Abstract
Transgenic mouse models have been an invaluable resource in elucidating the complex roles of Aβ and tau in Alzheimer’s disease. While many laboratories rely on qualitative or semi-quantitative techniques when investigating tau pathology, we have developed four Low-Tau Sandwich ELISAs that quantitatively assess different epitopes of tau relevant to Alzheimer’s disease: total tau, pSer-202, pThr-231, pSer-396/404. In this study, after comparing our assays to commercially available ELISAs, we demonstrate our assays high specificity and quantitative capabilities using brain homogenates from tau transgenic mice, htau, JNPL3, tau KO mice. All four ELISAs show excellent specificity for mouse and human tau, with no reactivity to tau KO animals. An age dependent increase of serum tau in both tau transgenic models was also seen. Taken together, these assays are valuable methods to quantify tau and phospho-tau levels in transgenic animals, by examining tau levels in brain and measuring tau as a potential serum biomarker.
Keywords: Alzheimer’s disease, tau-ELISA, tau transgenic mice, htau, JNPL3, sera
1. Introduction
Alzheimer’s disease (AD), the most common form of neurodegenerative disease, is characterized pathologically by the formation of two protein lesions: neuritic plaques composed of β-amyloid (Aβ) and neurofibrillary tangles (NFT) composed of the microtubule-associated protein tau. The connection between these two pathologies remains unclear. However, it seems that tau pathology more closely correlates with neuronal loss and severity of dementia (Arriagada et al., 1992; Bancher et al., 1993; Gomez-Isla et al., 1997; Guillozet et al., 2003). NFTs are mainly comprised of aggregated paired helical filaments (PHF-tau) (Grundke-Iqbal et al., 1986; Ihara et al., 1986), and are found in other forms of dementia collectively known as tauopathies some of which are due to mutations in the tau gene (Hutton et al., 1998; Poorkaj et al., 2001; Spillantini et al., 1998). With the recent push towards disease-modifying therapies, it is critical to further elucidate the roles of Aβ and tau in AD, and, ultimately, observe how potential therapies may affect the underlying mechanisms.
The formation of filamentous tau seems to occur in several stages, from pre-tangles to intracellular tangles to extracellular tangles, with a sequential phosphorylation pattern occurring as tau pathology develops (Augustinack et al., 2002; Kimura et al., 1996). There is also evidence, in vitro, that certain phospho-tau epitopes require a particular order of phosphorylation events. The AT100 epitope requires first an initial stimulatory phosphorylations Ser-199, Ser-202, and Thr-205 in any order, then at Thr-212, and finally at Ser-214 (Zheng-Fischhofer et al., 1998). Being able to quantitatively examine relevant site-specific phosphorylation as tau pathology progresses, or in contrast, is affected by disease-modifying therapies, is a key step in moving the field forward. Currently, few quantitative techniques are available for examining the mechanisms of tau hyperphosphorylation in mouse models, and investigators are required to rely on semi-quantitative methods such as immunohistochemistry or immunoblot analyses. There are commercially available enzyme-linked immunosorbent assays (ELISA) for quantitative analysis of tau, for example from Innotest or Invitrogen, that are mainly used in cerebrospinal fluid (CSF) for biomarker detection (Barten et al., 2011; Lachno et al., 2011; Vanderstichele et al., 2006). The high cost of these assays is prohibitive for their use by investigators at many academic institutions. Moreover, the availability of reliable and relevant phospho-tau epitope assays is very limited.
In this study, we have developed four different assays suitable for the detection of low levels of mouse and human tau, referred to as Low-Tau Sandwich ELISA. Taking advantage of newly characterized and previously established tau monoclonal antibodies, we were able to selectively focus on total tau (DA31) and phospho-tau epitopes pertinent in Alzheimer’s disease including pSer-202 (CP13) (Duff et al., 2000; Lewis et al., 2000), pThr-231 (RZ3) (Vingtdeux et al., 2011), and pSer-396/404 (PHF-1) (Greenberg et al., 1992; Otvos et al., 1994). In order to demonstrate the specific and quantitative qualities of the assays, brain homogenates from two different tau transgenic models were used: htau mice, which express all six isoforms of the normal human tau protein (Andorfer et al., 2003; Polydoro et al., 2009), and JNPL3 (P301L), which express 0N4R human tau with the P301L mutation (Lewis et al., 2000; Lin et al., 2003a; Lin et al., 2003b). Interestingly, our ELISAs demonstrate enough sensitivity to detect total tau and pSer-202 tau in the serum of these transgenic mice, with an age dependent increase of tau in serum. We also compared our total tau ELISA (DA31) to commercially available human ELISA kits from Invitrogen and Innotest demonstrating its versatility. Hence, these four Low-Tau Sandwich ELISAs are highly sensitive and practical assays to quantify total and phosphorylated tau levels in brain and serum of transgenic mice.
2. Materials and Methods
2.1. Production of Monoclonal Antibodies
Monoclonal tau antibodies DA9 and DA31 were generated as previously described (Davies, 1999). Briefly, four intraperitoneal injections of 0.2mL of purified paired helical filaments, PHF-tau, at a concentration of 1–2mg/mL, were administered to a cohort of tau KO mice, over an 8-week period. PHF-tau was isolated from severe human AD brain, as previously described (Jicha et al., 1999). These tau KO mice have a targeted disruption by the insertion of green fluorescent protein cDNA into exon one of tau (Tucker et al., 2001). The immunized mice were last injected 3 days before the spleen was removed. The spleen cells were fused with myeloma cells (NSO cells) in the presence of polyethylene glycol (PEG). The fusion products were plated in 96-well plates in selection medium containing hypoxanthine-aminopterin-thymidine (HAT) (Invitrogen). Positive clones were identified by assaying the culture media both by PHF-ELISA and by immunoblot. Clones with high specificity for tau were selected, cloned, expanded, and ultimately screened on the epitope mapping ELISA described below.
2.2. Immunoblotting
For initial characterization of the new total tau antibodies, DA9 and DA31, immunoblotting was performed. Recombinant tau, Tau441 (TauA), tau without exons 2 3 and 10 (TauD), Tau366 (amino acids 1–366), Tau270 (amino acids 1–270) and Tau190 (amino acids 1–190 (constructs are C-terminal deletions) were expressed using pcDNA vectors. Cos7 cells were transfected with a mixture containing 2µg of cDNA and Lipofectamine 2000 reagent (Invitrogen), in serum free medium for 6 hours at 37°C and homogenized after 48 hrs. Finally, cells were harvested in TBS-based homogenizing buffer: Tris-buffered saline (TBS), pH 7.4, containing 1 mM Na3VO4, 2 mM EGTA, and 10mM NaF and Complete Mini-EDTA Protease inhibitor cocktail (Roche). Cell homogenates were kept at −80°C until use. Cellular lysates together with brain homogenates obtained from wild type mice, tau KO mice (Tucker et al., 2001), htau mice (Andorfer et al., 2003), and a PHF-tau preparation were boiled for 5 min in Laemmli sample buffer for SDS-PAGE analysis. Immunoblotting for RZ3 characterization was run on homogenate samples of health control and confirmed AD patients, as well as homogenate from wild type mice, tau ko mice, and htau mice. Brain homogenate preparations are described in a following section. Membranes were probed with human specific total tau CP27 (aa130–170) (Duff et al., 2000; Lewis et al., 2000), DA9, DA31 at 1:50 dilution, and purified RZ3 was used at a concentration of 2µg/ml.
2.3. Epitope Mapping ELISA
The characterization of three new monoclonal tau antibodies, total-tau DA9, total-tau DA31, and RZ3 (pThr-231) was performed as previously described (Espinoza et al., 2008). Briefly, for RZ3 antibody, 96-well plates were coated with NeutrAvidin protein (ThermoScientfic) at a concentration of 2µg/ml overnight at 4°C in coating buffer (15mM KH2PO4, 25mM KH2PO4, 0.1M NaCl, 0.1M EDTA and 7.5mM NaN3, pH 7.2). Next plates were incubated with appropriate biotinylated phospho-peptides diluted in 2% bovine serum albumin (BSA) in 1xTBS, a final concentration of 2µg/ml, and incubated for 1 hr at room temperature. Synthetic phospho-tau peptides were made with the appropriate phospho-amino acid, plus 7 amino acids flanking each side of the phospho-site, and with a biotinylated N-terminus. For example, the peptide for a (pThr-231) positive antibody would be Biotin-KKVAVVR(phosphoT)PPKSPSS. RZ3 was serially diluted in 5% Milk/1xTBS and incubated for 1 hr at room temperature. Goat anti-mouse IgG1-HRP conjugated secondary antibody was used at a dilution of 1:500 in 5% Milk/1xTBS, for 1 hr at room temperature. Plates were visualized with Horseradish Peroxidase Substrate Kit (Biorad) and read with an Infinite m200 plate reader (Tecan) at 405 nm. The epitope mapping ELISAs for the total tau antibodies DA9 and DA31, was a similar protocol, except the non-phosphorylated amino acid sequences of tau used were not biotinylated. Therefore, the appropriate peptides were directly coated to the plates at a concentration of 1µg/ml.
2.4. Tau Monoclonal Antibodies used for Low-Tau Sandwich ELISA
The following purified monoclonal antibodies were used as capture antibodies in the Low-Tau Sandwich ELISA: total tau DA31 (aa150–190), PHF-1 (pSer-396/404) (Greenberg et al., 1992; Otvos et al., 1994), CP13 (pSer-202) (Duff et al., 2000; Lewis et al., 2000), and RZ3 (pThr-231) (previously called 2E12) (Vingtdeux et al., 2011). The detection antibody used for all ELISAs was purified DA9 (aa102–140) (Tremblay et al., 2010), which was labeled with HRP according to the Lightning Link-HRP protocol (InnovaBiosciences). Briefly, 150µg of purified antibody was incubated in 100µl of 50mM NaH2PO4, pH8.0. To this 10µl of LL-modifier was added, and the mixture was transferred to the vial containing 100µg LL-HRP. The reaction was allowed to proceed for 4 hours at room temperature, at which time 10µl of the LL-Quencher was added. After 30 minutes, the volume was made 1ml with (880ul) of sodium phosphate, pH 8.0. Aliquots of 100µl were made and stored at −80°C, and thawed as necessary. Once thawed DA9-HRP was stabilized with LifeXtend HRP conjugate stabilizer (InnovaBiosciences) using 900µl of stabilizing reagent to 100µl of antibody and kept at 4 °C.
2.5. Sample Preparations for ELISA
To determine the assay sensitivity and inter/intra plate variation, recombinant tau (Tau441) (rPeptide) was used for the DA31 capture ELISA. For the various phospho-tau capture ELISAs, sensitivities were measured with paired helical filaments (PHF-tau) isolated from human AD brain, as previously described (Jicha et al., 1999).
Mouse brain homogenates, used for the overall specificity of the Low-Tau Sandwich ELISA were prepared from the forebrain of wild type, tau KO (Tucker et al., 2001), 11 month htau, that express all six isoforms of the normal human tau protein (Andorfer et al., 2003; Polydoro et al., 2009), and 6 month female JNPL3 which express 0N4R human tau with the P301L mutation (Lewis et al., 2000; Lin et al., 2003b). To demonstrate the quantitative properties of these ELISAs, we used forebrains of wild type, 11 months htau, and whole brain minus the cerebellum of 6 months female JNPL3. The brain regions were extracted and homogenized in an appropriate volume of homogenizing buffer, a solution of Tris-buffered saline (TBS), pH 7.4 containing 10mM NaF, 1mM NaVO3, and 2mM EGTA, including a complete Mini protease inhibitor (Roche). Samples were stored in aliquots at −80°C until use. Prior to use, to obtain total tau (soluble) preparation homogenates were thawed and spun at 14,000 g for 10 min at 4°C, supernatants were collected and either Laemmli sample buffer, a solution of Tris-buffered saline (TBS), pH6.8, containing 4% SDS, 2% β-mercaptoethanol, 10% glycerol, and bromophenol blue, was added or not depending on the preparation. Samples containing Laemmli sample buffer were then boiled for 5 minutes. To obtain insoluble tau preparation samples were prepared as previously described (Chai et al., 2011). Briefly, 500µl of homogenate was thawed and spun at 6,000 g for 10 min at 4°C, supernatants was collected and spun at 200,000 g for 30 min at 25°C. Pellet was then re-suspended in 450µl of homogenizing buffer and spun again at 200,000 g for 30 min at 25°C. Finally, pellet was re-suspended in 200µl of 1× Laemmli sample buffer, and boiled for 5 minutes.
2.6. Low-Tau Sandwich ELISA
96-well plates were coated with the various purified monoclonal tau antibodies mentioned above, at a concentration of 6µg/ml in coating buffer. 100µl of the capture antibody was added to each well and incubated at least 48 hrs at 4°C. Plates were washed 3× in wash buffer, and blocked for 1 hr at room temperature using StartingBlock (ThermoScientific) to avoid non-specific binding. After the 1 hr block, each plate was washed this time 5×, and 50µl of appropriate sample was added in triplicate. All brain homogenates, rtau, and PHF-tau were diluted in 20% SuperBlock in 1xTBS (ThermoScientific). Once the samples were added, 50µl of the total tau detection antibody DA9-HRP diluted 1:50 in 20% SuperBlock in 1xTBS was added to the samples and tapped to combine. Plates were then incubated O/N at 4°C. Plates were washed 9× in wash buffer soaking for 15 sec in between. 100µl of 1-Step ULTRA TMB-ELISA (ThermoScientific) was added for 30 minutes at room temperature. Finally, 100µl of 2M H2SO4 was added to stop reaction, and plates were read with an Infinite m200 plate reader (Tecan) at 450 nm.
2.7. Mouse Serum ELISAs
Serum was collected from wild type, htau, and P301L mice, at various ages, and stored at −80°C until use. Upon sacrifice mice were bled, samples were collected and allowed to clot for 30 min at room temperature. After cooling for 15 min, samples were spun at 14,000 g for 10 min at 4°C; supernatants were collected and then re-spun at 14,000 g for 10 min at 4°C. The final supernatants obtained corresponded to the serum samples. To determine whether or not mouse serum interacts and affects the sensitivity of the Low-Tau Sandwich ELISA, wild type mouse serum was spiked with recombinant tau (rPeptide) and run on the DA31 Low-Tau Sandwich ELISAs. To detect levels of total tau and phosphorylated tau in serum, samples were diluted 1:5 in 20% SuperBlock in 1xTBS and ran on the DA31 or CP13 Low-Tau Sandwich ELISA, as already described.
2.8. Commercial total tau ELISA kits
Commercially available total tau ELISA kits were purchased from Invitrogen, called Tau (total) Human ELISA kit (#KHB0041) and Innogenetics called Innotest hTau Ag (#80226). The assays were used in accordance to the manufacturer’s instructions.
2.9. Statistical Analysis
All statistical analyses were performed using GraphPad Prism. An unpaired t-test was used for comparison of htau, and P301L brain and sera preparations to wild type samples. Intra and inter-assay variations, or the coefficients of variation (CV), were calculated as previously described (Lachno et al., 2011; Murray and Lawrence, 1993; Porstmann and Kiessig, 1992).
3. Results
3.1. DA9 and DA31 are tau sequence antibodies, while RZ3 is specific for pThr-231 tau
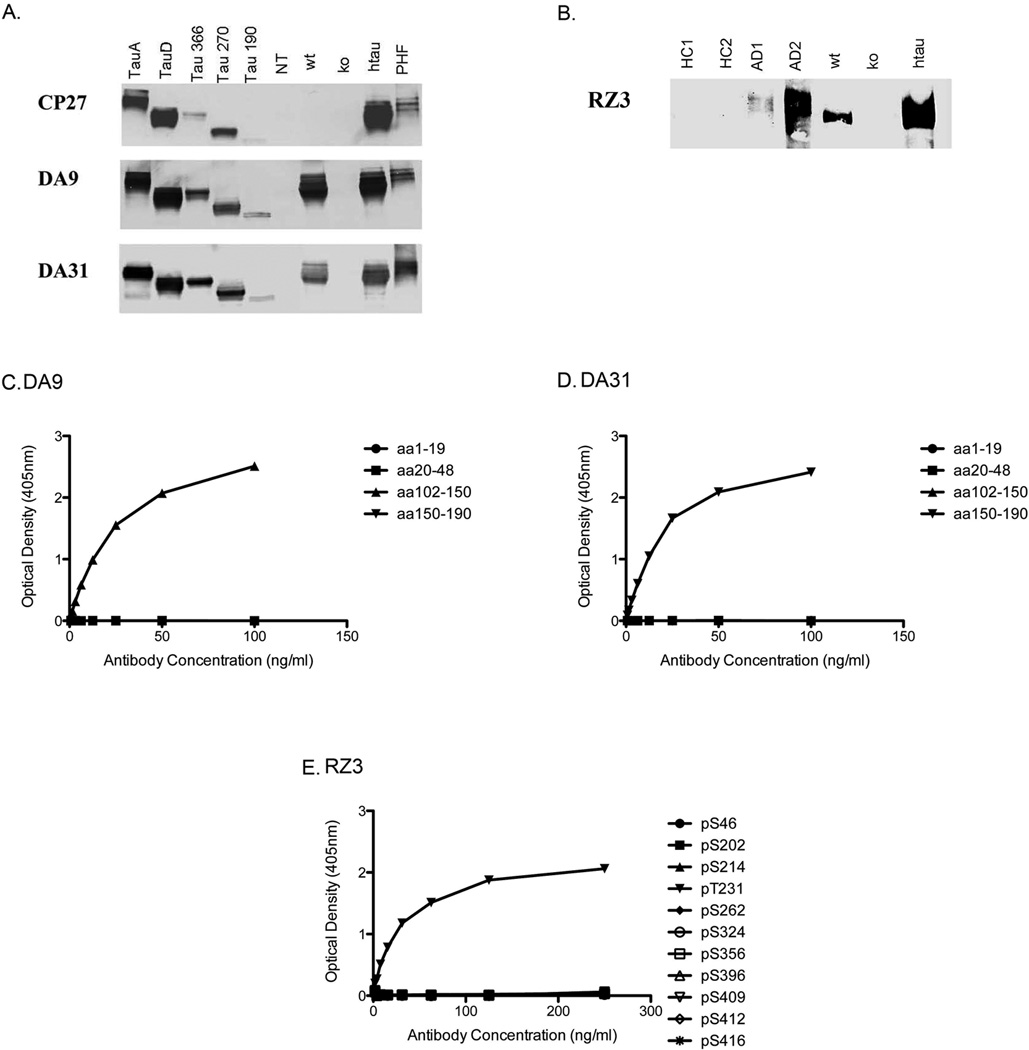
The hybridoma supernatants from DA9 and DA31 were first screened by ELISA in order to test the reactivity to PHF-tau preparation and to a bacterially expressed recombinant tau. The antibodies were reactive with both antigens (data not shown). Since recombinant tau was produced in a bacterial system lacking the most common post-translational modifications occurring in the brain, it was likely that both antibodies recognized tau sequence epitopes. In an attempt to determine the exact sequence of the epitopes, immunoblots were performed on a panel of tau constructs. Both DA9 and DA31 recognize TauA, TauD, Tau366, Tau270 and Tau190, signifying that the epitope was located within the first 190 amino acids of tau (Figure 1a). Moreover, since DA9 and DA31 were reactive to TauD, an isoform lacking exons 2 and 3 localized in the sequence aa48–101 of tau, it was likely that the epitopes were located within the residues aa1–47 or aa102–190. To further characterize the DA antibodies, we determined their reactivity in mouse brain homogenates: DA9 and DA31 were able to recognize both murine tau in wild type mouse brain and human tau in htau mouse brain. However, both antibodies were not reactive with tau KO mouse brain homogenate, suggesting an extreme specificity to tau (Figure 1a). To confirm the exact epitopes of DA9 and DA31, the regions of interest, aa1–47 or aa102–190, were run on an epitope mapping ELISA. DA9 reacts with the peptide correlating with aa102–150, with no reactivity to any other tau sequence peptide (Figure 1c). The aa140–150 region is different in human and mouse and DA9 recognizes both human and mouse tau. This suggests that the antibody maps to a homology region outside this sequence correlating to aa102–140. DA31 reacts with the aa150–190 peptide with no other reactivity (Figure 1d), therefore the epitope sequence for DA31 is aa150–190.
Figure 1. DA9 and DA31 are tau sequence antibodies, while RZ3 is specific for pThr-231 tau.
A. Immunoblots of new total tau antibodies DA9 and DA31 on a panel of cell lysates transfected with tau constructs. Both antibodies react with Tau441 (TauA), tau without exons 2, 3 and 10 (TauD), Tau366, Tau270 and Tau190 (C-terminal deletions). The antibodies also react with brain homogenates from wild type and htau mice, with no reactivity to the tau KO mouse. Therefore both DA9 and DA31 recognize a tau sequence between amino acids 1–47 or 102–190. B. Immunoblot of RZ3 demonstrates that the phospho-tau antibody reacts with homogenate samples from confirmed human AD patients, wild type and htau mice, with no reactivity to healthy controls patients or tau KO mice. C, D, E. Epitope mapping ELISAs to determine the exact epitope reactivity of DA9, DA31, and RZ3. C. DA9 only recognizes a tau amino acid sequence peptide from 102–150. D. DA31 only recognizes a tau amino acid sequence peptide from 105–190. E. RZ3 recognizes the pThr-231 site on a phospho-tau peptide with no reactivity to any other relevant phospho-tau site.
The characterization of RZ3 was more straightforward, since the antibody was made directly to a peptide with a phosphorylation at Thr-231. Initially, RZ3 was confirmed to have no reactivity with recombinant tau, and immunoblots were run with brain homogenates from confirmed healthy control and AD patients, as well as wild type, tau KO, and htau mice. RZ3 is able to recognize phospho-tau in AD patients, as well as both mouse phospho-tau in wild type mouse brain and human phospho-tau in htau mouse brain. However, this antibody was not reactive with healthy control patients and tau KO mouse brain homogenate, suggesting an extreme specificity to phosphorylated tau (Figure 1b). With this in mind, RZ3 was then analyzed against a library of phospho-tau peptides relevant to Alzheimer’s disease (Figure 1e). In this regard, RZ3 is extremely specific to pThr-231 on tau, with no additional reactivity to other relevant tau phosphorylation sites.
3.2. Sensitivity of Low-Tau Sandwich ELISAs
The purpose of developing this particular Low-Tau Sandwich ELISA was to allow accurate quantification of tau pathology in brain. With these newly characterized and highly specific antibodies as well as other established phospho-tau antibodies, we have developed four unique Low-Tau Sandwich ELISAs that accurately and reproducibly measure tau in an assortment of models.
The possible cross reactivity between the capture and detection antibodies is known to be critical with any method of quantification, especially if the antibodies have similar isotypes. Therefore, the simplest method was to conjugate HRP directly to the total tau detection antibody of choice, in this case DA9, while changing the capture antibody to detect different relevant epitopes of tau. The intra-assay variation for this Low-Tau ELISA was calculated to be 2%, while the inter-assay variation was calculated to be 4%, values that are well below the accepted 10% variation (Murray and Lawrence, 1993; Porstmann and Kiessig, 1992).
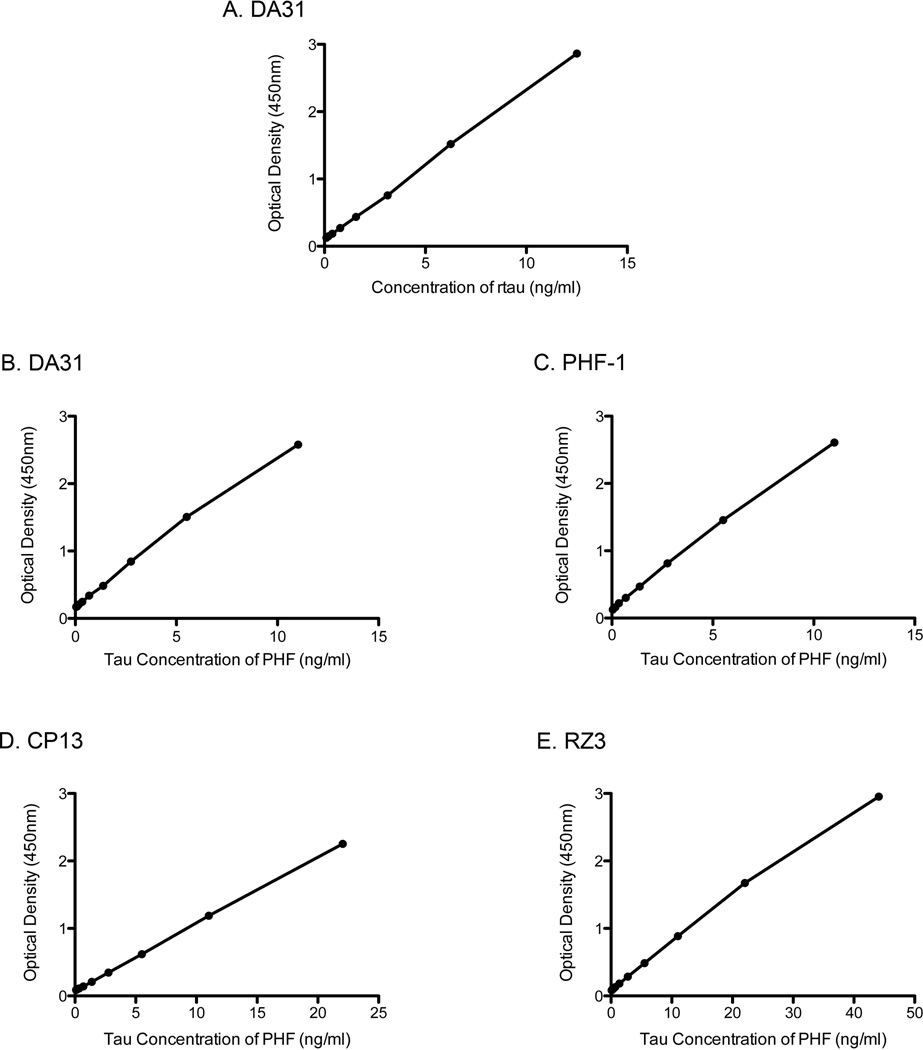
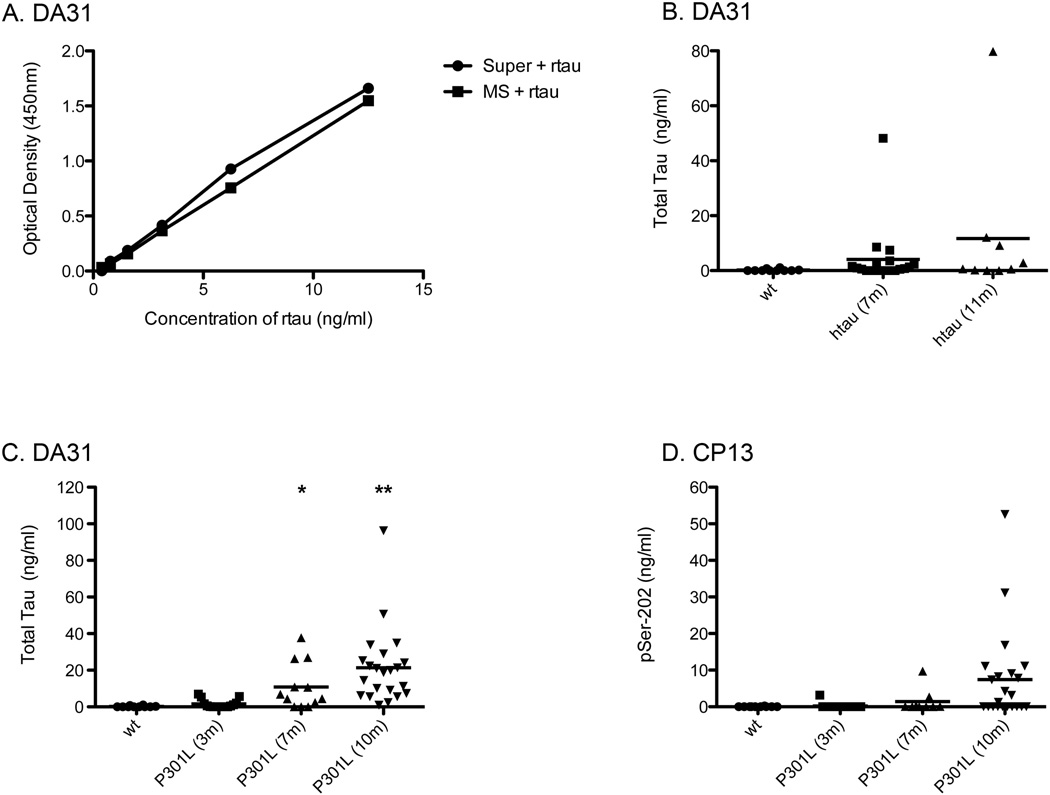
To examine the sensitivities of these four different ELISAs, recombinant tau or a sample of PHF-tau were used (Figure 2). Since there is a readily available supply of recombinant tau, with a known concentration, the absolute sensitivity of the DA31 capture ELISA was determined to be around 24pg/ml (Figure 2a). This was defined as the rtau concentration that gives an optical density signal greater than background plus two times the standard deviation of the background. It is not possible to determine the absolute sensitivity of the phospho-tau ELISAs, since there are no standard samples of tau available that are known to be fully phosphorylated at the appropriate site. With that in mind, all four ELISAs relative sensitivities were analyzed against PHF-tau (Figure 2b, c, d, e). The different ELISAs show similar sensitivity to PHF-tau. Both DA31 and PHF-1 required a PHF-tau concentration around 5ng/ml to obtain an optical density of around 1.0. Using CP13 or RZ3 as capture antibodies resulted in lower sensitivity to PHF-tau, required a tau concentration of approximately 10ng/ml. Overall, all four Low-Tau ELISAs examined are remarkably sensitive to PHF-tau.
Figure 2. Standard dilution curve for Low-Tau ELISAs.
A. Analysis of recombinant tau (rtau) dilution curve, with the total-tau antibody capture, DA31. B, C, D, E. Analyses of the relative sensitivities of total tau and phospho-tau ELISA to the PHF-tau preparation: DA31 (B), PHF-1 (C), CP13 (D), and RZ3 (E). Tau concentration of PHF-tau determined based on ng/ml of tau in PHF-tau sample, a direct comparison of the dilution curves of the same PHF-tau sample and rtau on DA31 capture ELISA.
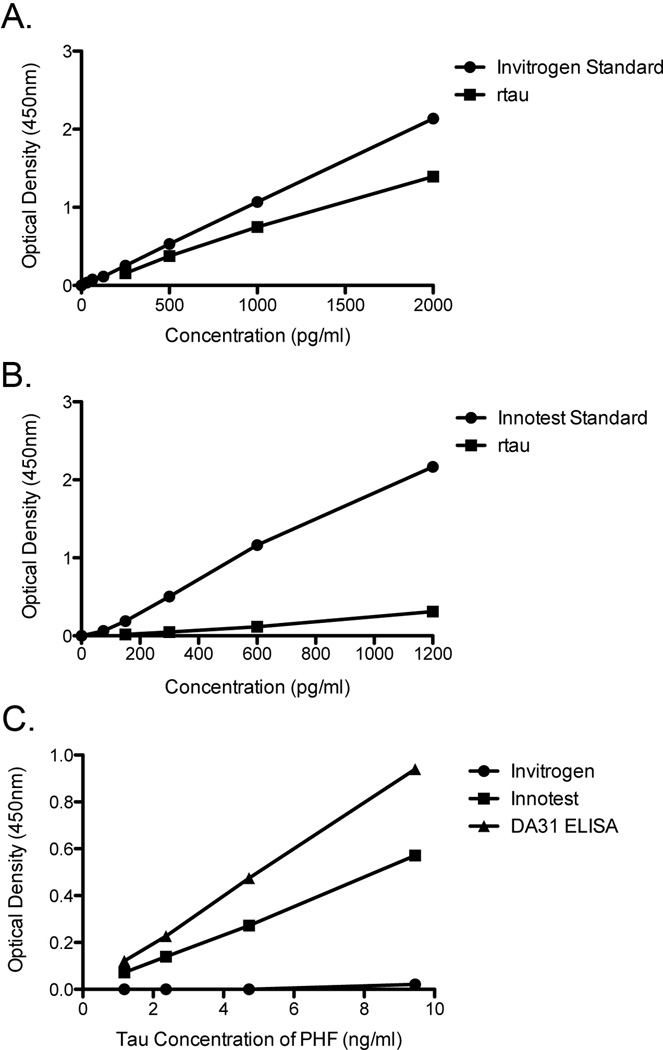
Two commercially available total tau ELISAs, from Innotest and Invitrogen that are commonly used for CSF-tau detection were compared to our total tau ELISA (DA31). Direct comparison between various protocols is extremely difficult, since each assay is optimized to different tau standards. Therefore, recombinant tau was used to compare relative sensitivities of these two assays with the provided standard. On the Invitrogen plate, the optical density values from the provided standard were comparable to rtau at the same concentrations (Figure 3a). Interestingly, when using the Innotest assay, the optical density values were much lower with rtau compared to the kit standard at the same apparent concentration (Figure 3b). To be able to confidently use commercial ELISA kits for the quantification of tau pathology in brain, it is important that the assays recognize PHF-tau. The PHF-tau preparation was analyzed with all three total tau assays. Surprisingly, Invitrogen’s total tau ELISA did not recognize PHF-tau, while the Innotest total tau ELISA did, but with lower sensitivity than the DA31 ELISA (Figure 3c). Overall, our Low-Tau ELISA has increased sensitivity to pathological PHF-tau, with similar sensitivity to recombinant tau when compared to the Invitrogen and Innotest ELISAs.
Figure 3. Comparison of commercial total tau ELISAs.
A. Analysis of the provided total tau standard and rtau on Invitrogen total tau ELISA kit demonstrates similar reactivity at the same concentrations. B. Analysis of rtau and provided total tau standard on Innotest total tau ELISA kit shows less reactivity to rtau at the same concentrations. C. A serial dilution of the same PHF-tau sample in the three different total tau ELISAs, show that DA31 ELISA has improved sensitivity to PHF-tau compared to the Innotest assay, while the Invitrogen ELISA does not recognize PHF-tau. Tau concentration of PHF-tau was determined based on ng/ml of tau in PHF-tau sample, using a direct comparison of the dilution curves of the same PHF-tau sample and rtau on the DA31 capture ELISA.
3.3. Specificity and quantification of ELISAs in tau transgenic brain homogenates
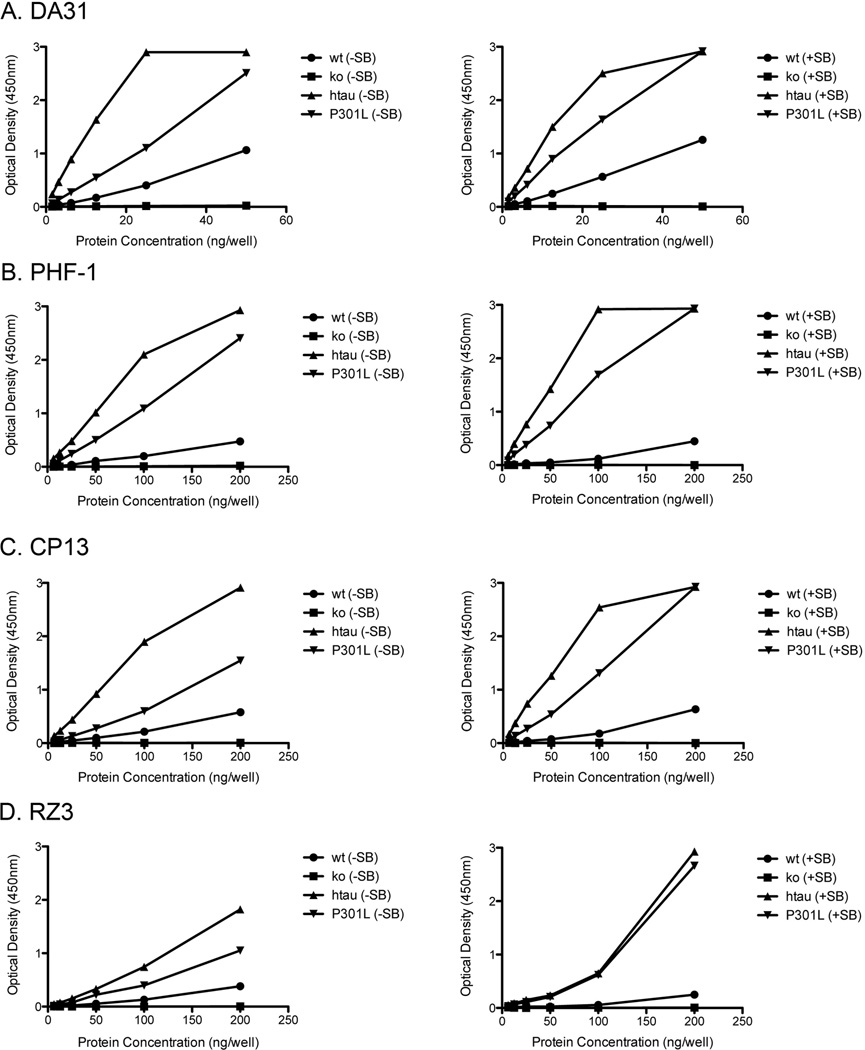
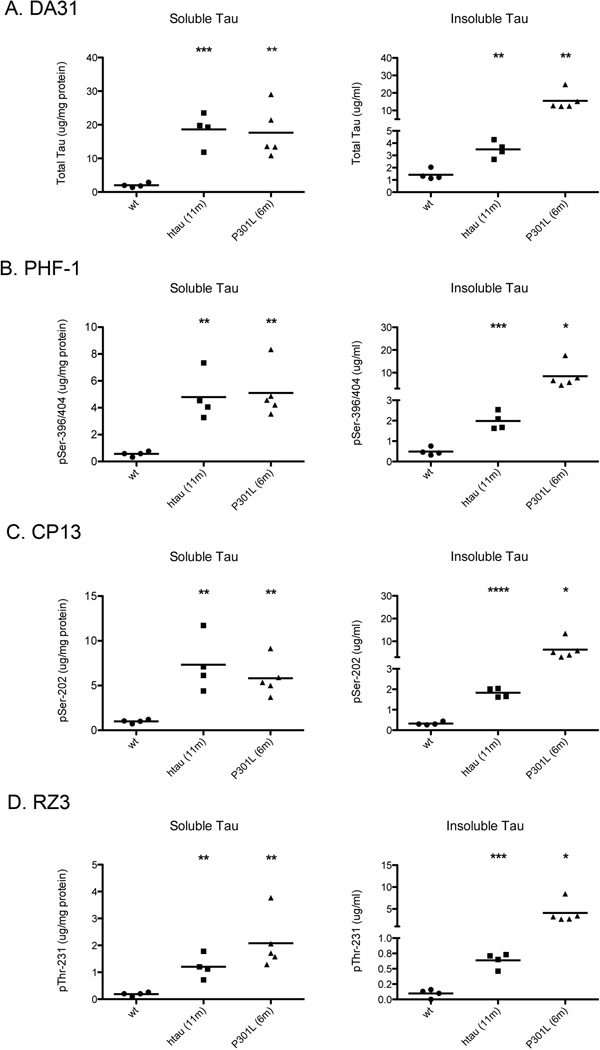
Since there are numerous well-established tau transgenic mouse models, the specificity of these Low-Tau Sandwich ELISAs were scrutinized against four different lines of mice, wild type, tau KO, htau and JNPL3. All four ELISAs are specific for mouse tau, normal human tau and the mutated P301L tau, while showing no reactivity to tau KO brain homogenates (Figure 4). Analyses were performed on the same forebrain lysates with (Figure 4, right column) or without (Figure 4, left column) denaturation in SDS/PAGE sample buffer. At the sample dilutions used, there is no interference from the β-mercapto-ethanol or SDS reagents contained in the sample buffer, and denaturing the protein elicits an increased response in these ELISAs, presumably by exposing more of the antibody epitopes. It is thus possible to prepare a single sample for both quantitative assays and qualitative studies by immunoblotting. To demonstrate the quantification potential of these ELISAs, total (soluble) and insoluble tau fractions were generated from wild type, htau at 11 months, and P301L at 6 months. Both tau transgenic models had increased levels of soluble and insoluble total tau (Figure 5a) and phosphorylated tau at pSer-396/404, pSer-202, and pThr-231 (Figure 5b, c, d) when compared to wild type mice. The four ELISAs demonstrate an accurate and reproducible method to measure tau pathology in the brains of various models. The Invitrogen and Innotest assay kits are designed for measurement of human tau, and assay of mouse tau requires purchase of separate kits. In contrast, the four assays we have developed are equally efficient at measurement of mouse and human tau.
Figure 4. Specificity of Low-Tau ELISAs on mouse brain homogenates.
A, B, C, D. DA31 (A), PHF-1 (B), CP13 (C) and RZ3 (D) capture ELISAs demonstrate specificity to mouse (wt) and human tau (htau and P301L), with no reactivity to a transgenic mouse lacking tau (tau KO). Same ELISAs also show tremendous versatility in the ability to use various sample preparations. All four ELISAs react with forebrain lysate prepared with (right panel) and without (left panel) Laemmli sample buffer.
Figure 5. Quantification of soluble and insoluble tau in tau transgenic mice.
A, B, C, D. Quantitative analysis of DA31 (A), PHF-1 (B), CP13 (C), and RZ3 (D) ELISAs on the soluble and insoluble tau preparations from brain homogenate of 11 month htau and 6 month P301L, compared to wild type mice. Both soluble and insoluble tau in the htau and P301L mice demonstrate expected increases in tau and phosphorylated tau at the observed ages. Unpaired t-test was used to calculate significance of soluble tau and insoluble tau transgenic brain homogenate when compared to wild type samples *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001.
3.4. Detection of total tau and phosphorylated tau in mouse serum
Since this Low-Tau Sandwich ELISA seems to be efficient at detecting lower levels of total tau with a calculated DA31 assay sensitivity around 24pg/ml, the sera samples from both tau transgenic mouse models were examined. First, spike experiments were performed in order to determine whether mouse serum interferes with the DA31 capture ELISA. Comparison of recombinant tau spiked in wild type mouse serum to recombinant tau in the normal diluent (20% Superblock) showed no difference in the standard dilution curve (Figure 6a). Using the DA31 and CP13 capture ELISAs, it is possible to detect measurable levels of total tau and pSer-202 in the serum of these transgenic mice. There is an increased trend of total tau amounts in the sera of the htau mice beginning at 7 months of age, when compared to wild type mice (Figure 6b). However, the analyses of pSer-202 on the same samples showed that only a couple of mice had a detectable level of pSer-202, which correlated with a larger amount of total tau in the serum (data not shown). The total tau sera levels in the P301L mice showed a significant age dependent increase of tau, from 3 to 10-month-old mice, when compared to wild type sera (Figure 6c). Analysis of the pSer-202 levels in the sera of the same P301L mice showed an increased trend beginning around 10 months of age (Figure 6d).
Figure 6. Total tau and phospho-tau is detectable in mouse serum.
A. Serum collected from wild type mice and spiked with rtau (MS + rtau) show similar reactivity when compared to rtau added to the normal diluent 20% Superblock (Super + rtau), analysis on DA31 ELISA. B. DA31 ELISA analysis of htau mouse sera at 7 months and 10 months of age illustrates an increase trend, however not significant, in the amount of total tau detected compared to wild type sera. C. DA31 analysis of sera from P301L mice shows a significant age-dependent increase of total tau, beginning at 7 months of age and increasing in 10-month mice. D. CP13 analysis of the same sera from P301L mice shows an increased trend of pSer-202 tau at around 10 months of age. Unpaired t-test was used to calculate significance of tau in the sera of htau and P301L when compared to wild type sera, *= p<0.05, **=p<0.01.
4. Discussion
Here we have examined newly developed tau monoclonal sandwich enzyme-linked immunosorbent assays (ELISA). These ELISAs demonstrate a sensitive and versatile method of quantitatively measuring the levels of total and phosphorylated tau in the brain or serum of transgenic mouse models. The possibility of expanding the capture antibody to other relevant tau monoclonal antibodies in order to examine other phosphorylation sites associated with AD is obvious.
Initially, this method of ELISA was constructed to be able to accurately quantify tau pathology without any interference from mouse IgG. By directly conjugating the horseradish peroxidase to the detection antibody, we are able to accurately measure tau levels in brain and serum without interference from endogenous IgG. The direct conjugation of HRP to a total tau antibody also made it possible to expand the library of possible antibody combinations. Tau monoclonal antibodies with the same isotype are now possible to use concurrently, without any cross reactivity from secondary antibodies.
Many recent studies have demonstrated that both active and passive immunization targeting tau reduces tau pathology in the brains of transgenic mice (Asuni et al., 2007; Boimel et al., 2010; Boutajangout et al., 2011; Boutajangout et al., 2010; Chai et al., 2011). Using immunization as a method to treat tau pathology offers a fascinating approach to induce clearance of tau pathology in the brain. It is crucial to dissect the mechanism of tau clearance as well as epitope specificity of various immunization treatments (Gu and Sigurdsson, 2011). Future experiments utilizing these ELISA protocols will examine tau levels in sera of immunized mice, in attempts to elucidate the mechanism of tau clearance.
Another important observation with these tau ELISAs is the fact that SDS/PAGE sample buffer, specifically SDS and β-mercapto-ethanol, do not interfere with the binding of brain homogenates to the capture antibody, because the assays’ high sensitivities allow these reagents to be diluted out. In fact, denaturing the proteins with sample buffer gives more of a complete picture of the actual amount of tau and phosphorylated tau in the brain homogenate, by exposing more of the available epitopes. For practical purposes, this is also significant because a laboratory could run two complementary methods of analyses, semi-quantitative immunblotting and quantitative ELISA, with one sample preparation. Experimentally in these transgenic mice, a dilution of 1:8000 or 1:1500 with DA31 and a dilution of 1:1000 or 1:5000 with the phospho-tau ELISAs were used respectively for the quantification of soluble or insoluble tau. By using this method of analysis to quantify the soluble total tau levels in these tau transgenic models, we demonstrate nearly a 10-fold increase for both htau and P301L mice when compared to wild type mice. This is in agreement with previous work done by immunoblot analysis that estimated that the total tau levels in the htau mice were increased approximately 5-fold (Andorfer et al., 2003). However, it is in contrast with previous analysis of the P301L mice, in which the total tau levels are shown to be similar to the wild type tau levels (Lewis et al., 2000). Future experiments will be needed to examine the observed differences between these two complementary methods in the P301L mice. Another relevant observation is that these assays require around 10ug of tissue to elicit a signal. This low tissue requirement allows other potential applications for these assays, for example in experiments utilizing regional tissue dissection. By altering the capture antibody with antibodies against four different tau epitopes, and by varying the type of sample preparations, this tau ELISA is a sensitive and versatile method of quantifying tau pathology in a variety of experiments.
The majority of commercially available tau ELISAs are developed and optimized for the detection of tau in human CSF samples, with different tau standards used. Making direct comparisons between ELISA protocols is very difficult. The absolute sensitivities of each of the assays were within similar ranges: 24pg/ml (DA31), 59pg/ml (Innotest) and 12pg/ml (Invitrogen). All three total tau ELISAs had similar reactivity to the htau and P301L mice. One significant advantage of the Low-Tau ELISAs is that all four capture antibodies were developed for and initially screened against PHF-tau. Therefore, it is not unexpected that the DA31 ELISA has an enhanced signal against PHF-tau, compared to the commercial assays examined. With the lower cost and the availability of reliable phospho-tau antibodies that can also be used in complementary techniques, these ELISAs provide enhanced methods to quantitatively examine tau pathology in the brain.
To our knowledge, this is the first method developed that detects measurable levels of total tau and phosphorylated tau in the sera of transgenic mice. Since a large concentration of total tau in the serum is required to detect any signal of tau at the phosphorylated epitope, it would appear that tau in serum is phosphorylated only at a low level. The observed serum tau increases in tau transgenic mice are preliminary observations, and a great deal work is required for these ELISAs to translate to a potential biomarker of AD in patients. Interestingly, when examining the tau levels in the serum, it seems that there is a higher concentration and more consistent amount of serum tau in P301L mice compared to htau. This difference might be due simply to the transgene promoters; the htau transgene is derived from human PAC, H1 haplotype (Duff et al., 2000) and should be expressed only in neurons. In contrast, the JNLP3 line uses the mouse prion promoter that is expressed in neurons as well as other cells (Borchelt et al., 1996). Another possibility for the difference between tau models is the ages examined in this study. Serum tau levels may be comparable to CSF tau levels, in that total tau is a biomarker for neuronal degeneration, based on previous studies demonstrating that there is an increase of total tau and not phospho-tau in the CSF of patients that suffer acute stroke (Hesse et al., 2001) or Creutzfeldt-Jakob disease (Riemenschneider et al., 2003). The htau mice examined in this study were relatively young, given that the on onset of neurodegeneration in these mice occurs at approximately 14 months of age (Andorfer et al., 2005). In contrast, P301L mice exhibit neuronal loss around 5 months of age (Lewis et al., 2000). Future experiments are needed to determine whether tau in the serum correlates to the extent of NFT or neuronal loss in the brain of these animals.
As mentioned previously, there are many well-established ELISA protocols for quantifying total tau and phosphorylated tau in human CSF, and tau in the CSF has been shown to be elevated in AD patients (Blennow and Hampel, 2003; Hampel et al., 2010). CSF collection is possible in mouse models without contamination from blood or brain tissue (Barten et al., 2011; Liu and Duff, 2008). A recent study reports that, in three different tau transgenic lines, there is an age dependent increase of fragmented tau in the CSF (Barten et al., 2011). However, CSF collection methods for mice are notoriously difficult to reproduce, and only small volumes of CSF can be obtained, around 10µl. Taken with our findings of tau in the serum of transgenic mice, these Low-Tau Sandwich ELISAs might be a useful technique to connect CSF tau and serum tau while examining the mechanisms of tau as a biomarker in mouse or possibly larger animal models.
In summary, we have developed a series of sensitive and versatile Low-Tau Sandwich ELISAs that are a useful method to objectively quantify the soluble and insoluble levels of total tau and phosphorylated tau in transgenic models. These assays will be invaluable when attempting to understand the role that tau exerts in Alzheimer’s disease and other tauopathies. Antibodies used in these assays are available on request from pdavies@nshs.edu.
Acknowledgements
This work was supported by NIMH38623 and NIH-AG022102.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25(22):5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86(3):582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–9129. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103(1):26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Bancher C, Braak H, Fischer P, Jellinger KA. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer's and Parkinson's disease patients. Neurosci Lett. 1993;162(1–2):179–182. doi: 10.1016/0304-3940(93)90590-h. [DOI] [PubMed] [Google Scholar]
- Barten DM, Cadelina GW, Hoque N, DeCarr LB, Guss VL, Yang L, Sankaranarayanan S, Wes PD, Flynn ME, Meredith JE, Ahlijanian MK, Albright CF. Tau transgenic mice as models for cerebrospinal fluid tau biomarkers. J Alzheimers Dis. 2011;24(Suppl 2):127–141. doi: 10.3233/JAD-2011-110161. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2(10):605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224(2):472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13(6):159–163. doi: 10.1016/s1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118(4):658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30(49):16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O'Neill MJ, Hutton ML, Citron M. Passive immunization with anti-tau antibodies in two transgenic models: Reduction of tau pathology and delay of disease progression. J Biol Chem. 2011 doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. Characterization and use of monoclonal antibodies to tau and PHF-tau. Alzheimer's Disease: Methods and Protocols Methods in Molecular Medicine. 1999:361–374. doi: 10.1385/1-59259-195-7:361. [DOI] [PubMed] [Google Scholar]
- Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M, Malester B, Hutton M, Adamson J, Goedert M, Burki K, Davies P. Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol Dis. 2000;7(2):87–98. doi: 10.1006/nbdi.1999.0279. [DOI] [PubMed] [Google Scholar]
- Espinoza M, de Silva R, Dickson DW, Davies P. Differential incorporation of tau isoforms in Alzheimer's disease. J Alzheimers Dis. 2008;14(1):1–16. doi: 10.3233/jad-2008-14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41(1):17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Greenberg SG, Davies P, Schein JD, Binder LI. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992;267(1):564–569. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Sigurdsson EM. Immunotherapy for Tauopathies. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60(5):729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer's disease. Exp Gerontol. 2010;45(1):30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, Blennow K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297(3):187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Nukina N, Miura R, Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem. 1986;99(6):1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Jicha GA, O'Donnell A, Weaver C, Angeletti R, Davies P. Hierarchical phosphorylation of recombinant tau by the paired-helical filament-associated protein kinase is dependent on cyclic AMP-dependent protein kinase. J Neurochem. 1999;72(1):214–224. doi: 10.1046/j.1471-4159.1999.0720214.x. [DOI] [PubMed] [Google Scholar]
- Kimura T, Ono T, Takamatsu J, Yamamoto H, Ikegami K, Kondo A, Hasegawa M, Ihara Y, Miyamoto E, Miyakawa T. Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dementia. 1996;7(4):177–181. doi: 10.1159/000106875. [DOI] [PubMed] [Google Scholar]
- Lachno DR, Romeo MJ, Siemers ER, Vanderstichele H, Coart E, Konrad RJ, Zajac JJ, Talbot JA, Jensen HF, Sethuraman G, Demattos RB, May PC, Dean RA. Validation of ELISA Methods for Quantification of Total Tau and Phosporylated-Tau181 in Human Cerebrospinal Fluid with Measurement in Specimens from Two Alzheimer's Disease Studies. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110296. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am J Pathol. 2003a;162(1):213–218. doi: 10.1016/S0002-9440(10)63812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol. 2003b;32(9):1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- Liu L, Duff K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp. 2008;21 doi: 10.3791/960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Lawrence GP. How should the repeatability of clinical measurements be analysed? An assessment of analysis techniques with data from cardiovascular autonomic function tests. Q J Med. 1993;86(12):831–836. [PubMed] [Google Scholar]
- Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39(6):669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Polydoro M, Acker CM, Duff K, Castillo PE, Davies P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci. 2009;29(34):10741–10749. doi: 10.1523/JNEUROSCI.1065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P, Kas A, D'Souza I, Zhou Y, Pham Q, Stone M, Olson MV, Schellenberg GD. A genomic sequence analysis of the mouse and human microtubule-associated protein tau. Mamm Genome. 2001;12(9):700–712. doi: 10.1007/s00335-001-2044-8. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Kiessig ST. Enzyme immunoassay techniques. An overview. J Immunol Methods. 1992;150(1–2):5–21. doi: 10.1016/0022-1759(92)90061-w. [DOI] [PubMed] [Google Scholar]
- Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, Vanmechelen E, Forstl H, Kurz A. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8(3):343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A. 1998;95(13):7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MA, Acker CM, Davies P. Tau phosphorylated at tyrosine 394 is found in Alzheimer's disease tangles and can be a product of the Abl-related kinase, Arg. J Alzheimers Dis. 2010;19(2):721–733. doi: 10.3233/JAD-2010-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4(1):29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, De Vreese K, Blennow K, Andreasen N, Sindic C, Ivanoiu A, Hampel H, Burger K, Parnetti L, Lanari A, Padovani A, DiLuca M, Blaser M, Olsson AO, Pottel H, Hulstaert F, Vanmechelen E. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer's disease and dementia with Lewy bodies. Clin Chem Lab Med. 2006;44(12):1472–1480. doi: 10.1515/CCLM.2006.258. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Davies P, Dickson DW, Marambaud P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies. Acta Neuropathol. 2011;121(3):337–349. doi: 10.1007/s00401-010-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng-Fischhofer Q, Biernat J, Mandelkow EM, Illenberger S, Godemann R, Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur J Biochem. 1998;252(3):542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]