Circadian rhythms in mammals: Formal properties and environmental influences. In: Kryger MH, Roth T, Dement W, editors. Principles and Practise of Sleep Medicine. 5th ed. W.B. Saunders Co; Philadelphia: 2011. pp. 363–75. [Google Scholar]
- 2.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus - The Mind's Clock. Oxford University Press; New York: 1991. [Google Scholar]
- 3.Meijer JH, Rietveld WJ. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev. 1989 Jul;69(3):671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- 4.Aschoff J, Fatranska M, Giedke H, Doerr P, Stamm D, Wisser H. Human circadian rhythms in continuous darkness: Entrainment by social cues. Science. 1971;171:213–5. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- 5.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233(4764):667–71. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 6.Borbely AA. Sleep: circadian rhythm vs. recovery process. In: Koukkou ML, D. Angst J, editors. Functional States of the Brain: the determinants. Elsevier/North-Holland; Amsterdam: 1980. [Google Scholar]
- 7.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993 Mar;13(3):1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007;9(3):333–42. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007 May;114(2):222–32. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997 Feb;54(2):145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 11.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009 Aug 15;168(3):259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Benca R, Duncan MJ, Frank E, McClung C, Nelson RJ, Vicentic A. Biological rhythms, higher brain function, and behavior: Gaps, opportunities, and challenges. Brain Res Rev. 2009 Sep 18; doi: 10.1016/j.brainresrev.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci. 2000 Nov;25(5):446–58. [PMC free article] [PubMed] [Google Scholar]
- 14.Yamadera W, Sasaki M, Itoh H, Ozone M, Ushijima S. Clinical features of circadian rhythm sleep disorders in outpatients. Psychiatry Clin Neurosci. 1998 Jun;52(3):311–6. doi: 10.1046/j.1440-1819.1998.00395.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamei Y, Urata J, Uchiyaya M, Hayakawa T, Ozaki S, Shibui K, et al. Clinical characteristics of circadian rhythm sleep disorders. Psychiatry Clin Neurosci. 1998 Apr;52(2):234–5. doi: 10.1111/j.1440-1819.1998.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 16.ICSD-2 . In: The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Sateia M, editor. American Academy of Sleep Medicine; Westchester, IL: 2005. [Google Scholar]
- 17.Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res. 1993 Mar;2(1):51–5. doi: 10.1111/j.1365-2869.1993.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 18.Czeisler CA, Richardson GS, Coleman RM, Zimmerman JC, Moore-Ede MC, Dement WC, et al. Chronotherapy: resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep. 1981;4(1):1–21. doi: 10.1093/sleep/4.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Hirata FC, Lima MC, de Bruin VM, Nobrega PR, Wenceslau GP, de Bruin PF. Depression in medical school: the influence of morningness-eveningness. Chronobiol Int. 2007;24(5):939–46. doi: 10.1080/07420520701657730. [DOI] [PubMed] [Google Scholar]
- 20.Ong JC, Huang JS, Kuo TF, Manber R. Characteristics of insomniacs with self-reported morning and evening chronotypes. J Clin Sleep Med. 2007 Apr 15;3(3):289–94. [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi Y, Hohjoh H, Matsuura K. Predisposing factors in delayed sleep phase syndrome. Psychiatry Clin Neurosci. 2000 Jun;54(3):356–8. doi: 10.1046/j.1440-1819.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 22.Shirayama M, Shirayama Y, Iida H, Kato M, Kajimura N, Watanabe T, et al. The psychological aspects of patients with delayed sleep phase syndrome (DSPS). Sleep Med. 2003 Sep;4(5):427–33. doi: 10.1016/s1389-9457(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 23.Dagan Y, Stein D, Steinbock M, Yovel I, Hallis D. Frequency of delayed sleep phase syndrome among hospitalized adolescent psychiatric patients. J Psychosom Res. 1998 Jul;45(1):15–20. doi: 10.1016/s0022-3999(97)00299-7. Spec No. [DOI] [PubMed] [Google Scholar]
- 24.Abe T, Inoue Y, Komada Y, Nakamura M, Asaoka S, Kanno M, et al. Relation between morningness-eveningness score and depressive symptoms among patients with delayed sleep phase syndrome. Sleep Med. 2011 Aug;12(7):680–4. doi: 10.1016/j.sleep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness-morningness” dimension in “depressive” college students. J Affect Disord. 1999 Jan-Mar;52(1-3):19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 26.Randler C. Association between morningness-eveningness and mental and physical health in adolescents. Psychol Health Med. 2011 Jan;16(1):29–38. doi: 10.1080/13548506.2010.521564. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, et al. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol Int. 2010 Oct;27(9-10):1797–812. doi: 10.3109/07420528.2010.516705. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, Olavarrieta-Bernardino S, Ramos-Platon MJ, Bixler EO, et al. Nighttime sleep and daytime functioning correlates of the insomnia complaint in young adults. J Adolesc. 2009 Mar 26; doi: 10.1016/j.adolescence.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Sateia M, editor. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2 ed. American Academy of Sleep Medicine; Westchester IL: 2005. [Google Scholar]
- 30.DSM-IV(TR) Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Test Revision ed. American Psychiatric Association; Washington D. C.: 2000. [Google Scholar]
- 31.First M MB, Spitzer M RL, Gibbon M M, Williams D JBW. User's Guide For the Structured Clinical Interview for DSM-IV Axis 1 Disorders, SCID-1, Clinical Version. American Psychiatric Association; Washington, DC: 1997. [Google Scholar]
- 32.Horne J, Ostberg O. A Self-Assessment Questionnaire to Determine Morningness-Eveningness in Human Circadian Rhythms. International Journal of Chronobiology. [Journal] 1976 Jun;4:97–110. [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991 Dec;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 35.Chervin RD, Aldrich MS, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J Psychosom Res. 1997 Feb;42(2):145–55. doi: 10.1016/s0022-3999(96)00239-5. [DOI] [PubMed] [Google Scholar]
- 36.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994 Dec;17(8):703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 37.Weaver T, Laizner A, Evans L, Maislin G, Chugh D, Lyon K, et al. An Instrument to Measure Functional Status Outcomes for Disorders of Excessive Sleepiness. Sleep. [Journal] 1997 May;20(10):835–43. [PubMed] [Google Scholar]
- 38.Dessau RB, CB P. “R”--project for statistical computing. Ugeskr Laeger. 2008;170:328–30. [PubMed] [Google Scholar]
- 39.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005 Jun;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avery DH, Dahl K, Savage MV, Brengelmann GL, Larsen LH, Kenny MA, et al. Circadian temperature and cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression. Biol Psychiatry. 1997 Jun 1;41(11):1109–23. doi: 10.1016/S0006-3223(96)00210-7. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Rex KM, Nievergelt CM, Kelsoe JR, Kripke DF. Delayed sleep phase syndrome is related to seasonal affective disorder. J Affect Disord. 2011 Oct;133(3):573–9. doi: 10.1016/j.jad.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray G, Allen NB, Trinder J. Seasonality and circadian phase delay: prospective evidence that winter lowering of mood is associated with a shift towards Eveningness. J Affect Disord. 2003 Sep;76(1-3):15–22. doi: 10.1016/s0165-0327(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 43.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003 Jun 18;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 44.Harford TC, Grant BF, Yi HY, Chen CM. Patterns of DSM-IV alcohol abuse and dependence criteria among adolescents and adults: results from the 2001 National Household Survey on Drug Abuse. Alcohol Clin Exp Res. 2005 May;29(5):810–28. doi: 10.1097/01.alc.0000164381.67723.76. [DOI] [PubMed] [Google Scholar]
- 45.Randolph ME, Torres H, Gore-Felton C, Lloyd B, McGarvey EL. Alcohol use and sexual risk behavior among college students: understanding gender and ethnic differences. Am J Drug Alcohol Abuse. 2009;35(2):80–4. doi: 10.1080/00952990802585422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1-2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 47.Wittmann M, Paulus M, Roenneberg T. Decreased psychological well-being in late ‘chronotypes’ is mediated by smoking and alcohol consumption. Subst Use Misuse. 2010;45(1-2):15–30. doi: 10.3109/10826080903498952. [DOI] [PubMed] [Google Scholar]
- 48.Urban R, Magyarodi T, Rigo A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiol Int. 2011 Apr;28(3):238–47. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002 Sep;11(3):191–9. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 50.Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J Sleep Res. 2006 Jun;15(2):162–6. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 51.Goulet G, Mongrain V, Desrosiers C, Paquet J, Dumont M. Daily light exposure in morning-type and evening-type individuals. J Biol Rhythms. 2007 Apr;22(2):151–8. doi: 10.1177/0748730406297780. [DOI] [PubMed] [Google Scholar]
- 52.Reid KJ, Jaksa A, Carter B, Lu B, Zee PC. Habitual Activity and Light exposure in Subjects with Delayed Sleep Phase Syndrome. Sleep. 2008;31(Abstract Suppl):A163. [Google Scholar]
- 53.Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Kogan JN, Sachs GS, et al. Intensive psychosocial intervention enhances functioning in patients with bipolar depression: results from a 9-month randomized controlled trial. Am J Psychiatry. 2007 Sep;164(9):1340–7. doi: 10.1176/appi.ajp.2007.07020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005 Sep;62(9):996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 55.Frank E, Soreca I, Swartz HA, Fagiolini AM, Mallinger AG, Thase ME, et al. The role of interpersonal and social rhythm therapy in improving occupational functioning in patients with bipolar I disorder. Am J Psychiatry. 2008 Dec;165(12):1559–65. doi: 10.1176/appi.ajp.2008.07121953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozaki N, Iwata T, Itoh A, Kogawa S, Ohta T, Okada T, et al. Body temperature monitoring in subjects with delayed sleep phase syndrome. Neuropsychobiology. 1988;20(4):174–7. doi: 10.1159/000118495. [DOI] [PubMed] [Google Scholar]
- 57.Campbell SS, Murphy PJ. Delayed sleep phase disorder in temporal isolation. Sleep. 2007 Sep 1;30(9):1225–8. doi: 10.1093/sleep/30.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001 Aug;115(4):895–9. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 59.Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003 Jun 15;26(4):413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 60.Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001 Apr;2(4):342–6. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hohjoh H, Takasu M, Shishikura K, Takahashi Y, Honda Y, Tokunaga K. Significant association of the arylalkylamine N-acetyltransferase (AA-NAT) gene with delayed sleep phase syndrome. Neurogenetics. 2003 Apr;4(3):151–3. doi: 10.1007/s10048-002-0141-9. [DOI] [PubMed] [Google Scholar]
- 62.Iwase T, Kajimura N, Uchiyama M, Ebisawa T, Yoshimura K, Kamei Y, et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002 Mar 15;109(2):121–8. doi: 10.1016/s0165-1781(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 63.Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. A single-nucleotide polymorphism in the 5'-untranslated region of the hPER2 gene is associated with diurnal preference. J Sleep Res. 2005 Sep;14(3):293–7. doi: 10.1111/j.1365-2869.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 64.Carpen JD, von Schantz M, Smits M, Skene DJ, Archer SN. A silent polymorphism in the PER1 gene associates with extreme diurnal preference in humans. J Hum Genet. 2006;51(12):1122–5. doi: 10.1007/s10038-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 65.Pereira DS, Tufik S, Louzada FM, Benedito-Silva AA, Lopez AR, Lemos NA, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep. 2005 Jan 1;28(1):29–32. [PubMed] [Google Scholar]
- 66.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998 Jul 30;17(14):1623–34. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]