Abstract
Light is electromagnetic radiation that can convert its energy into different forms (e.g., heat, chemical energy, and acoustic waves). This property has been exploited in phototherapy (e.g., photothermal therapy and photodynamic therapy) and optical imaging (e.g., fluorescence imaging) for therapeutic and diagnostic purposes. Light-controlled therapies can provide minimally or non-invasive spatiotemporal control as well as deep tissue penetration. Nanotechnology provides a numerous advantages, including selective targeting of tissues, prolongation of therapeutic effect, protection of active payloads, and improved therapeutic indices. This review explores the advances that nanotechnology can bring to light-based therapies and diagnostics, and vice versa, including photo-triggered systems, nanoparticles containing photoactive molecules, and nanoparticles that are themselves photoactive. Limitations of light-based therapies such as photic injury and phototoxicity will be discussed.
1 Introduction
In this review we will illustrate some general strategies by which light can be applied in nanomedicine, and problems in phototherapy that can be addressed by nanotechnology. The mechanisms, principles and progress in technology relating to photochemistry and nanotechnology will be overviewed in brief. We will discuss various nanoparticles (NPs) used in phototherapy or light-triggered drug delivery. Some challenges in the design and fabrication of light-triggered nanomaterials for potential clinical translation will be featured.
1.1 Light
Light is the narrow spectral region of electromagnetic radiation perceived by human vision (wavelengths 390–750 nm);1 however that term is often extended to ultraviolet (UV) and infrared (IR) rays in photochemistry and photobiology (Figure 1a). Light, consisting of photons, is a form of energy with dual wave-particle properties.2 The energy of light is inversely proportional to light wavelength (E = hν), i.e. UV light has more energy than the same number of photons of IR light.
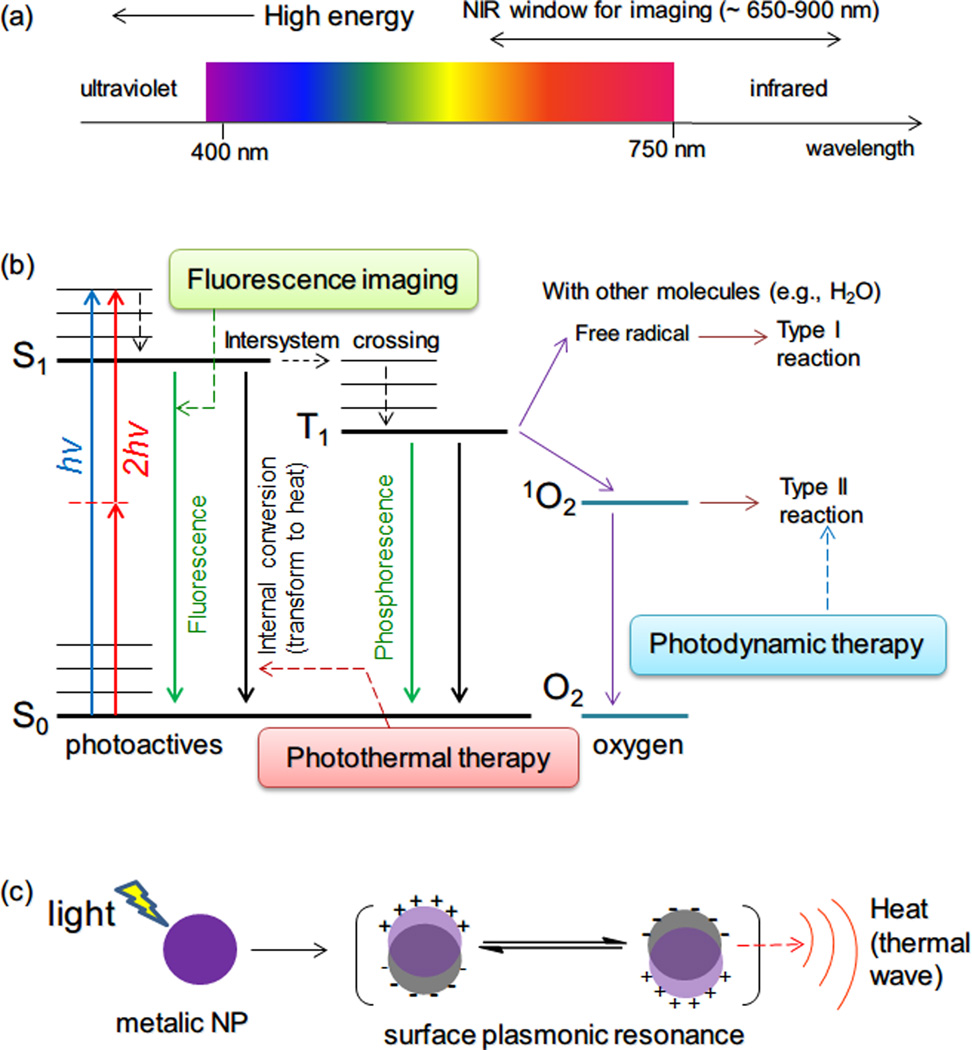
Figure 1.
(a) Schematic of the electromagnetic spectrum of UV, visible and IR light, and of the NIR window for in vivo imaging. (b) Energy diagram for conventional organic photoactives, molecular oxygen and related photophysical and photochemical processes. Abbreviation: hv, one-photon absorption; 2hv, two-photon absorption; S0, ground state; S1, singlet state; T1, triplet state. Fluorescence, photothermal therapy, and photodynamic therapy are related to different transformation processes of the absorbed energy. (c) Schematic illustration of surface plasmon resonance of a metallic NP, which can absorb visible or NIR light by surface plasmonic resonance, and dissipate the absorbed light energy as heat (photothermal effect).
Light has been used as a therapy for more than three thousand years. The ancient Egyptians, Indians, and Chinese used light to treat various diseases, including psoriasis, rickets, vitiligo, and skin cancer. However, a systematic understanding of photochemical and photophysical processes was only established in the last century. Modern phototherapy was developed by Finsen,3 who used UV light to treat cutaneous tuberculosis and used red light to prevent the formation and discharge of smallpox pustules (for which he received the 1903 Nobel Prize). The direct use of light as a therapeutic agent has been important in the treatment of vitamin D deficiency, neonatal jaundice, autoimmune diseases, manic depression, and other conditions.4
Light has several useful properties. Absorbed light can induce changes in administered or endogenous chemicals. (In this review all such compounds are referred to as photoactive molecules or photoactives.) Subsequently, these compounds can undergo photochemical or photophysical processes leading to therapeutic or diagnostic uses. These processes include: photochemical reaction of the agent itself (e.g., photocleavage or photoswitching, see sections 2.5 and 2.6) or of other endogenous molecules (e.g., generation of cytotoxic singlet oxygen from O2 in photodynamic therapy, see section 2.2), emission of light at different wavelengths (e.g., fluorescence), or transfer of energy to other forms (e.g., heat for photothermal therapy, see section 2.1, and acoustic waves for photoacoustic imaging see section 2.4). The photochemical processes for photodynamic therapy, photothermal therapy and fluorescence imaging using small-molecule photoactive agents are shown in Figure 1b.
1.2 Access to tissues: the near infrared (NIR) window and novel light sources
Upon interaction with a tissue, light can be reflected, scattered, transmitted, or absorbed depending on the optical features of the tissue (absorption coefficient etc.). Light propagation in the tissue is affected by scattering due to tissue heterogeneity, and by absorbance by water and endogenous dyes such as hemoglobin.5 The maximum skin permeability to light occurs in the range ca. 650–900 nm (the so-called near infrared light window, Figure 1a).6 NIR light can propagate through tissues with less attenuation compared to that for visible light: penetration depths greater than 3 cm can be achieved in muscle and brain, and greater than 10 cm in less-attenuating organs such as the human breast.5,7 The use of NIR light therefore has significant advantages for phototherapy and optical imaging within deep tissues over UV and visible light.
Fiberoptic devices provide another minimally invasive means of conveying light deep within the body, and have become increasingly versatile with progressive miniaturization; 8 they are also useful for transmitting images and performing medical procedures.9 Flexible optical fiber-bundle miniature endoscope have been manufactured with ~200–300 µm diameters, which are well suited for fluorescence imaging deep within tissues, as well as the delivery of light for phototherapy (e.g., for photodynamic therapy).10
The commercial availability of two novel light sources, light-emitting diodes and femtosecond solid-state lasers, can be readily adapted for clinical applications (e.g. for endoscopy) with visible or NIR output.4,11 The light-emitting diode is a semiconductor light source that emits in a narrow light bandwidth (5–10 nm) and high power output (up to hundreds of mW/cm2 over an area of 20 cm2).11 Being inexpensive and compact, light-emitting diodes can be arranged to cover complex anatomic topographies.11 Femtosecond solid state lasers that can generate high intensity light with pulse durations around100 femtoseconds are useful for in vivo two-photon excitation12 (see section 2.3). Both of these light sources should be applicable via fiberoptics.
1.3 Light toxicity
Light can induce photic injury in a manner dependent on the irradiation power density, spot size, irradiation time, the manner of exposure (e.g., the frequency of the target being irradiated), and wavelength. The most significant type of photic injury is photothermal damage, where tissues are heated by absorbed light energy. On a cellular and molecular level, increases in temperature cause the denaturation of proteins, loss of molecular tertiary structure, and fluidization of membranes. Depending on the extent of damage induced by the rise in thermal energy, cells may undergo apoptosis (55–58 °C), apoptosis and necrosis (60–68 °C), and immediate cell death (72 °C or greater).13 This property is useful for surgical incision and ablation of tissue parts. Light can also cause photochemical injury, where it generates free radicals by interacting with endogenous chromophores (e.g., photoreceptors in eyes, heme proteins and flavoproteins etc.).13 The free radicals can oxidize proteins and cell membrane lipids. Photochemical damage is associated with long durations of exposure and high energy (or low wavelength, ca. UV) light exposure. As examples of photochemical injury, prolonged exposure to UV light can result in painful eye injury, premature skin aging, and/or skin cancer, in addition to skin burning. Furthermore, high-energy light (megawatts or terawatts / cm2) can cause photomechanical damage, applying compressive or tensile forces to tissues, even if tissue is irradiated for as short as a period nanoseconds to picoseconds.13
American National Standards Institute publishes maximum permissible exposures for different light sources,14 assuming ocular irradiation. However, photic injury can be delayed in onset, occurring long after treatment is over.15 Consequently, optical imaging and treatment using light should be mindful of phototoxicity. In considering light-activated systems, it is also important to bear in mind the ambient state of environmental irradiation (e.g. daylight) that can lead to non-specific activation of photoactives.
1.4 Photochemical properties of photoactives
The dissipation of photonic energy (light) can proceed by more than one pathway. For example, a photodynamic sensitizer can be also fluorescent. The selection of photoactives for specific treatments is therefore important and can be guided by an appreciation of each agent’s photophysical properties, including the extinction coefficient, photostability, quantum yield (the probability of a particular photochemical process following the absorption of a photon) and absorption cross section (the probability of absorption, taking light scattering into consideration). In general, agents for photodynamic therapy require high molar extinction coefficients and high-energy and long-lived triplet states (longer than singlet state), in order to generate singlet oxygen with a high quantum yield. A photoactive with a high molar extinction coefficient, very low quantum yield of fluorescence, and a short-lived and low-energy triplet state (pico-second range, no tendency for the photodynamic route) is best for photothermal therapy, as its absorbed light energy can be primarily converted to heat. For fluorescence imaging for in vivo applications, the agent should have low toxicity, good solubility in aqueous media, a high fluorescence quantum yield and a longer fluorescence life time than the components of the biological samples under study (e.g., tryptophan residues in proteins).4
1.5 Nanomedicine and light-triggered drug delivery
Nanomedicine refers to the application of nanotechnology for the diagnosis, monitoring, prevention and treatment of clinical conditions.16 Nanomedicine can enhance therapeutics and diagnostics in many ways, as has been reviewed.17 This is well demonstrated in the case of nanoparticulate drug delivery systems. For example, cellular uptake of hydrophobic drugs in NPs can be considerably enhanced over that of free drug,18 which can be advantageous in treating resistant infections, developing vaccines, or treating resistant tumors.17a,19 In cancer chemotherapy the nanoscale enables the preferential delivery of drugs to tumors owing to the enhanced permeability and retention (EPR) effect whereby NPs are preferentially taken up by the leakier vasculature in tumor beds and are retained because of the tortuous lymphatics.14,20 Several nanoparticulate therapeutics, e.g., DoxilTM (~100 nm PEGylated liposome loaded with doxorubicin) and AbraxaneTM (~130 nm paclitaxel albumin-stabilized NPs), have been approved for use by the FDA, and have shown improved pharmacokinetics and reduced adverse effects compared to their parent drugs.17a In general, NPs with sizes below 200 nm are suitable for systemic (usually intravenous) distribution, as larger ones can cause embolic phenomena.14
One significant drawback of commercially available drug delivery NPs is that drugs are released at a predetermined rate irrespective of patient needs or changing physiological circumstances. Light has been investigated as an external active control element to achieve on-demand triggered drug delivery. Such drug delivery systems would allow repeated on-demand dosing that would be adaptable to the patients’ regimen, and allow multiple dosages from a single administration.14 Modulation of the amplitude and duration of irradiation allows the desired biological effect to be achieved while minimizing tissue damage. Many NPs loaded with photoactive chromophores (e.g. photocaging groups or photoswitching groups) have been reported to undergo light-triggerable physicochemical changes (e.g., alteration in size, surface, assembly structure, drug release rate) allowing on-demand drug delivery.14 It bears noting that, although it may not be necessary for clinical purposes, triggering by light allows sub-nanometer spatial resolution, sub-micrometer visualization, and sub-millisecond temporal resolution and control; this may be of interest in research applications.
1.6 Nanoparticulate delivery of photoactive compounds
An important limiting factor in phototherapy is the availability of suitable photoactive agents. Small-molecule photoactives often have circulation half-life in vivo that is too brief to be useful; the concentration of these agents at diseased sites may be insufficient for therapeutic effect.21 Consequently, the light dosages needed to achieve therapeutic effect in many clinical studies are close to levels that cause photothermal and photomechanical damage by lasers.22 These problems have been addressed by incorporating photoactives into NPs, to take advantage of the enhancements to drug delivery afforded by nanoencapsulation outlined above.23 Photoactives can be confined within NPs at high concentration and protected from degradation and photobleaching.24 NPs can prolong the compounds’ circulation times and optimize the distribution of photoactives in vivo: e.g. by enhancing their accumulation in leaky vasculatures like those of tumors due to the EPR effect (Figure 2).25 For example, the biodistribution of poly(lactic acid)-PEG NPs containing the photosensitizer hexadecafluoro zinc phthalocyanine has been studied in tumor-baring mice: the NPs exhibited tumor accumulation of the photoactive and sensitivity to photodynamic therapy for 24 hours, while free photoactives dispersed in Cremophor-EL only showed photosensitization in tumors for 8 hours.26 Similarly, sub-100 nm micelles containing zinc porphyrin for photodynamic therapy selectively accumulated in choroidal neovasculation lesions for at least 24 hours, and was effective treatment.27 Free photosensitizer was completely cleared during that period, and was less effective.
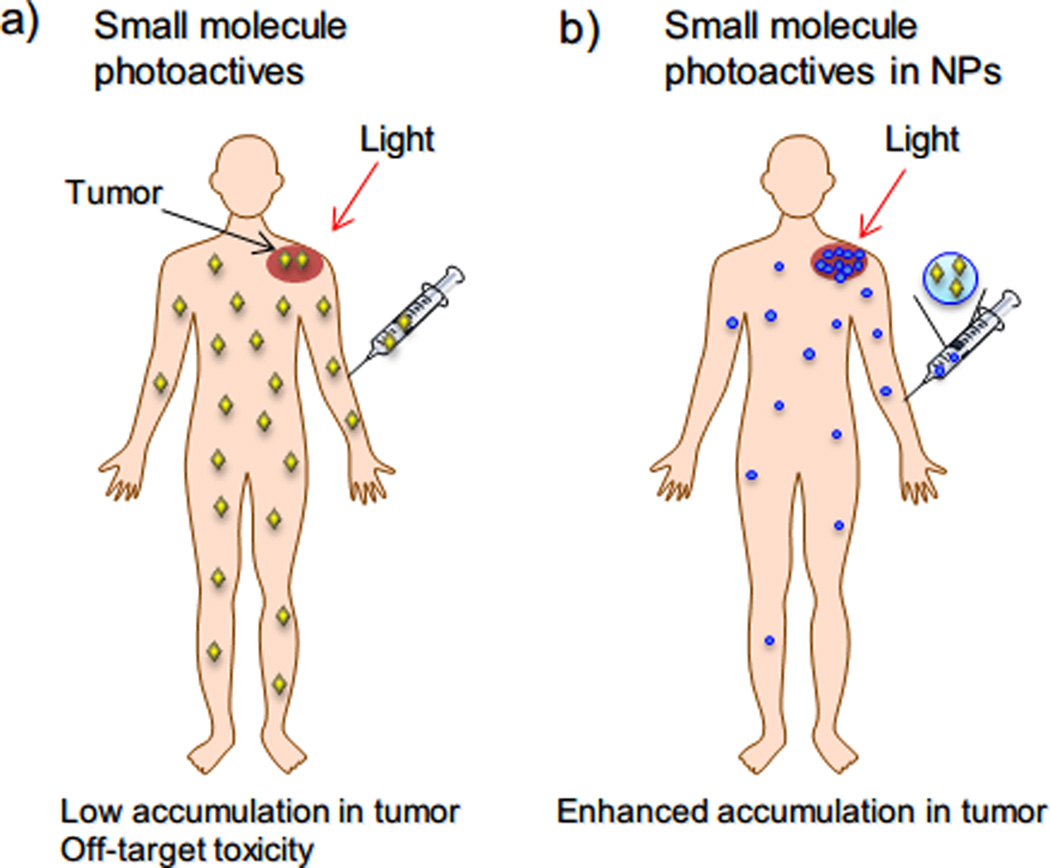
Figure 2.
Advantages of using NPs in phototherapy. (a) Administered free photoactives have low accumulation in tumor and distribute to the whole body. Photoactives in healthy tissue may be activated by ambient visible light and cause off-target toxicity. (b) NPs can enhance accumulation of photoactives in disease sites (e.g., tumors) by EPR effect to improve phototherapy efficacy.
1.7 Nanoparticles as photoactive agents
An important advance in both phototherapy and nanotechnology was the finding that some NPs can themselves act as photoactives.23
Metallic NPs with surface plasmon resonance (e.g., gold NPs) can efficiently absorb light and act as photoactives for photothermal therapy.28 Surface plasmon resonance is a phenomenon whereby light (an electromagnetic wave) induces collective oscillations of conductive metal electrons at the particle surface, which can give rise to a sharp and intense absorption band (Figure 1c). The surface electron oscillation decays non-radiatively by conversion of the absorbed light energy to heat, known as the photothermal effect. The photophysics of such NPs (e.g., tunability of the absorption band, scattering properties) can be pre-determined by NP size, shape or geometry.29 The surface plasmon absorption bands of NPs can be also changed if their shape is changed by laser irradiation.30 The optical behavior of NPs can therefore be controlled and tailored for in vivo applications with appropriate lasers, minimizing phototoxicity towards non-target tissues. One such formulation is entering clinical trials to ablate tumors (AuroShellTM) and other promising formulations are under investigation.31
Some NPs can act as photoactives by generating singlet oxygen for photodynamic therapy (e.g., TiO2 NPs, ZnO NPs, and fullerenes).32 Other NPs can achieve unconventional photophysical phenomena. For example, specific NPs can absorb NIR light and emit UV or visible light (see section 3.2);33 upon NIR irradiation such “upconversion NPs” can act as UV or visible light sources to activate photoactive moieties in the immediate (nanoscale) vicinity of the NPs which cannot be directly excited by NIR light.
2 Strategies in photochemistry and phototherapy
2.1 Photothermal therapy
In photothermal therapy, small-molecule photoactive agents are administered to a patient; upon irradiation of a target location, the photoactives are excited and then undergo internal conversion to the ground state (converting photonic energy to heat (Figure 1b).34 For tissues like tumors, that have an inadequate supply of blood and oxygen, the resulting hyperthermia can cause irreversible cell damage at 42–46°C over tens of minutes.35 The higher the temperature the shorter the required treatment time (e.g. the induced cytotoxicity takes 240 min at 43 °C, equivalent to heating for 1 s at 54 °C)36 This approach has been used successfully to ablate tumors, or enhance drug delivery efficiency by increasing blood flow and tumor vessel permeability.37
Photothermal therapies in current clinical practice lack tumor selectivity in that surrounding healthy tissues can also be damaged.34,38 The lack of selectivity arises in part from the relative inefficiency of photothermal agents such as naphthalocyanines and metal porphyrins in absorbing and converting light (photonic energy) into heat,34 which leads to an increase in the required dosage of light. Furthermore, the small-molecule actives do not have a natural tropism toward tumor tissue. As noted above, NPs can take advantage of EPR to accumulate in tumors.39 This characteristic is particularly useful in treating complex tumor margins or disseminated tumors. NPs can also protect encapsulated photoactives from photobleaching, so that they can be triggered to reach a target temperature multiple times following a single administration.40 This cannot be readily realized with free photoactives.
Plasmonic NPs (e.g., gold NPs) can absorb light (photonic energy) effectively and convert it efficiently into heat energy41 (efficiency ~ 1 for gold NPs). 39b,42 By this means, NIR irradiation of gold NPs can induce local hyperthermia for thermal ablation in vivo,39b,42 without small molecule photoactives.28 (The application of gold NPs for photothermal therapy is detailed in section 3.1).
2.2 Photodynamic therapy
Photodynamic therapy (PDT) is a noninvasive photochemistry-based method of treating tumors or other diseases such as macular degeneration (Table 1).43 PDT generates highly reactive singlet oxygen (1O2, the excited state of molecular oxygen) which results in the oxidative destruction of cellular targets, the occlusion of blood vessels,44 and inflammation that can activate an immune response against targeted cells45 (e.g., tumor-specific T cells).43a,46 PDT requires three components to generate singlet oxygen: a photosensitizer (here defined as a molecule that generates singlet oxygen in response to light)47, light of an appropriate wavelength and power, and molecular oxygen. A photosensitizer in its ground singlet state (electrons paired) is first promoted to an excited singlet state when irradiated by light of a specific wavelength (usually ~ 600–900 nm in PDT to avoid absorption by endogenous chromophores). The photosensitizer in the excited singlet state moves to a lower-energy excited triplet state (electrons unpaired) and generates singlet oxygen in the presence of molecular oxygen (Figure 1b),48 instead of undergoing thermal decay or emitting fluorescence.
Table 1.
Clinical application of photodynamic therapy for various diseases.
| Disease | Photosensitizers | Disease | Photosensitizers |
|---|---|---|---|
| Cervical cancer | Photofrin | Cutaneous skin cell lesions | Pc4* |
| Basal-cell carcinoma | Levulan, Metvix, Photochlor* | Barrett’s esophagus | Photofrin, Photochlor* |
| Brain tumor | Photofrin*, BOPP* | Myopic maculopathy | Photolon |
| Actinic keratosis | Levulan, Metvix | Gastric cancer | Photofrin |
| Gastrointestinal tract cancer | Benzvix* | Coronary artery disease | Lutex* |
| Head and neck cancer | Foscan | Early lung cancer | Talaporfub, Photofrin |
| Bladder cancer | Photofin*, Hexvix*, Levulan* | Advanced lung cancer | Photofrin |
| Prostate cancer | Foscan, Lutex, Tookad*, Purlytin* | Metastatic breast cancer | Purlytin* |
| Skin and mucosa tumors | Photolon | Kaposi's sarcoma | Purlytin* |
| Age-related macular degeneration | Visudyne, Photolon, Photosens* | Sterilization of blood | Pc4* |
| Cutaneous T-cell lymphoma | Pc4* | Atherosclerosis | Antrin* |
| Acne vulgaris | Photofrin* | Hepatocellular carcinoma | Talaporfin* |
| Penile cancer | Photofrin* | Gliomas | Photofrin* |
Photosensitizers with * indications are in clinical trials; the photosensitizers without * are approved.43a,47,141
List of photosensitizers’ chemical composition and corresponding treating light wavelength: Photofrin (630nm): mixture of oligomers formed by ether and ester linkages of up to eight porphyrin units. Levulan (635nm): δ-aminolevulinic acid (inducing synthesis of protoporphyrin IX). Metvix (635nm): methyl aminolevulinate (inducing synthesis of protoporphyrin IX). Photochlor (665nm): 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a. BOPP (630nm): boronated porphyrin. Benzvix (635nm): 5-benzyl aminolevulinate (inducing synthesis of protoporphyrin IX). Hexvix (375–400nm): 5-hexyl aminolevulinate (inducing synthesis of protoporphyrin IX). Foscan (or Temoporfin, 652nm): m-tetrahydroxyphenylchlorin (mTHPC). Lutex (732nm): lutetium texaphyrin. Tookad (763nm): Pd-bacteriopheophorbide. Purlytin (664 nm): tin ethyl etiopurpurin. Photolon (or Fotolon, 660–670 nm): trisodium salt Chlorin e6 and polyvinylpyrrolidone. Visudyne (693nm): a liposomal formulation of Verteporfin (a benzoporphyrin derivative). Pc4 (670 nm): phthalocyanine-4. Talapofin (664nm): mono-L-aspartyl chlorine. Antrin (732nm): motexafin lutetium.
There are several barriers to the effectiveness of PDT that can be addressed by NPs. Most photosensitizers bind to normal cells as well as to cancer cells, leading to unwanted off-target activation from environmental (ambient) light.24,49 NPs have the advantage of prolonging circulation half-lives and increasing the accumulation of PDT sensitizers in tumor tissues or other leaky vasculatures via EPR.
The generation of singlet oxygen by many small-molecule PDT sensitizers is limited by self-inactivation in aqueous media, due to their large hydrophobic aromatic domains forming ground-state stable aggregates.50 Encapsulation in NPs can prevent this aggregation. For example, the confinement of the photosensitizer zinc porphyrin at the center of ionized dendrimers can greatly increase the efficiency of 1O2 generation for PDT.27,51
NPs loaded with photosensitizers can also act as activatable PDT agents. For example, many photosensitizers that are completely self-inactivated when assembled in close proximity inside hydrophobic pockets of NPs can regain phototoxicity upon cell internalization and subsequent release.24,27,47 Another approach to enhance photodynamic efficacy is to co-localize (e.g., conjugate) photosensitizers with NPs (e.g. by conjugation); NPs with efficient light-absorbing properties can transfer the photonic energy to activate the photosensitizer, generating 1O2.52
2.3 Two-photon excitation
Two-photon excitation is a new optical technique wherein two photons of a given wavelength that encounter a photoactive simultaneously combine their energies to promote the molecule to its excited state, as if they were a single photon of half the wavelength (twice the energy; Figure 1b). Thus for example, two-photon excitation allows a photoactive agent having single-photon absorption in the UV or visible region to be excited with a stream of strongly focused pulses of NIR laser light. Two-photon excitation is several orders of magnitude weaker than that of conventional one-photon excitation, and therefore requires pulsed high-intensity lasers (MW·cm−2 to GW·cm−2) as light sources.53 This technique has broad applications, including in vivo fluorescence imaging,54 PDT,12,55 and photocaging 56 (see section 2.5).Two-photon excitation using NIR light is promising for in vivo treatment: it can activate photosensitizers for PDT at a depth of 2 cm to ablate tumors.57 Of note, due to the extensive scattering of the light in deep tissues, two-photon excitation for fluorescence microscopy using NIR laser can only excite specimens up to one millimeter deep within tissues54 with spatial resolution down to sub-100 nm.58
Since two-photon excitation applies high-intensity light,59 the NIR lasers should be applied in short bursts with durations of about 100 femtoseconds at about 100 MHz,54 which will reduce the average energy over time, minimizing tissue damage. The selection of photoactive agents for two-photon excitation is also important to prevent tissue damage: many chromophores in current use have a low efficiency for activation by two-photon excitation (low two-photon cross-sections) and therefore require undesirably high energy densities.13,22,60 For use in two-photon excitation in PDT, 60–61 photosensitizers should have a high two-photon cross-section62 (larger than 100 Goeppert–Mayer units, 1 Goeppert–Mayer units = 10−50 cm4·s per photon), in order to prevent tissue damage.63 Inorganic NPs with enhanced two-photon excitation processes have been developed: the two-photon cross-section of 5 nm quantum dot - silicon phthalocyanine conjugates is two orders of magnitude better than that of free silicon phthalocyanine.64 Such advances may provide means to efficiently use light energy and minimize photic injury when applying two-photon excitation in clinical practice.
Two-photon excitation technologies using high energy laser sources are not optimal for use over prolonged periods (e.g., over minutes to hours), as tissue damage and photobleaching of imaging agents may ensue.8 They are also not ideal for use over large tissue volumes (e.g., for small-animal whole body imaging) since excitation only happens at the focal point of the laser. These spatial and/or temporal limitations in imaging may be overcome by use of other imaging technologies such as second-harmonic generation (whereby two photons are scattered by the target and combined to produce a new photon with the sum of two photon’s energy without energy loss),8,65 optical coherence tomography (based on the superposition of the scattered light, especially useful for ophthalmology)66 or photoacoustic imaging (see section 2.4; comparison of different imaging techniques in Table 2). These technologies can be sometimes combined for imaging and are reviewed elsewhere.66c,67
Table 2.
Overview of imaging methods in preclinical biomedical research
| Imaging method |
Type of wave |
Tissue penetration depth |
Acquisition time |
Resolution | Labeling required or not |
Labeling agents |
Multi- channel or nota |
|---|---|---|---|---|---|---|---|
| Computed tomography | X-ray | No limit | Minutes | 50 µm | No | Iodine | No |
| Magnetic resonance imaging | Radio-frequency wave | No limit | Minutes to hours | 10–100 µm | No | Magnetic and paramagtetic | No |
| Ultrasound | Sound | Centimeters | Minutes to hours | 50 µm | No | Microbubbles | No |
| Positron emission tomography | Gamma ray | No limit | Minutes to hours | 1–2 mm | Yes | Radionuclide (e.g., 18F) | No |
| Single-photon emission computed tomography | Gamma ray | No limit | Minutes to hours | 1–2 mm | Yes | Radionuclide (e.g., 99Tc) | No |
| Confocal fluorescence microscopy | Visible or NIR light | ~ 50 µm | Seconds to minutes | < 1 µm | Yes | Fluorophore | Yes |
| Fluorescence-mediated tomography | visible or NIR light | <10 cm | Minutes to hours | 1 mm | Yes | Visible and NIR fluorophore | Yes |
| Bio-luminescence imaging | Visible light | Centimeters | Minutes | ~1–10 mm | Yes | Lucifrin | No |
| Two-photon fluorescence microscopy | NIR light | < 1 mm | Seconds to minutes | <1 µm | Limited | Visible and NIR fluorophore | Yes |
| Optical coherence tomography | NIR light | 1–2 mm | Seconds to hours | 1–20 µm | No | NIR Fluorophore and NPs (e.g., gold NPs) | Yes |
| Photoacoustic imaging | Visible or NIR light and sound | Centimeters | Seconds to hours | ~ 5 µm | No | NIR Fluorophore and NPs (e.g., gold NPs) | Yes |
Multichannel: simultaneous acquisition of samples through different detectors, e.g., measurement of fluorescence light intensities at different emission light wavelengths in fluorescence microscopy.
2.4 Photoacoustic imaging
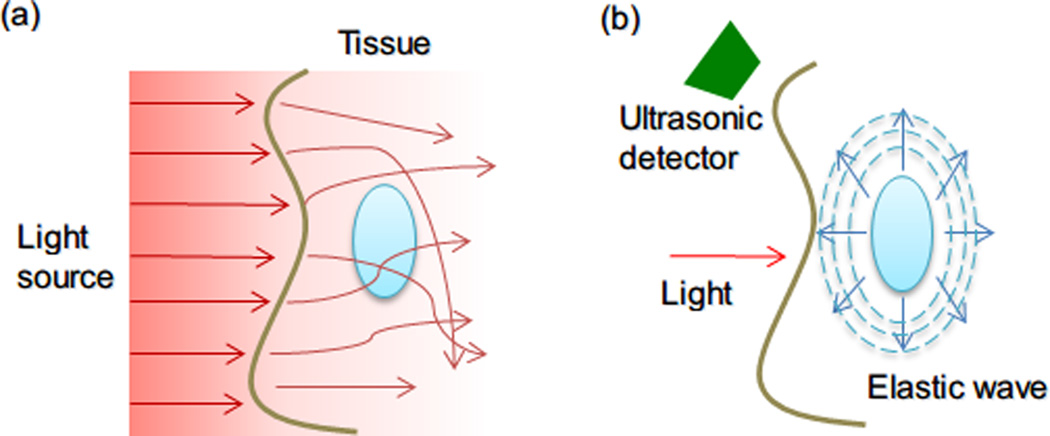
Photoacoustic (or optoacoustic) imaging is an ultrasonic imaging technique, in which wide-band ultrasonic waves can be induced by a pulsatile excitation laser (NIR laser) due to thermo-elastic expansion of tissues. Photoacoustic imaging for biological applications was first reported in the 1970s 68 but it was only recently that photoacoustic small-molecule probes have been explored as imaging agents.69 Photoacoustic imaging with NIR light can stimulate several centimeters of tissue; the loss of signal in photoacoustic imaging is negligible compared to other optical imaging techniques, since acoustic waves have 2–3 orders of magnitude less scattering in tissue than light (Figure 4).69a Small-molecule chromophores for photoacoustic imaging, however, suffer from fast clearance times and relatively small optical absorption cross sections. Inorganic NPs (e.g., carbon nanotubes and gold NPs70) have recently been shown to be improved contrast agents for photoacoustic imaging, with better photophysical properties and prolonged circulation times than small-molecule agents. The combination of photoacoustic tomography imaging techniques with the potential therapeutic effects from metallic NPs (e.g., photothermal therapy) may provide a strategy for simultaneous diagnosis and treatment of cancers.71 For example, hollow gold nanospheres surface-modified with cyclic arginine-glycine-aspartic acid (RGD) peptide were injected intravenously and targeted murine glioma tumors with overexpressed integrins. Tumors with accumulated hollow gold nanospheres could be imaged using photoacoustic technology.70c Accurate and efficient ablation of tumor by photothermal therapy were achieved by simply switching laser power from a power suitable for photoacoustic imaging level (50 mW/cm2) to one for photothermal therapy (16 W/cm2, 3 minutes).70c
Figure 4.
(a) Conventional optical imaging methods suffer from scattering in biological tissues. (b) Propagation of ultrasound in tissue. Using laser pulses to generate elastic pressure waves (ultrasound) allows high-resolution optical information to be obtained since ultrasonic scattering is two to three orders of magnitude lesser than optical scattering.
2.5 Photocaging
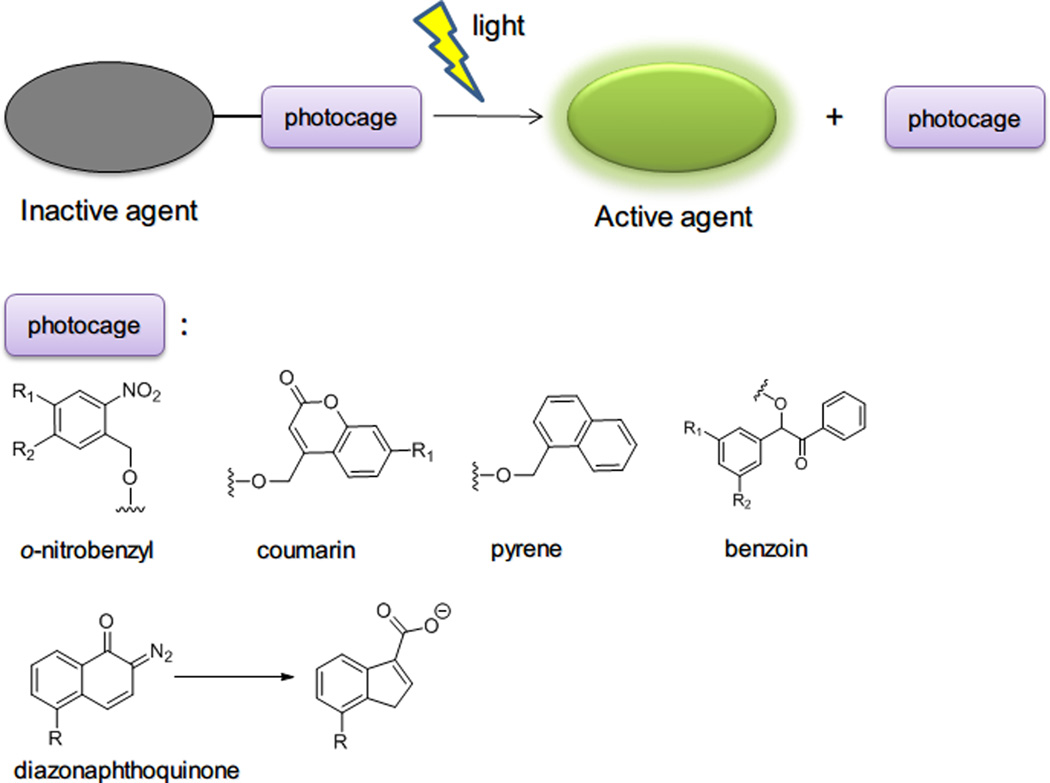
Photocaging refers to the temporary inactivation of a therapeutically or biologically active molecule by covalent linking with a photosensitive protection group.72 Light irradiation of the photosensitive moiety liberates the caged molecule in its active form at a desired site and time by a process called ‘photolysis’ or ‘photocleavage’. Several classes of photocaging groups have been developed73 including o-nitrobenzyl, coumarin-4-yl-methyl, p-hydroxyphenacyl, and 7-nitroindoline derivatives 74 with ester, amide, carbonate, carbamate, and phosphate linkages for photolysis (Figure 5). Many molecules including drugs, neurotransmitters, proteins and DNA, have been photocaged and then released, their dosages being precisely controlled by the applied light.75 The photocaging strategy has been further extended to light-triggered drug delivery: NPs loaded with drugs have been decorated with photocaging moieties. Drugs are released through disruption of particle structure (e.g., change of the particle hydrophilicity or ionic charge) or breaking a linkage between drugs and particles by photocleavage (Table 3).
Figure 5.
General photocaging strategy and commonly used photocaging protection groups.
Table 3.
Representative studies using photocaging and photoswitching groups for light-triggered drug delivery.
| NPs | Light | Photocaging group | Study goal | References |
|---|---|---|---|---|
| NaYF4:TmYb upconversion NPs | 980 nm | o-Nitrobenzyl group | Disrupt micelles to release payload | 117c |
| NaYF4:TmYb upconversion NPs | 980 nm | 1-(2-Nitrophenyl)ethyl group | Phototriggered release of D-luciferin | 117a |
| NaYF4:TmYb upconversion NPs | 980 nm | 3′, 5′-Di(carboxymethoxy)benzoin group | Phototriggered release of acetic acid | 117b |
| Gold NPs | 365 nm | o-Nitrobenzyl group | Phototriggered release of 5-fluorouracil | 110a |
| Gold NPs | 350 nm | o-Nitrobenzyl group | Phototriggrered release DNA by disrupting gold/DNA ionic complex | 110b |
| MSNPs | 413 nm | Azobenzene photoswitching linker | Phototriggered release by light-driven 'valve' | 122,142 |
| MSNPs with gold NPs | 365 nm | o-Nitrobenzyl group | Change gold NPs surface hydrophilicity to uncap Si NPs and released entrapped drugs | 123c |
| MSNPs capped with cyclodextrin | 350 nm | o-Nitrobenzyl group | Phototriggered uncap of the moieties blocking NP pores to release drugs | 123b |
| MSNPs capped with cyclodextrin | 400 nm or 800 nm | 7-Amino-courmarin group | Phototriggered release of chlorambucil | 124 |
| MSNPs | UV light >310 nm | 7-[(3-Triethoxysilyl)propoxy] coumarin group | Photocontrolled storage and release | 123a |
| PAMAM dendrimer | 360 nm | o-Nitrobenzyl group | Release doxorubicin | 133f |
| Photoresponsive cationic vesicles | 365 nm | Azobenzene photoswitching | Phototriggered release of DNA | 143 |
| NPs comprising amphiphilic polymers | 365 nm, 405 nm or 795 nm | Diazonaphthoquinone group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 77a,133d,e |
| NPs comprising amphiphilic polymers | 313 / >500 nm | Dithienylethene photoswitching group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 133c,133g |
| NPs comprising amphiphilic polymers | 365nm / 620 nm | Spiropyran photoswitching group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 133b |
| NPs comprising amphiphilic polymers | 360nm / 440 nm | Azobenzene photoswitching group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 133a,133m,133o |
| NPs comprising amphiphilic polymers | 375 nm | Pyrene group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 133h |
| NPs comprising amphiphilic polymers | 794 nm or 365 nm | Coumarin group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 77b |
| NPs comprising amphiphilic polymers | 365 nm or 700 nm | o-Nitrobenzyl group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 133i,133m,n |
| NPs comprising amphiphilic polymers | 365 nm | Dialkoxycyanostilbene photoswitching group | Disrupt NPs by change hydrophobic polymer to hydrophilic | 133j |
| NPs with coumarin crosslinker | 260 / 310 nm | Reversible photocrosslinking coumarin | Light induced crosslinking/ degradation | 133k |
| NPs with cinnamic acid crosslinker | >260 nm / < 260 nm or 254 nm/ 280 nm | Reversible photocrosslinking cinnamic acid | Light induced crosslinking/ degradation | 86,133l |
| Polyester NP containing a quinone−methide self-immolative moiety | 750 nm | o-Nitrobenzyl group | Photocleave the cage group to trigger the self-immolative group to degrade polymer by two-photon excitation | 134 |
| Polystyrene carboxylate NPs with caged peptide ligand | 365 nm | o-Nitrobenzyl group | Photocleave the cage group to activate NPs with targeting ligand for cell binding | 76 |
| Spiropyran/lipid-PEG NPs | 365nm/500–600nm | Reversible photoswitching spiropyran | Photoswitchable NPs size change for on-demand drug delivery and enhanced tissue penetration | 88 |
Abbreviations: NP, nanoparticle. MSNP, mesoporous silica NPs. PEG, polyethylene glycol. PAMAM, poly(amido amine).
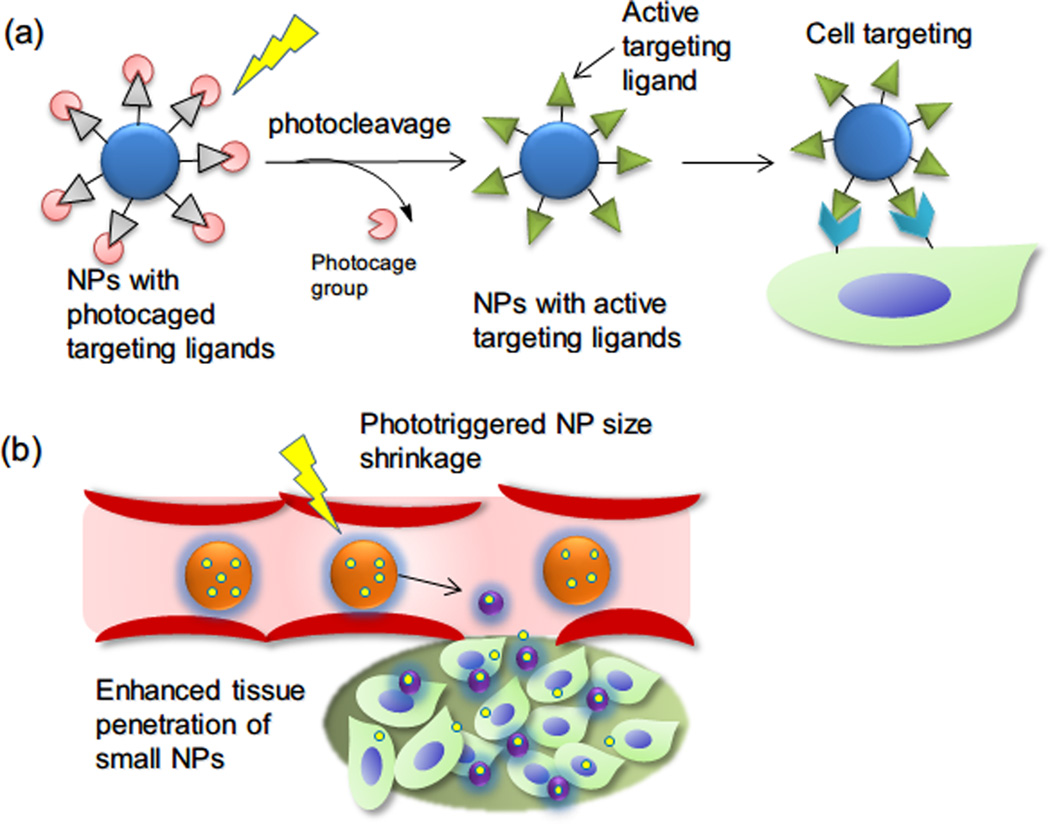
Recently we demonstrated a simple proof-of-concept whereby NPs can selectively target any tissue upon illumination (Figure 6a). NPs were functionalized with targeting peptide ligands (in this case, a peptide targeting highly prevalent integrins) that were inactivated by photocaging groups. Upon illumination the caging groups were released by photcleavage, revealing the targeting peptide ligands and allowing binding to cells. This approach could be useful for selectively depositing NPs at sites targeted by light.76 It also has the useful property of allowing nanoparticle targeting in the absence of a specific ligand to a particular tissue.
Figure 6.
Three commonly used photoswitching reactions in light-triggered drug delivery system.
Most photocaging groups, however, suffer a serious drawback for in vivo application: they require high-energy UV or visible light as the triggering source, but these cannot penetrate deeply into most tissues compared to NIR light. Two-photon excitation with NIR light may provide a practical solution to these issues for specific photocaging groups77, but many photocaging groups do not have large enough two-photon cross sections to be activated by NIR light78. Another solution is to employ NIR-absorbing particles that can emit UV light, which has sufficient energy to activate the photocaging groups. Upconversion NPs are good candidates for converting NIR laser light into different wavelengths of UV and visible light and therefore can drive the liberation of photocaging groups in NPs (see section 3.2 and Table 3).
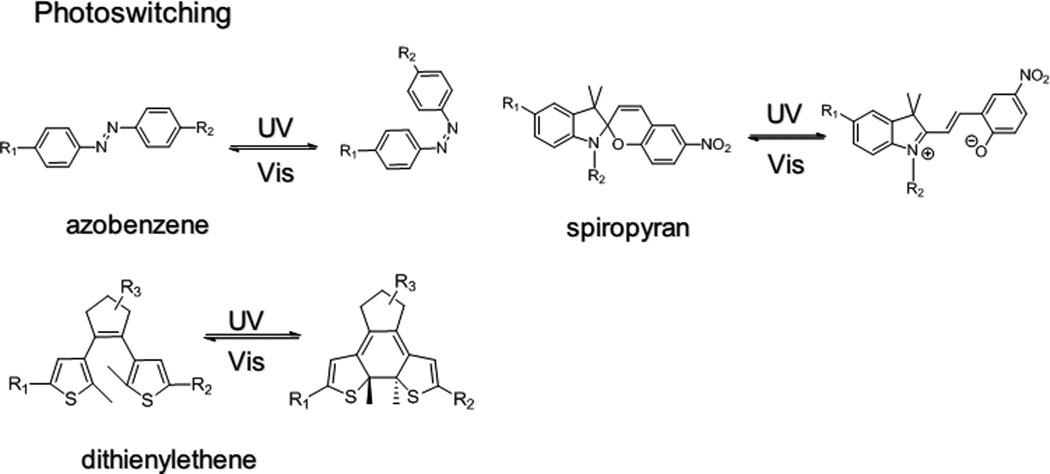
2.6 Photoswitching
Photoswitching refers to light-induced reversible structural changes between two isomers with different properties (molecular geometry, dipole, charge etc.).79 The reversible conversion between two isomers is usually controlled by UV or visible light,79a although recently two-photon technology using NIR light has been used for this purpose instead of UV light.80 Commonly used photoswitching molecules include azobenzenes, spiropyran, dithienylethene, and stilbene (Figure 7).
Figure 7.
(a) Schematic of photo-targeted polymeric NPs. Peptide targeting ligands (grey triangle) are inactivated by photocaging with o-nitrobenzyl groups (pink circle). Illumination leads to cleavage of the photocaged group and reveals the active targeting ligand (green triangle), with subsequent binding of the targeted NPs to the irradiated tissue sites. (b) Photoswitchable NPs (150 nm, orange spheres) can shrink to 40 nm (purple spheres) upon light illumination. The smaller NPs will have enhanced tissue penetration. Release of drugs (bright yellow spheres) loaded in the NPs is simultaneously triggered by light.
Light-induced molecular structural changes in photoswitching moieties can often be magnified in polymer networks or nanomaterials, leading to macroscopic shape deformation (contraction,81 bending,82 rotation,83 swimming,84 ciliary motion85), or physical property changes86 (such as hydrophilicity, viscosity and permeability etc.). NPs with photoswitching moieties have been investigated as light-triggerable drug delivery systems87 which can release drugs repetitively. We recently developed a spiropyran-based drug delivery NP that exhibited enhanced tissue penetration properties upon light irradiation, presumably because of a light-induced reversible volume change from 150 to 40 nm (Figure 6b).88 The volume change of the monodisperse NPs also enabled repetitive drug release.
3 Photo-excited NPs
3.1 Gold NPs
Gold NPs can strongly absorb light in the visible and NIR range with absorption coefficients orders of magnitude larger than those of most small molecule dyes that are used as sensors and contrast agents. (Gold NPs extinction coefficients are ~ 107–109 M−1·cm−1; many small molecule photoactives have coefficients ~ 104–105 M−1·cm−1). This advantageous optical property is derived from the localized surface plasmon resonance of gold NPs, which differs from the light-absorbing mechanism of organic dyes.89 Due to this strong absorption, gold NPs and their bioconjugates have been found to be excellent sensors90 for applications including genomics,91 immunoassays,92 detection of microorganisms,93 and clinical chemistry94 etc. In addition gold NPs are also been applied as novel contrast agents for biomedical diagnosis in various technologies, e.g., polarized resonance scattering,95 optical coherence tomography,96 two-photon luminescence,97 surface enhanced Raman scattering imaging98 and photoacoustic techniques.69b,70d Furthermore, since the light absorbed by gold NPs can be efficiently converted into heat on a picosecond time scale,29 gold NPs can be used as photoactive agents in photothermal therapy for cancer treatment.99
The optical properties of gold NPs change dramatically depending on their size, shape and geometry6b. Consequently, various gold nanostructures with absorption peaks in the NIR range have been developed for photothermal therapy, including nanoshells,100 nanorods,101 nanocages,102 etc.103 Spherical gold NPs with absorption peaks in the visible region of the spectrum have also been studied for cancer research.42a Gold NPs can be also used as the heat source for thermal-responsive drug delivery systems. In those cases thermoresponsive polymers were coated on gold nanostructure surfaces, 104 and loaded drugs were released in response to light-induced changes in the structure of the polymer/gold assemblies. The use of light to trigger drug release from NPs has been extensively reviewed.14
Low intensity laser irradiation of gold NPs for 20–30 min can generate sufficient reactive oxygen species to induce cytotoxicity. Importantly, this can be achieved without photothermal effects.105 At high laser intensity, it is likely that both non-thermal mechanism and photothermal mechanism contribute to cell death.
The photothermal properties of gold NPs are also useful in rendering conventional drug delivery nanocarriers (e.g., liposomes) light-triggerable. Upon irradiation, gold NPs increase the temperature in their immediate environment and induce micro-bubble formation, leading to the disruption of liposomes and drug release. The method by which gold NPs are coupled to the nanocarriers affects the drug release rate. For example, tethering of gold nanoshells to liposomes via a lipid-PEG-thiol linker enhanced release in response to a photic stimulus compared to that for gold NPs free outside of liposomes or encapsulated inside liposomes.106
The photothermal properties of gold NPs have been utilized to enhance the accumulation of subsequently administered conventional nanocarriers in tumors. Systemically administered gold NPs were triggered by NIR light to photothermally disrupt tumor vessels. Tumor heating by the same NPs initiated extravascular coagulation leading to local overexpression of fibrin. A second group of NPs, which were surface-modified with fibrin-binding peptide, were administered 72 hours later to target the induced over-expression of fibrin. The accumulation of the second NPs in tumor was thereby enhanced tenfold over only dosing with second NPs.107 This enhanced accumulation in tumor led to improved efficacy in a mouse xenograft tumor: treatment with NIR-triggered gold nanorods followed by liposomes containing doxorubicin, was more effective than treatment with the individual NPs.107–108
Gold NPs for photothermal therapy can be integrated with other treatments (see Table 4). For example, gold NPs complexed with photosensitizers for PDT can be used for combined PDT and photothermal therapy.109 Gold NPs surfaces can be also modified with photocaging moieties for light-triggered drug or gene delivery (see Table 3).110
Table 4.
Representative studies of NPs used in phototherapy
| NPs | Light | Modality examined | Notes | References |
|---|---|---|---|---|
| Surface enhanced Raman scattering gold nanorod | 810 nm | Photothermal therapy and multimodal imaging | Raman scattering-active molecules coated onto PEG-NRs | 98 |
| Gold nanoshell | 754 nm | Photothermal therapy | Monocytes containing NS infiltrate the tumor spheroids | 144 |
| Gold NPs with liposome | 830 nm | Photoinduced heating to form transient vapor bubbles | Disrupt liposome to release payload | 145 |
| Gold nanoshells with liposome | 800 nm | Photoinduced heating to form transient vapor bubbles | Disrupt liposome to release payload | 106 |
| Gold nano-popcorn | 785 nm | Photothermal therapy | Surfaced coated with Raman scattering-active molecule for targeted sensing | 146 |
| Gold nanocages with thermal-sensitive PNIPAM | NIR Ti:sapphire laser | Photothermal therapy to trigger drug release | PNIPAM collapsed once heated to released payload | 104 |
| Gold nanorod with photosensitizer | 810 nm | Photothermal therapy, PDT and imaging | Photosensitizers is quenched on gold nanorod surface until they are released | 109 |
| Magnetic Fe3O4 with gold nanoshell | 800 nm | MRI and photothermal therapy | 147 | |
| Gold nanorod | 800 nm and 1100nm | Selective melting of nanorods by different wavelength laser irradiation | Selective delivery and release multiple DNAs | 148 |
| FeCo graphitic-shell nanocrystal | 808nm | Photothermal therapy | 149 | |
| Gold nanorod | 810nm | Photothermal therapy | Reduce xenograft tumor | 39b,150 |
| Gold nanoshell | 808nm | Photoacoustic imaging and phothermal therapy | Reduce xenograft tumor | 70c,151 |
| Gold nanorods + targeted NPs loaded with drugs | 810nm | Photothermal heating tumor to induce coagulation, which second NPs with peptide ligand will target | Reduce xenograft tumor | 107b,108 |
| Silica NPs entrapping photosensitizer | 650 nm | Imaging and PDT | Kill cancer cells | 152 |
| CuS NPs | 808 nm | Photothermal therapy and PET/CT imaging | Reduce xenograft tumor | 130 |
| Photosensitizer-core dendrimer with DNA/cationic peptide complex | 689nm | Selective photodamage of the endosome | Gene delivery | 27,51a,153 |
| Dendrimer encapsulating photosensitizer, complex with cationic polymer | 400–700nm xenon lamp | PDT | Kill cancer cells | 51b |
| PLGA NPs encapsulating photosensitizer | 650nm | PDT | Reduce tumor | 24 |
| Porphyrin lipsome conjugates | 673 nm | PDT and imaging | Reduced tumor | 136 |
| F3 peptide functionalized, polyacrylamide NP loaded with photofrin and iron oxide | 630 nm | PDT | Reduced tumor | 154 |
| Polyallyamine NPs loaded with indocyanine green | 808 nm | Photothermal therapy | 40 | |
| porous Si NPs | Halogen lamp with IR filter | PDT, generation of singlet oxygen | Kill cancer cells | 155 |
Abbreviation: NP, nanoparticle. PDT, photodynamic therapy. NIR, near-infrared. PNIPAM, poly(N-isopropylacrylamide). PLGA, poly(lactic-co-glycolic acid). PET, positron emission tomography. CT, X-ray computed tomography. MRI, magnetic resonance imaging.
The biocompatibility and biodistribution of gold NPs remain incompletely understood; they have been extensively investigated with contradictory conclusions.111 This heterogeneity in reporting occurs partly because gold NPs with different sizes, shapes, and surface chemistries have different biological effects in vitro and in vivo.18b,111
3.2 Upconversion NPs
Most fluorophores emit light at a longer wavelength (lower energy) than that of their excitation wavelength (so-called ‘downconverting photoluminescence’, or Stokes emission). In contrast, upconverting NPs can be excited with continuous-wave (power is constant over the time, in contrast to pulsed lasers) NIR light (900–1000 nm) to emit at shorter wavelengths such as visible and UV light.33a,112 The detailed physical mechanism of the upconversion process (anti-Stokes emission) was discovered in the 1960s and has been reviewed elsewhere.33a
Upconverting NPs are usually NaYF4 NPs doped with trivalent rare-earth ions (Yb3+, Tm3">3+, Er3+, Ho3+ etc.). Such upconversion NPs have attracted considerable attention in bioimaging applications due to their large anti-Stokes shifts (> 400 nm), sharp emission bandwidths, high resistance to photobleaching, stable emission, ability to be detected deep within tissue (using NIR light), and ability to undergo surface modification with biomolecules.33b Importantly, relative low power density NIR lasers (~500 mW·cm−2) can be used for small animal whole-body upconverting imaging, which is much less than the energy required in two-photon excitation imaging (~106–109 W·cm−2).113 Tissue overheating (and associated phototoxicity) can occur when using upconverting NPs because 980 nm light is strongly absorbed by water. Such potential photic injury can be minimized by reducing the wavelength from 980 nm to 915 nm, using NaYbF4 upconversion NPs co-doped with Yb3+ and other lanthanide ions.114 Upconverting luminescence imaging systems are laboratory-based techniques at this time;115 none are yet commercially available. Preliminary studies using NaYF4 upconversion NPs coated with PEG or polyacrylic acid have been found to have no apparent toxicity or adverse effects in vitro and in vivo in mice.116
Upconverting photoluminescence can be coupled with photocaging to create photocaged upconverting NPs: the upconversion of NIR light to UV light can trigger the uncaging process and liberate the encapsulated payload. This can be especially useful since most photocaging groups require the higher energy of UV light to activate (Figure 3). Representative studies of this type are listed in Table 3, including upconverting NPs using nitrobenzyl and benzoin-type caging groups.117
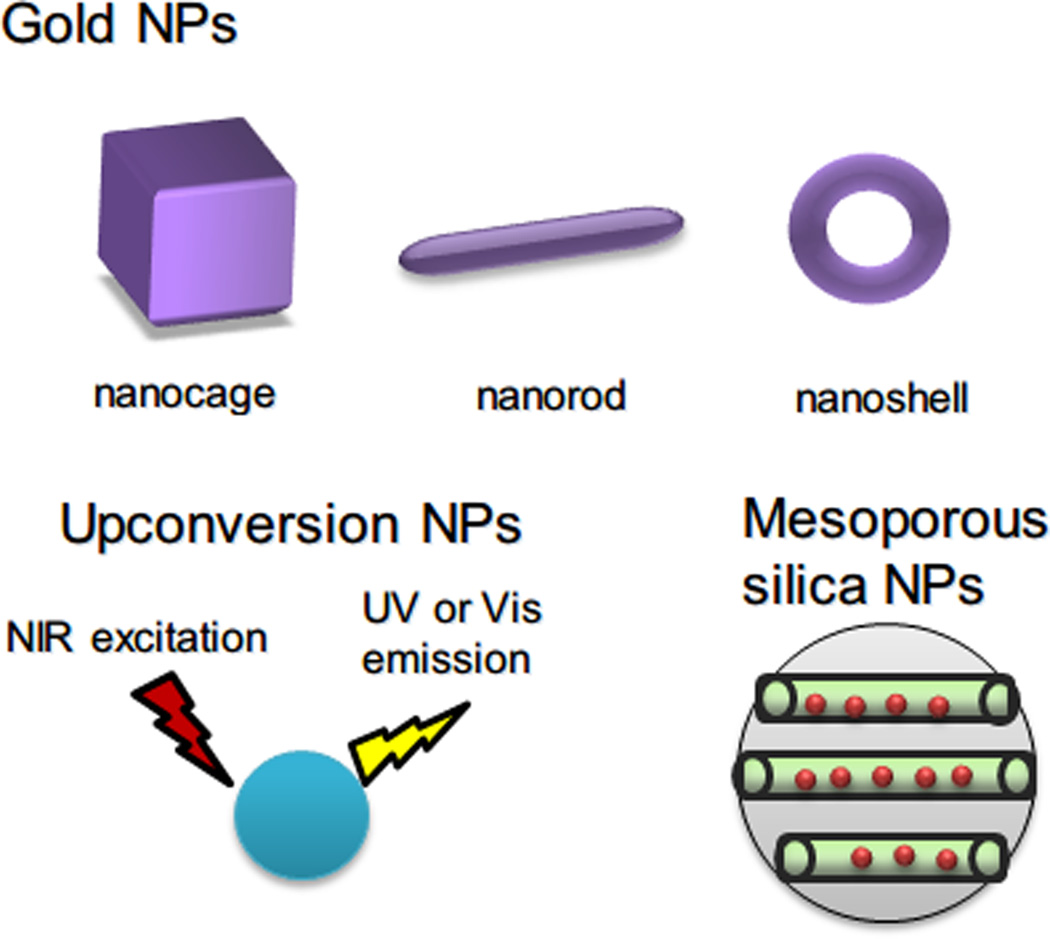
Figure 3.
Sample inorganic NPs used in phototherapy: gold NPs with various shapes and sizes, upconversion NPs, which can be excited by NIR light to emit UV or visible light, and mesoporous silica NPs, which contains porous nanostructure to encapsulate drugs inside.
Upconverting NPs have been coated with other inorganic nanocomposites (e.g., mesoporous silica)118 or polymeric materials (e.g., PEG or polylactide-PEG)119 containing photosensitizers for PDT. The NPs are irradiated with NIR then emit light at a wavelength equal to that of the excitation band of the photosensitizers incorporated in the polymer or silica nanocomposites. Upconverting NPs have been coated with Ag nanocomposites so that NIR “upconverted” to shorter wavelengths will trigger heating from the silver shell, which has surface plasmonic resonance absorption in the visible spectrum region (500–600 nm).120
3.3 Silica NPs
Mesoporous silica nanoparticles (MSNPs) 121 have a honeycomb-like porous structure with hexagonal channels (diameters vary ~ 2–30 nm) which enables physical absorption or encapsulation of therapeutic agents. As a result of this desirable property, MSNPs have been explored as nanocarriers for drug delivery. MSNPs are also optically transparent, which is advantageous for photic control and spectroscopic monitoring of encapsulated chromogenic species.
Light has been used to modulate drug release from MSNPs by opening and closing the pores. This has been achieved by grafting the pores with molecules that can be photoactivated such as photoswitching azobenzene groups 122, and reversible photodimerization coumarin derivatives (Table 3). Photocaging o-nitrobenzyl derivatives have been also used as photo-removable ‘caps’ to regulate pore opening in MSNPs.123
Two-photon NIR light excitation has been used to modulate release of an anticancer drug from MSNPs. The walls of MSNPs were functionalized with 7-amino-coumarin, which capped the pores of MSNPs to prevent the release of the anticancer drug chlorambucil. The coumarin derivative had a sufficiently high two-photon absorption cross section to allow efficient and precisely regulated release of the drug upon NIR two-photon excitation with 800 nm light. Light-dependent cytotoxicity was observed in cancer cells, indicating light-triggered release of the anticancer drug.124
MSNPs may cause toxicity when administered in large doses,125 perhaps because intravascular coagulation. A comprehensive investigation of the safety of practical dosages is necessary for future clinical application of these particles.126
3.4 Other inorganic NPs
Potential toxicity is a major concern for many inorganic NPs, due to the toxicity of heavy metal or rare earth metal in NPs. Toxicity can be mitigated by modifying their composition or by optimizing NPs clearance from the body. There are two primary routes of clearance of NPs from the body: renal filtration with excretion into urine, and hepatobiliary (liver, bladder and bile duct) processing with excretion into bile. Inorganic NPs larger than the renal excretion limit (< ~8–10 nm in diameter) have a tropism for hepatobiliary processing, which is slower and increases the risk of toxicity of heavy metals.127 Potentially toxic inorganic NPs should be made especially small128 so that they can be excreted via the kidney.129 Therefore, a major challenge with such inorganic NPs-based molecular probes is synthesizing agents that exhibit the unique photophysical features of NPs (e.g., brightness) but can be also excreted via the urinary tract to minimize potential toxicity.
CuS NPs are a new class of photoactives for photothermal therapy that have absorption peaks in the NIR region (900–1100 nm). These NPs have a surface plasmon absorption band in the NIR region similar to that of gold nanostructures, but the NPs are much smaller (<15 nm), which makes them more likely to reach their targets and more readily cleared by the renal system. 64Cu-labeled CuS NPs can be used for photothermal therapy and also permit PET imaging and radiotherapy (Table 4).130
Quantum dots, containing heavy metals such as cadmium and selenium, were initially proposed as imaging agents.131 The major concern limiting their use is the toxicity of cadmium. Efforts to make non-heavy metal quantum dots for biological application are ongoing.127,128b
3.5 Organic NPs
Optically active inorganic NPs have not yet achieved broad clinical implementation, possibly stemming from (i) drug loading typically being limited to the NP surface and (ii) concerns regarding long-term safety and biocompatibility. In contrast, organic NPs such as liposomes, polymeric micelles, polymersomes, and dendrimers have already found many human therapeutic applications because of their favorable biocompatibility and excellent drug-loading capacity, by encapsulation or conjugation.132 Drugs inside such NPs can be protected from degradation until they reach the targeted disease sites.17a,17c
One disadvantage in using organic NPs for phototherapy is that the majority of current organic NPs do not intrinsically absorb light. One strategy to remedy this shortcoming is to impart photoactive moieties to organic NPs. For example, the incorporation of photocleavable or photoswitching groups can either disrupt or deform the assembly structure of NPs, so that light can be used to precisely control the release of loaded drugs or genes.77,86,133 In many cases, photocaging and photoswitching moieties can only be activated by UV or visible light; the use of two-photon excited photocaging groups in organic NPs may allow the use of NIR for this purpose.
In a recent example of triggering organic NPs with two-photon NIR light, particles were made of a quinone-methide self-immolative polymeric backbone modified with two-photon excited photocaging groups. Upon NIR irradiation, the caging groups were cleaved and the polymer degraded automatically to release loaded dyes.134 The use of organic NPs to combine photothermal therapy with chemotherapy has recently garnered interest. Photothermal therapy may potentially improve the chemotherapy efficacy of organic NPs containing drugs.135 For example, nanoliposomes composed of lipid conjugates of pyropheophorbide (a chlorin analogue) can efficiently absorb and transfer light energy into heat for photothermal therapy, with an extinction coefficient of ~109 M−1·cm−1.136 The liposomal nanocarrier with pyropheophorbide can also deliver a large amount of doxorubicin. The effectiveness of laser ablation of tumors is improved over that of NPs without laser treatment. In this example, both systemic drug delivery and light-triggered phototherapy were combined a rationally designed organic NP having a photothermal efficiency comparable to that of gold NPs. The photothermal efficiency of these liposomal NPs is attributable to the fact that the pyropheophorbide inside liposomal bilayers dissipated the absorbed photonic energy as heat rather than as fluorescence emission or photo-generation of singlet oxygen, as occurs with free pyropheophorbide. As the above example suggests, the photochemistry of photoactives may differ whether they are in the free molecule state or encapsulated within NPs. Therefore the selection and/or design of the photoactive agents and their organic NPs are important.34,137
4. Conclusion and outlook
Nanomedicine is one of the most rapidly growing fields of translational medicine,17e and can have marked impacts on the toxicity and efficacy of therapies. The convergence of phototherapy and nanomedicine may allow the development of patient-individualized treatments (e.g. on demand drug delivery) and provide new therapeutic modalities (e.g. new nano-photosensitizer formulations) that are easy to apply throughout the body in a targeted manner. Progress in the field will depend on a fundamental understanding of photophysics, chemistry, materials science, electronics, biology and clinical practice to allow rational design of optimized formulations, tools for delivering them and/or light, and measure outcomes (e.g. by imaging). Advances in nanoscience will also obviously play a key role in advances at the interface between nanotechnology and phototherapy, and will impact many aspects of therapy, including targeting, biodistribution (pharmacokinetics)138 and NP penetration into diseased tissues.139 Biocompatibility and phototoxicity will remain important issues for new nano-photosensitizers.25,140
Acknowledgments
The work was supported by a grant from Sanofi-Aventis, and NIH (R21DC009986).
References
- 1.Starr C, Evers CA, Starr L. Biology: concepts and applications. 7 ed. Brooks Cole; 2007. [Google Scholar]
- 2.Einstein A. Ann. Phys.-Berlin. 1905;17:132. [Google Scholar]
- 3.Finsen NR. Phototherapy. London: Edward Arnold; 1901. [Google Scholar]
- 4.Szaciłowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G. Chemical Reviews. 2005;105:2647. doi: 10.1021/cr030707e. [DOI] [PubMed] [Google Scholar]
- 5.Ntziachristos V, Ripoll J, Weissleder R. Opt. Lett. 2002;27:333. doi: 10.1364/ol.27.000333. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Nat Biotech. 2005;23:313. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]; (b) Weissleder R. Nat Biotech. 2001;19:316. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan S, Pogue BW, Jiang S, Dehghani H, Kogel C, Soho S, Gibson JJ, Tosteson TD, Poplack SP, Paulsen KD. Proceedings of the National Academy of Sciences. 2003;100:12349. doi: 10.1073/pnas.2032822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry S, Burke R, Brown E. Annals of Biomedical Engineering. 2012;40:277. doi: 10.1007/s10439-012-0512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung ELM, Schnitzer MJ. Nat Meth. 2005;2:941. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yelin D, Rizvi I, White WM, Motz JT, Hasan T, Bouma BE, Tearney GJ. Nature. 2006;443:765. doi: 10.1038/443765a. [DOI] [PubMed] [Google Scholar]
- 11.Brancaleon L, Moseley H. Lasers in Medical Science. 2002;17:173. doi: 10.1007/s101030200027. [DOI] [PubMed] [Google Scholar]
- 12.Bhawalkar JD, Kumar ND, Zhao CF, Prasad PN. Journal of clinical laser medicine & surgery. 1997;15:201. doi: 10.1089/clm.1997.15.201. [DOI] [PubMed] [Google Scholar]
- 13.Youssef PN, Sheibani N, Albert DM. Eye. 2011;25:1. doi: 10.1038/eye.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timko BP, Dvir T, Kohane DS. Adv Mater. 2010;22:4925. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 15.(a) Parver LM, Auker CR, Fine BS. Ophthalmology. 1983;90:964. doi: 10.1016/s0161-6420(83)80024-4. [DOI] [PubMed] [Google Scholar]; (b) Tso MOM, Woodford BJ. Ophthalmology. 1983;90:952. doi: 10.1016/s0161-6420(83)80023-2. [DOI] [PubMed] [Google Scholar]; (c) Reichel E. N. Engl. J. Med. 1994;330:1320. doi: 10.1056/NEJM199405053301822. [DOI] [PubMed] [Google Scholar]
- 16.Moghimi SM, Hunter AC, Murray JC. The FASEB Journal. 2005;19:311. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 17.(a) Jain RK, Stylianopoulos T. Nat. Rev. Clin. Oncol. 2010;7:653. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wagner V, Dullaart A, Bock AK, Zweck A. Nat. Biotechnol. 2006;24:1211. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]; (c) Langer R. Nature. 1998;392:5. [PubMed] [Google Scholar]; (d) Cai W, Chen X. Small. 2007;3:1840. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]; (e) Weldon C, Tian B, Kohane DS. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2011;3:223. doi: 10.1002/wnan.128. [DOI] [PubMed] [Google Scholar]
- 18.(a) Farokhzad OC, Langer R. ACS Nano. 2009;3:16. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]; (b) Chithrani BD, Ghazani AA, Chan WCW. Nano Letters. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 19.(a) Adair BM. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;1:405. doi: 10.1002/wnan.45. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim PS, Read SW. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2010;2:693. doi: 10.1002/wnan.118. [DOI] [PubMed] [Google Scholar]; (c) Sheng W-Y, Huang L. Pharm. Res. 2011;28:200. doi: 10.1007/s11095-010-0258-8. [DOI] [PubMed] [Google Scholar]; (d) Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Nat. Med. 2010;16:1035. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) De Temmerman M-L, Rejman J, Demeester J, Irvine DJ, Gander B, De Smedt SC. Drug Discov. Today. 2011;16:569. doi: 10.1016/j.drudis.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Proceedings of the National Academy of Sciences. 1998;95:4607. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Nostrum CF. Advanced Drug Delivery Reviews. 2004;56:9. doi: 10.1016/j.addr.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Samkoe KS, Clancy AA, Karotki A, Wilson BC, Cramb DT. Journal of Biomedical Optics. 2007;12:034025. doi: 10.1117/1.2750663. [DOI] [PubMed] [Google Scholar]
- 23.Some recent reviews on the application of nanoparticles in phototherapy: Sortino S. Photochemical & Photobiological Sciences. 2008;7:911. doi: 10.1039/b807353h. Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T. Advanced Drug Delivery Reviews. 2010;62:1094. doi: 10.1016/j.addr.2010.09.002. Sortino S. Journal of Materials Chemistry. 2012;22:301.
- 24.McCarthy JR, Perez JM, Brückner C, Weissleder R. Nano Letters. 2005;5:2552. doi: 10.1021/nl0519229. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee DK, Fong LS, Zhang Y. Advanced Drug Delivery Reviews. 2008;60:1627. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Allémann E, Rousseau J, Brasseur N, Kudrevich SV, Lewis K, van Lier JE. International Journal of Cancer. 1996;66:821. doi: 10.1002/(SICI)1097-0215(19960611)66:6<821::AID-IJC19>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Ideta R, Tasaka F, Jang W-D, Nishiyama N, Zhang G-D, Harada A, Yanagi Y, Tamaki Y, Aida T, Kataoka K. Nano Letters. 2005;5:2426. doi: 10.1021/nl051679d. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Jain P, El-Sayed I, El-Sayed M. Lasers in Medical Science. 2008;23:217. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 29.Link S, El-Sayed MA. International Reviews in Physical Chemistry. 2000;19:409. [Google Scholar]
- 30.Link S, Burda C, Nikoobakht B, El-Sayed MA. The Journal of Physical Chemistry B. 2000;104:6152. [Google Scholar]
- 31.Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. Chemical Society Reviews. 2012 doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Gao R, Zhou F, Selke M. Journal of Materials Chemistry. 2004;14:487. [Google Scholar]
- 33.(a) Auzel F. Chemical Reviews. 2003;104:139. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]; (b) Wang F, Liu X. Chemical Society Reviews. 2009:38. doi: 10.1039/b809132n. [DOI] [PubMed] [Google Scholar]
- 34.Jori G, Spikes JD. Journal of Photochemistry and Photobiology B: Biology. 1990;6:93. doi: 10.1016/1011-1344(90)85078-b. [DOI] [PubMed] [Google Scholar]
- 35.(a) Overgaard J, Bentzen SM, Overgaard J, Gonzalez Gonzalez D, Hulshof MCCM, Arcangeli G, Dahl O, Mella O. The Lancet. 1995;345:540. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]; (b) Svaasand LO, Gomer CJ, Morinelli E. Lasers in Medical Science. 1990;5:121. [Google Scholar]; (c) Overgaard J. International Journal of Radiation Oncology, Biology, Physics. 1989;16:535. doi: 10.1016/0360-3016(89)90470-7. [DOI] [PubMed] [Google Scholar]
- 36.Sapareto SA, Dewey WC. International Journal of Radiation Oncology, Biology, Physics. 1984;10:787. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 37.(a) Rosenberg C, Puls R, Hegenscheid K, Kuehn J, Bollman T, Westerholt A, Weigel C, Hosten N. American Journal of Roentgenology. 2009;192:785. doi: 10.2214/AJR.08.1425. [DOI] [PubMed] [Google Scholar]; (b) Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SRH, Atri M, Wilson BC, Fenster A, Trachtenberg J. The Journal of Urology. 2009;182:1371. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 38.Camerin M, Rello S, Villanueva A, Ping X, Kenney ME, Rodgers MAJ, Jori G. European Journal of Cancer. 2005;41:1203. doi: 10.1016/j.ejca.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 39.(a) Fiedler VU, Schwarzmaier H-J, Eickmeyer F, Müller FP, Schoepp C, Verreet PR. Journal of Magnetic Resonance Imaging. 2001;13:729. doi: 10.1002/jmri.1101. [DOI] [PubMed] [Google Scholar]; (b) von Maltzahn G, Park J-H, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Cancer Research. 2009;69:3892. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Javier D, Yaseen MA, Nitin N, Richards-Kortum R, Anvari B, Wong MS. Journal of the American Chemical Society. 2010;132:1929. doi: 10.1021/ja908139y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson HH, Carlson MT, Tandler PJ, Hernandez P, Govorov AO. Nano Letters. 2009;9:1139. doi: 10.1021/nl8036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.(a) El-Sayed IH, Huang X, El-Sayed MA. Cancer Letters. 2006;239:129. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]; (b) Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, Stafford J, Olson T, Zhang JZ, Li C. Molecular Cancer Therapeutics. 2008;7:1730. doi: 10.1158/1535-7163.MCT-08-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.(a) Dolmans DEJGJ, Fukumura D, Jain RK. Nat Rev Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]; (b) Dougherty TJ, Dougherty TJ, Gomer CJ, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Journal of the National Cancer Institute. 1998;90:889. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolmans DEJGJ, Kadambi A, Hill JS, Waters CA, Robinson BC, Walker JP, Fukumura D, Jain RK. Cancer Research. 2002;62:2151. [PubMed] [Google Scholar]
- 45.(a) Gollnick SO, Vaughan L, Henderson BW. Cancer Research. 2002;62:1604. [PubMed] [Google Scholar]; (b) Korbelik M, Dougherty GJ. Cancer Research. 1999;59:1941. [PubMed] [Google Scholar]; (c) Castano AP, Mroz P, Wu MX, Hamblin MR. Proceedings of the National Academy of Sciences. 2008;105:5495. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.(a) MacDonald IJ, Dougherty TJ. J. Porphyr. Phthalocyanines. 2001;5:105. [Google Scholar]; (b) Castano AP, Mroz P, Hamblin MR. Nat. Rev. Cancer. 2006;6:535. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Nat Med. 2011;17:1685. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovell JF, Liu TWB, Chen J, Zheng G. Chemical Reviews. 2010;110:2839. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 48.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Chemical Reviews. 2010;110:2795. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.(a) Vrouenraets MB, Visser GWM, Snow GB, van Dongen G. Anticancer Res. 2003;23:505. [PubMed] [Google Scholar]; (b) Carcenac M, Dorvillius M, Garambois V, Glaussel F, Larroque C, Langlois R, Hynes NE, van Lier JE, Pelegrin A. Br J Cancer. 2001;85:1787. doi: 10.1054/bjoc.2001.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Vrouenraets MB, Visser GWM, Stewart FA, Stigter M, Oppelaar H, Postmus PE, Snow GB, van Dongen GAMS. Cancer Research. 1999;59:1505. [PubMed] [Google Scholar]
- 50.Grossweiner LI, Patel AS, Grossweiner JB. Photochemistry and Photobiology. 1982;36:159. doi: 10.1111/j.1751-1097.1982.tb04358.x. [DOI] [PubMed] [Google Scholar]
- 51.(a) Nishiyama N, Iriyama A, Jang W-D, Miyata K, Itaka K, Inoue Y, Takahashi H, Yanagi Y, Tamaki Y, Koyama H, Kataoka K. Nat Mater. 2005;4:934. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]; (b) Jang W-D, Nishiyama N, Zhang G-D, Harada A, Jiang D-L, Kawauchi S, Morimoto Y, Kikuchi M, Koyama H, Aida T, Kataoka K. Angewandte Chemie International Edition. 2005;44:419. doi: 10.1002/anie.200461603. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, Ohulchanskyy TY, Pudavar HE, Pandey RK, Prasad PN. Journal of the American Chemical Society. 2007;129:2669. doi: 10.1021/ja0680257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denk W, Strickler J, Webb W. Science. 1990;248:73. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 54.Helmchen F, Denk W. Nat Meth. 2005;2:932. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 55.Starkey JR, Rebane AK, Drobizhev MA, Meng FQ, Gong AJ, Elliott A, McInnerney K, Spangler CW. Clinical Cancer Research. 2008;14:6564. doi: 10.1158/1078-0432.CCR-07-4162. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, Zheng Q, Dakin K, Xu K, Martinez ML, Li W-H. Journal of the American Chemical Society. 2004;126:4653. doi: 10.1021/ja036958m. [DOI] [PubMed] [Google Scholar]
- 57.Starkey JR, Rebane AK, Drobizhev MA, Meng F, Gong A, Elliott A, McInnerney K, Spangler CW. Clinical Cancer Research. 2008;14:6564. doi: 10.1158/1078-0432.CCR-07-4162. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez EJ, Novotny L, Xie XS. Physical Review Letters. 1999;82:4014. doi: 10.1103/PhysRevLett.90.095503. [DOI] [PubMed] [Google Scholar]
- 59.König K. Journal of Microscopy. 2000;200:83. doi: 10.1046/j.1365-2818.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 60.Collins HA, Khurana M, Moriyama EH, Mariampillai A, Dahlstedt E, Balaz M, Kuimova MK, Drobizhev M, YangVictor XD, Phillips D, Rebane A, Wilson BC, Anderson HL. Nat Photon. 2008;2:420. [Google Scholar]
- 61.(a) Ogawa K, Ohashi A, Kobuke Y, Kamada K, Ohta K. Journal of the American Chemical Society. 2003;125:13356. doi: 10.1021/ja035056i. [DOI] [PubMed] [Google Scholar]; (b) Drobizhev M, Stepanenko Y, Dzenis Y, Karotki A, Rebane A, Taylor PN, Anderson HL. Journal of the American Chemical Society. 2004;126:15352. doi: 10.1021/ja0445847. [DOI] [PubMed] [Google Scholar]; (c) Drobizhev M, Stepanenko Y, Dzenis Y, Karotki A, Rebane A, Taylor PN, Anderson HL. The Journal of Physical Chemistry B. 2005;109:7223. doi: 10.1021/jp044261x. [DOI] [PubMed] [Google Scholar]; (d) Kuimova MK, Hoffmann M, Winters MU, Eng M, Balaz M, Clark IP, Collins HA, Tavender SM, Wilson CJ, Albinsson B, Anderson HL, Parker AW, Phillips D. Photochemical & Photobiological Sciences. 2007:6. doi: 10.1039/b700557a. [DOI] [PubMed] [Google Scholar]
- 62.Pawlicki M, Collins HA, Denning RG, Anderson HL. Angewandte Chemie International Edition. 2009;48:3244. doi: 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]
- 63.(a) Patrice T, Le Bodic M-F, Le Bodic L, Spreux T, Dabouis G, Hervouet L. Cancer Research. 1983;43:2876. [PubMed] [Google Scholar]; (b) Marchesini R, Melloni E, Fava G, Pezzoni G, Savi G, Zunino F, Docchio F. Lasers in Surgery and Medicine. 1986;6:323. doi: 10.1002/lsm.1900060306. [DOI] [PubMed] [Google Scholar]; (c) Lenz P. Photochemistry and Photobiology. 1995;62:333. doi: 10.1111/j.1751-1097.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 64.Dayal S, Burda C. Journal of the American Chemical Society. 2008;130:2890. doi: 10.1021/ja0781285. [DOI] [PubMed] [Google Scholar]
- 65.Zoumi A, Yeh A, Tromberg BJ. Proceedings of the National Academy of Sciences. 2002;99:11014. doi: 10.1073/pnas.172368799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.(a) Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE. Nat Med. 2009;15:1219. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee EC, de Boer JF, Mujat M, Lim H, Yun SH. Opt. Express. 2006;14:4403. doi: 10.1364/oe.14.004403. [DOI] [PubMed] [Google Scholar]; (c) Vakoc BJ, Fukumura D, Jain RK, Bouma BE. Nat Rev Cancer. 2012;12:363. doi: 10.1038/nrc3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weissleder R, Pittet MJ. Nature. 2008;452:580. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosencwaig A. Science. 1973;181:657. doi: 10.1126/science.181.4100.657. [DOI] [PubMed] [Google Scholar]
- 69.(a) Wang LV. Nat Photon. 2009;3:503. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Nat Biotech. 2003;21:803. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]; (c) Zhang HF, Maslov K, Stoica G, Wang LV. Nat Biotech. 2006;24:848. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 70.(a) Li M-L, Wang JC, Schwartz JA, Gill-Sharp KL, Stoica G, Wang LV. Journal of Biomedical Optics. 2009;14:010507. doi: 10.1117/1.3081556. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim C, Cho EC, Chen J, Song KH, Au L, Favazza C, Zhang Q, Cobley CM, Gao F, Xia Y, Wang LV. ACS Nano. 2010;4:4559. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lu W, Melancon MP, Xiong C, Huang Q, Elliott A, Song S, Zhang R, Flores LG, Gelovani JG, Wang LV, Ku G, Stafford RJ, Li C. Cancer Research. 2011;71:6116. doi: 10.1158/0008-5472.CAN-10-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kim J-W, Galanzha EI, Shashkov EV, Moon H-M, Zharov VP. Nature Nanotechnology. 2009;4:688. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melancon MP, Zhou M, Li C. Accounts of Chemical Research. 2011;44:947. doi: 10.1021/ar200022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelliccioli AP, Wirz J. Photochemical & Photobiological Sciences. 2002:1. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 73.Cosa G, Lukeman M, Scaiano JC. Accounts of Chemical Research. 2009;42:599. doi: 10.1021/ar8001969. [DOI] [PubMed] [Google Scholar]
- 74.San Miguel Vn, Bochet CG, del Campo An. Journal of the American Chemical Society. 2011;133:5380. doi: 10.1021/ja110572j. [DOI] [PubMed] [Google Scholar]
- 75.(a) Ellis-Davies GCR. Nat Meth. 2007;4:619. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Young DD, Deiters A. Organic & Biomolecular Chemistry. 2007:5. doi: 10.1039/b616410m. [DOI] [PubMed] [Google Scholar]; (c) Riggsbee CW, Deiters A. Trends in Biotechnology. 2010;28:468. doi: 10.1016/j.tibtech.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dvir T, Banghart MR, Timko BP, Langer R, Kohane DS. Nano Letters. 2010;10:250. doi: 10.1021/nl903411s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.(a) Goodwin AP, Mynar JL, Ma Y, Fleming GR, Fréchet JMJ. Journal of the American Chemical Society. 2005;127:9952. doi: 10.1021/ja0523035. [DOI] [PubMed] [Google Scholar]; (b) Babin J, Pelletier M, Lepage M, Allard J-F, Morris D, Zhao Y. Angewandte Chemie International Edition. 2009;48:3329. doi: 10.1002/anie.200900255. [DOI] [PubMed] [Google Scholar]
- 78.Denk W. Proceedings of the National Academy of Sciences. 1994;91:6629. doi: 10.1073/pnas.91.14.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.(a) Tamai N, Miyasaka H. Chemical Reviews. 2000;100:1875. doi: 10.1021/cr9800816. [DOI] [PubMed] [Google Scholar]; (b) Minkin VI. Chem. Rev. 2004;104:2751. doi: 10.1021/cr020088u. [DOI] [PubMed] [Google Scholar]
- 80.(a) Marriott G, Mao S, Sakata T, Ran J, Jackson DK, Petchprayoon C, Gomez TJ, Warp E, Tulyathan O, Aaron HL, Isacoff EY, Yan YL. P Natl Acad Sci USA. 2008;105:17789. doi: 10.1073/pnas.0808882105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu MQ, Zhang GF, Li C, Aldred MP, Chang E, Drezek RA, Li ADQ. Journal of the American Chemical Society. 2011;133:365. doi: 10.1021/ja106895k. [DOI] [PubMed] [Google Scholar]
- 81.Finkelmann H, Nishikawa E, Pereira GG, Warner M. Physical Review Letters. 2001;87:015501. doi: 10.1103/PhysRevLett.87.015501. [DOI] [PubMed] [Google Scholar]
- 82.(a) Yu Y, Nakano M, Ikeda T. Nature. 2003;425:145. doi: 10.1038/425145a. [DOI] [PubMed] [Google Scholar]; (b) Kondo M, Yu Y, Ikeda T. Angewandte Chemie International Edition. 2006;45:1378. doi: 10.1002/anie.200503684. [DOI] [PubMed] [Google Scholar]
- 83.Yamada M, Kondo M, Mamiya J-i, Yu Y, Kinoshita M, Barrett CJ, Ikeda T. Angewandte Chemie International Edition. 2008;47:4986. doi: 10.1002/anie.200800760. [DOI] [PubMed] [Google Scholar]
- 84.Camacho-Lopez M, Finkelmann H, Palffy-Muhoray P, Shelley M. Nat Mater. 2004;3:307. doi: 10.1038/nmat1118. [DOI] [PubMed] [Google Scholar]
- 85.van Oosten CL, Bastiaansen CWM, Broer DJ. Nat Mater. 2009;8:677. doi: 10.1038/nmat2487. [DOI] [PubMed] [Google Scholar]
- 86.Lendlein A, Jiang H, Junger O, Langer R. Nature. 2005;434:879. doi: 10.1038/nature03496. [DOI] [PubMed] [Google Scholar]
- 87.Sumaru K, Ohi K, Takagi T, Kanamori T, Shinbo T. Langmuir. 2006;22:4353. doi: 10.1021/la052899+. [DOI] [PubMed] [Google Scholar]
- 88.Tong R, Hemmati HD, Langer R, Kohane DS. Journal of the American Chemical Society. 2012 doi: 10.1021/ja211888a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daniel M-C, Astruc D. Chemical Reviews. 2003;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 90.(a) Rosi NL, Mirkin CA. Chemical Reviews. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]; (b) Stewart ME, Anderton CR, Thompson LB, Maria J, Gray SK, Rogers JA, Nuzzo RG. Chemical Reviews. 2008;108:494. doi: 10.1021/cr068126n. [DOI] [PubMed] [Google Scholar]
- 91.Taton TA, Mirkin CA, Letsinger RL. Science. 2000;289:1757. doi: 10.1126/science.289.5485.1757. [DOI] [PubMed] [Google Scholar]
- 92.(a) Lyon LA, Musick MD, Natan MJ. Analytical Chemistry. 1998;70:5177. doi: 10.1021/ac9809940. [DOI] [PubMed] [Google Scholar]; (b) Liu X, Dai Q, Austin L, Coutts J, Knowles G, Zou J, Chen H, Huo Q. Journal of the American Chemical Society. 2008;130:2780. doi: 10.1021/ja711298b. [DOI] [PubMed] [Google Scholar]; (c) Gupta S, Huda S, Kilpatrick PK, Velev OD. Analytical Chemistry. 2007;79:3810. doi: 10.1021/ac062341m. [DOI] [PubMed] [Google Scholar]
- 93.Phillips RL, Miranda OR, You C-C, Rotello VM, Bunz UHF. Angewandte Chemie International Edition. 2008;47:2590. doi: 10.1002/anie.200703369. [DOI] [PubMed] [Google Scholar]
- 94.Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. Analytical and Bioanalytical Chemistry. 2008;391:943. doi: 10.1007/s00216-007-1768-z. [DOI] [PubMed] [Google Scholar]
- 95.Aaron J, de la Rosa E, Travis K, Harrison N, Burt J, José-Yacamán M, Sokolov K. Opt. Express. 2008;16:2153. doi: 10.1364/oe.16.002153. [DOI] [PubMed] [Google Scholar]
- 96.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Nano Letters. 2007;7:1929. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 97.Park J, Estrada A, Sharp K, Sang K, Schwartz JA, Smith DK, Coleman C, Payne JD, Korgel BA, Dunn AK, Tunnell JW. Opt. Express. 2008;16:1590. doi: 10.1364/oe.16.001590. [DOI] [PubMed] [Google Scholar]
- 98.von Maltzahn G, Centrone A, Park J-H, Ramanathan R, Sailor MJ, Hatton TA, Bhatia SN. Advanced Materials. 2009;21:3175. doi: 10.1002/adma.200803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Advanced Drug Delivery Reviews. doi: 10.1016/j.addr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 100.(a) Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Chemical Physics Letters. 1998;288:243. [Google Scholar]; (b) Bardhan R, Lal S, Joshi A, Halas NJ. Accounts of Chemical Research. 2011;44:936. doi: 10.1021/ar200023x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.(a) Jana NR, Gearheart L, Murphy CJ. Advanced Materials. 2001;13:1389. [Google Scholar]; (b) Nicewarner-Peña SR, Freeman RG, Reiss BD, He L, Peña DJ, Walton ID, Cromer R, Keating CD, Natan MJ. Science. 2001;294:137. doi: 10.1126/science.294.5540.137. [DOI] [PubMed] [Google Scholar]
- 102.(a) Sun Y, Xia Y. Science. 2002;298:2176. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]; (b) Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK. Accounts of Chemical Research. 2011;44:914. doi: 10.1021/ar200061q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.(a) Liang Z, Susha A, Caruso F. Chemistry of Materials. 2003;15:3176. [Google Scholar]; (b) Kim F, Connor S, Song H, Kuykendall T, Yang P. Angewandte Chemie International Edition. 2004;43:3673. doi: 10.1002/anie.200454216. [DOI] [PubMed] [Google Scholar]
- 104.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. Nat Mater. 2009;8:935. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krpetić Ze, Nativo P, Sée V, Prior IA, Brust M, Volk M. Nano Letters. 2010;10:4549. doi: 10.1021/nl103142t. [DOI] [PubMed] [Google Scholar]
- 106.Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. Journal of the American Chemical Society. 2008;130:8175. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.(a) Park J-H, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Nat Mater. 2009;8:331. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) von Maltzahn G, Park J-H, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nat Mater. 2011;10:545. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park J-H, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor MJ. Proceedings of the National Academy of Sciences. 2010;107:981. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jang B, Park J-Y, Tung C-H, Kim I-H, Choi Y. ACS Nano. 2011;5:1086. doi: 10.1021/nn102722z. [DOI] [PubMed] [Google Scholar]
- 110.(a) Agasti SS, Chompoosor A, You C-C, Ghosh P, Kim CK, Rotello VM. Journal of the American Chemical Society. 2009;131:5728. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Han G, You C-C, Kim B-j, Turingan RS, Forbes NS, Martin CT, Rotello VM. Angewandte Chemie International Edition. 2006;45:3165. doi: 10.1002/anie.200600214. [DOI] [PubMed] [Google Scholar]
- 111.Khlebtsov N, Dykman L. Chemical Society Reviews. 2011:40. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 112.(a) Bloembergen N. Physical Review Letters. 1959;2:84. [Google Scholar]; (b) Liu Q, Sun Y, Yang T, Feng W, Li C, Li F. Journal of the American Chemical Society. 2011;133:17122. doi: 10.1021/ja207078s. [DOI] [PubMed] [Google Scholar]; (c) Wang F, Liu X. Journal of the American Chemical Society. 2008;130:5642. doi: 10.1021/ja800868a. [DOI] [PubMed] [Google Scholar]
- 113.Miller MJ, Wei SH, Parker I, Cahalan MD. Science. 2002;296:1869. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 114.Zhan Q, Qian J, Liang H, Somesfalean G, Wang D, He S, Zhang Z, Andersson-Engels S. ACS Nano. 2011;5:3744. doi: 10.1021/nn200110j. [DOI] [PubMed] [Google Scholar]
- 115.(a) Xiong L, Chen Z, Tian Q, Cao T, Xu C, Li F. Analytical Chemistry. 2009;81:8687. doi: 10.1021/ac901960d. [DOI] [PubMed] [Google Scholar]; (b) Salthouse C, Hilderbrand S, Weissleder R, Mahmood U. Opt. Express. 2008;16:21731. doi: 10.1364/oe.16.021731. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xu CT, Axelsson J, Andersson-Engels S. Applied Physics Letters. 2009;94:251107. [Google Scholar]
- 116.(a) Nyk M, Kumar R, Ohulchanskyy TY, Bergey EJ, Prasad PN. Nano Letters. 2008;8:3834. doi: 10.1021/nl802223f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xiong L, Yang T, Yang Y, Xu C, Li F. Biomaterials. 2010;31:7078. doi: 10.1016/j.biomaterials.2010.05.065. [DOI] [PubMed] [Google Scholar]; (c) Zhou J, Sun Y, Du X, Xiong L, Hu H, Li F. Biomaterials. 2010;31:3287. doi: 10.1016/j.biomaterials.2010.01.040. [DOI] [PubMed] [Google Scholar]; (d) Cheng L, Yang K, Shao M, Lu X, Liu Z. Nanomedicine. 2011;6:1327. doi: 10.2217/nnm.11.56. [DOI] [PubMed] [Google Scholar]
- 117.(a) Yang Y, Shao Q, Deng R, Wang C, Teng X, Cheng K, Cheng Z, Huang L, Liu Z, Liu X, Xing B. Angewandte Chemie International Edition. 2012 doi: 10.1002/anie.201107919. n/a. [DOI] [PubMed] [Google Scholar]; (b) Carling C-J, Nourmohammadian F, Boyer J-C, Branda NR. Angewandte Chemie International Edition. 2010;49:3782. doi: 10.1002/anie.201000611. [DOI] [PubMed] [Google Scholar]; (c) Yan B, Boyer J-C, Branda NR, Zhao Y. Journal of the American Chemical Society. 2011;133:19714. doi: 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- 118.Qian HS, Guo HC, Ho PC-L, Mahendran R, Zhang Y. Small. 2009;5:2285. doi: 10.1002/smll.200900692. [DOI] [PubMed] [Google Scholar]
- 119.(a) Shan J, Budijono SJ, Hu G, Yao N, Kang Y, Ju Y, Prud'homme RK. Advanced Functional Materials. 2011;21:2488. [Google Scholar]; (b) Wang C, Tao H, Cheng L, Liu Z. Biomaterials. 2011;32:6145. doi: 10.1016/j.biomaterials.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 120.Dong B, Xu S, Sun J, Bi S, Li D, Bai X, Wang Y, Wang L, Song H. Journal of Materials Chemistry. 2011;21:6193. [Google Scholar]
- 121.(a) Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Chemical Society Reviews. 2012 doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]; (b) Ambrogio MW, Thomas CR, Zhao Y-L, Zink JI, Stoddart JF. Accounts of Chemical Research. 2011;44:903. doi: 10.1021/ar200018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Angelos S, Choi E, Vögtle F, De Cola L, Zink JI. The Journal of Physical Chemistry C. 2007;111:6589. [Google Scholar]
- 123.(a) Mal NK, Fujiwara M, Tanaka Y. Nature. 2003;421:350. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]; (b) Park C, Lee K, Kim C. Angewandte Chemie International Edition. 2009;48:1275. doi: 10.1002/anie.200803880. [DOI] [PubMed] [Google Scholar]; (c) Vivero-Escoto JL, Slowing II, Wu C-W, Lin VSY. Journal of the American Chemical Society. 2009;131:3462. doi: 10.1021/ja900025f. [DOI] [PubMed] [Google Scholar]
- 124.Lin Q, Huang Q, Li C, Bao C, Liu Z, Li F, Zhu L. Journal of the American Chemical Society. 2010;132:10645. doi: 10.1021/ja103415t. [DOI] [PubMed] [Google Scholar]
- 125.Hudson SP, Padera RF, Langer R, Kohane DS. Biomaterials. 2008;29:4045. doi: 10.1016/j.biomaterials.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Small. 2010;6:1794. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Nat Biotech. 2007;25:1165. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.(a) Kim S-W, Zimmer JP, Ohnishi S, Tracy JB, Frangioni JV, Bawendi MG. Journal of the American Chemical Society. 2005;127:10526. doi: 10.1021/ja0434331. [DOI] [PubMed] [Google Scholar]; (b) Zimmer JP, Kim S-W, Ohnishi S, Tanaka E, Frangioni JV, Bawendi MG. Journal of the American Chemical Society. 2006;128:2526. doi: 10.1021/ja0579816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Longmire M, Choyke PL, Kobayashi H. Nanomedicine. 2008;3:703. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C. Journal of the American Chemical Society. 2010;132:15351. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chan WCW, Nie S. Science. 1998;281:2016. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 132.(a) Davis ME, Chen Z, Shin DM. Nat Rev Drug Discov. 2008;7:771. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]; (b) Petros RA, DeSimone JM. Nat. Rev. Drug Discov. 2010;9:615. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 133.(a) Wang G, Tong X, Zhao Y. Macromolecules. 2004;37:8911. [Google Scholar]; (b) Lee H-i, Wu W, Oh JK, Mueller L, Sherwood G, Peteanu L, Kowalewski T, Matyjaszewski K. Angewandte Chemie International Edition. 2007;46:2453. doi: 10.1002/anie.200604278. [DOI] [PubMed] [Google Scholar]; (c) Lim S-J, Carling C-J, Warford CC, Hsiao D, Gates BD, Branda NR. Dyes and Pigments. 2011;89:230. [Google Scholar]; (d) Chen C-J, Liu G-Y, Shi Y-T, Zhu C-S, Pang S-P, Liu X-S, Ji J. Macromolecular Rapid Communications. 2011;32:1077. doi: 10.1002/marc.201100196. [DOI] [PubMed] [Google Scholar]; (e) Tian F, Yu Y, Wang C, Yang S. Macromolecules. 2008;41:3385. [Google Scholar]; (f) Ki Choi S, Thomas T, Li M-H, Kotlyar A, Desai A, Baker JJR. Chemical Communications. 2010:46. doi: 10.1039/b927215c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen Z, He Y, Wang Y, Wang X. Macromolecular Rapid Communications. 2011;32:977. doi: 10.1002/marc.201100142. [DOI] [PubMed] [Google Scholar]; (h) Jiang J, Tong X, Zhao Y. Journal of the American Chemical Society. 2005;127:8290. doi: 10.1021/ja0521019. [DOI] [PubMed] [Google Scholar]; (i) Jiang J, Tong X, Morris D, Zhao Y. Macromolecules. 2006;39:4633. [Google Scholar]; (j) Menon S, Thekkayil R, Varghese S, Das S. Journal of Polymer Science Part A: Polymer Chemistry. 2011;49:5063. [Google Scholar]; (k) Jiang J, Qi B, Lepage M, Zhao Y. Macromolecules. 2007;40:790. [Google Scholar]; (l) Shi D, Matsusaki M, Kaneko T, Akashi M. Macromolecules. 2008;41:8167. [Google Scholar]; (m) Park C, Lim J, Yun M, Kim C. Angewandte Chemie International Edition. 2008;47:2959. doi: 10.1002/anie.200705271. [DOI] [PubMed] [Google Scholar]; (n) Kostiainen MA, Smith DK, Ikkala O. Angewandte Chemie International Edition. 2007;46:7600. doi: 10.1002/anie.200701200. [DOI] [PubMed] [Google Scholar]; (o) Zhu Y, Fujiwara M. Angewandte Chemie International Edition. 2007;46:2241. doi: 10.1002/anie.200604850. [DOI] [PubMed] [Google Scholar]
- 134.Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. Journal of the American Chemical Society. 2010;132:9540. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kong G, Braun RD, Dewhirst MW. Cancer Research. 2001;61:3027. [PubMed] [Google Scholar]
- 136.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WCW, Cao W, Wang LV, Zheng G. Nat Mater. 2011;10:324. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- 137.Detty MR, Gibson SL, Wagner SJ. Journal of Medicinal Chemistry. 2004;47:3897. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 138.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Proceedings of the National Academy of Sciences. 2007;104:15549. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Minchinton AI, Tannock IF. Nat Rev Cancer. 2006;6:583. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 140.Kohane DS, Langer R. Chemical Science. 2010;1:441. [Google Scholar]
- 141.(a) Huang Z. Technol. Cancer Res. Treat. 2005;4:283. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Josefsen LB, Boyle RW. Metal-Based Drugs. 2008:2008. doi: 10.1155/2008/276109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) O’Connor AE, Gallagher WM, Byrne AT. Photochemistry and Photobiology. 2009;85:1053. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]; (d) Juarranz Á, Jaén P, Sanz-Rodríguez F, Cuevas J, González S. Clinical and Translational Oncology. 2008;10:148. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]; (e) Juzeniene A, Peng Q, Moan J. Photochemical & Photobiological Sciences. 2007;6:1234. doi: 10.1039/b705461k. [DOI] [PubMed] [Google Scholar]; (f) Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roneucci G. Lasers in Surgery and Medicine. 2006;38:468. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 142.Lu J, Choi E, Tamanoi F, Zink JI. Small. 2008;4:421. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y-C, Le Ny A-LM, Schmidt J, Talmon Y, Chmelka BF, Lee CT. Langmuir. 2009;25:5713. doi: 10.1021/la803588d. [DOI] [PubMed] [Google Scholar]
- 144.Choi M-R, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. Nano Letters. 2007;7:3759. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 145.Volodkin DV, Skirtach AG, Möhwald H. Angewandte Chemie International Edition. 2009;48:1807. doi: 10.1002/anie.200805572. [DOI] [PubMed] [Google Scholar]
- 146.Lu W, Singh AK, Khan SA, Senapati D, Yu H, Ray PC. Journal of the American Chemical Society. 2010;132:18103. doi: 10.1021/ja104924b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim J, Park S, Lee JE, Jin SM, Lee JH, Lee IS, Yang I, Kim J-S, Kim SK, Cho M-H, Hyeon T. Angewandte Chemie International Edition. 2006;45:7754. doi: 10.1002/anie.200602471. [DOI] [PubMed] [Google Scholar]
- 148.(a) Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K. ACS Nano. 2008;3:80. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]; (b) Chen C-C, Lin Y-P, Wang C-W, Tzeng H-C, Wu C-H, Chen YC, Chen C-P, Chen L-C, Wu Y-C. Journal of the American Chemical Society. 2006;128:3709. doi: 10.1021/ja0570180. [DOI] [PubMed] [Google Scholar]
- 149.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, Dai H. Nat Mater. 2006;5:971. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 150.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Journal of the American Chemical Society. 2006;128:2115. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 151.O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Cancer Letters. 2004;209:171. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 152.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. Journal of the American Chemical Society. 2003;125:7860. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 153.Nishiyama N, Jang W-D, Kataoka K. New Journal of Chemistry. 2007:31. [Google Scholar]
- 154.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo Y-EL, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Clinical Cancer Research. 2006;12:6677. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 155.Xiao L, Gu L, Howell SB, Sailor MJ. ACS Nano. 2011;5:3651. doi: 10.1021/nn1035262. [DOI] [PMC free article] [PubMed] [Google Scholar]