Abstract
We have previously described an approach whereby antibody Fc fragments harboring a single C-terminal selenocysteine residue (Fc-Sec) are directed against a variety of targets by changing the peptide or small molecule to which they are conjugated. In the present work we describe methodology for improving the efficacy of these Fc-Sec conjugates by incorporating cytotoxic drugs. The Fc-Sec protein is first programmed to target specific tumor cell types by attachment of a bifunctional linker that contains a “clickable” handle (e.g. cyclobutane or cyclooctyne) in addition to a tumor cell-binding peptide or small molecule. Following Fc-Sec conjugation, a cytotoxic warhead is then attached by cycloaddition reactions of tetrazine or azide-containing linker. To validate this approach we used a model system in which folic acid (FA) is the targeting moiety and a disulfide-linked biotin moiety serves as a cytotoxic drug surrogate. We demonstrated successful targeting of Fc-Sec proteins to folate-receptor expressing tumor cells. Tetrazine ligation was found to be an efficient method for biotin “arming” of the folate-targeted Fc-Sec proteins. We also report novel bioconjugation methodologies that use [4+2] cycloaddition reactions between tetrazines and cyclootynes.
The therapeutic value of monoclonal antibodies (mAbs) in cancer therapy is well established. One reason for the success of mAbs is that they intrinsically exhibit many ideal drug properties, including high target specificity and affinity and long circulatory half-life. However, therapeutic mAbs often lack the potency and tissue penetration (particularly against solid tumors) required to eradicate target cell populations.1, 2 A common strategy for improving antibody potency involves attaching powerful cytotoxic drugs that are too poisonous to be safely administered as single agents.3 Antibody-drug conjugates (ADCs) function as “smart bombs” that allow the toxin to bypass healthy tissue and hit only the targets of interest. The remaining problem with mAbs of poor solid tumor penetration has spurred research into alternative antibody-like proteins that retain high affinity and selectivity for tumor cells but are smaller than full-size antibodies.4 Therapeutic proteins based on antibody Fc (fragment crystallizable) fragments are attractive examples of this latter class, since they often exhibit serum half-lives close to those of full-size antibodies, yet they are much smaller in size.5 An additional property of Fc proteins is that they can recruit elements of the immune system to attack targets of interest via interaction with immune effector cells (antibody-dependent cellular cytotoxicity, ADCC) and complement (complement-dependent cytotoxicity, CDC).6
Once they are separated from their antigen binding (Fab) regions, Fc proteins lose their ability to attack specific cell populations. One method for restoring antigen-recognition to the Fc moiety involves genetically fusing tumor cell-binding peptides or proteins.7 While this can be an effective strategy (five Fc-fusion proteins are currently on the market5), introducing specificity for each new target requires its own protein engineering project. We have previously described an alternative approach for grafting antigen-binding sites onto Fc proteins that does not rely on genetic engineering.8, 9 This involves initial protein engineering to introduce a single selenocysteine (Sec) residue into the C-terminus of an IgG Fc fragment (Fc-Sec). A tumor cell-binding peptide or small molecule can then be attached using standard protein alkylation methodologies (e.g. via maleimide-containing linkers) to generate conjugates having a 1:1 ratio of targeting agent to protein. This approach allows a single generic Fc-Sec protein to be directed against a wide variety of targets by simply changing the peptide or small molecule to which it is conjugated.
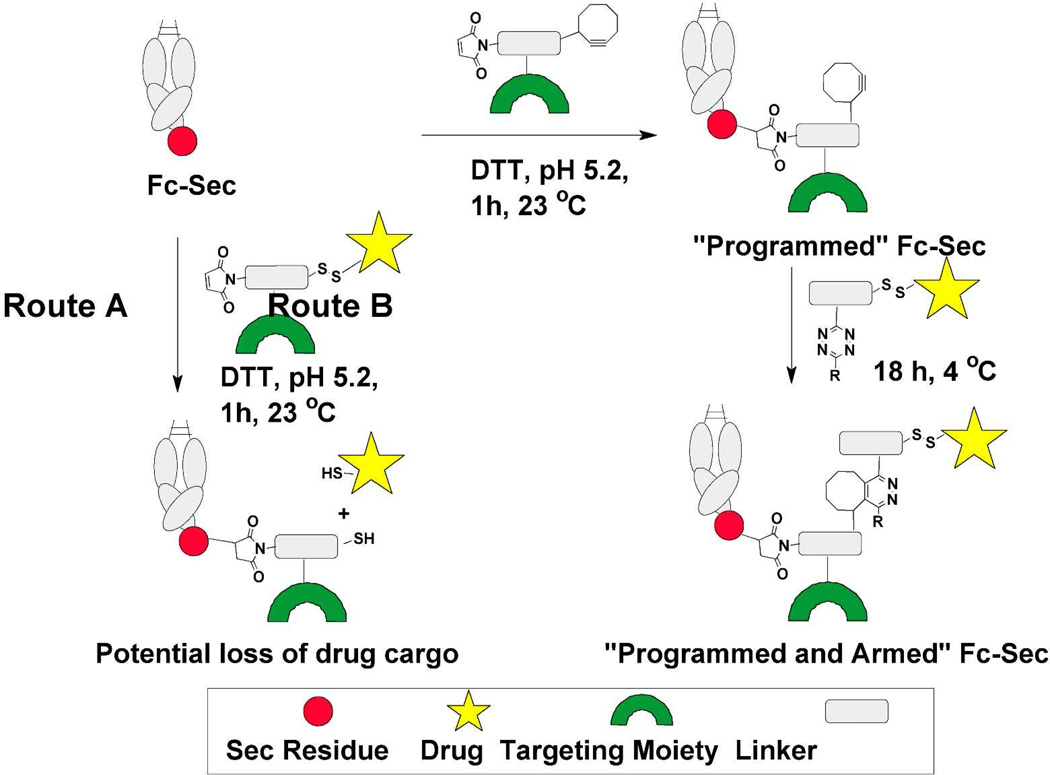
Although targeted Fc-Sec proteins may be therapeutically valuable in their own right, their efficacy may be improved by incorporating cytotoxic drugs. Several drug conjugate linker systems are designed to release their cargos under acidic (e.g. hydrazones) or reductive (e.g. disulfides) conditions. However, because Fc-Sec-conjugates are prepared under just such conditions, the use of these linker modalities is potentially contraindicated. In order to avoid the loss of drug cargo during conjugation to Fc-Sec proteins (Figure 1, Route A), we are examining two-step “program and arm” approaches (Figure 1, Route B). Here the Fc-Sec proteins are first programmed to target specific tumor cell types by attachment of bifunctional linkers that contain “clickable” handles (e.g. cyclobutane or cyclooctyne) in addition to tumor cell-binding peptides or small molecules. These linkers are conjugated to Fc-Sec proteins using previously established conditions (Figure 1).8 Following appropriate Fc-Sec conjugation, a tetrazine or azide-containing linker can be used to attach a cytotoxic warhead by means of cycloaddition reactions. Our current paper presents a model version of this “program and arm” concept in which folic acid (FA) serves as the targeting moiety, and a disulfide-linked biotin group is employed as a cytotoxic drug surrogate. We also report the use of [4+2] cycloaddition reactions between tetrazines and cyclootynes as novel bioconjugation methodologies.
Figure 1.
Two methods of producing Fc-Sec drug conjugates. Route A; a one-step approach that may result in loss of drug cargo and Route B; a two-step program and arm protocol that preserves drug cargo.
Since most previously prepared FA-drug conjugates rely on disulfide linkages for intracellular drug release,10 we chose this type of linkage for our Fc-Sec conjugates, although it was not clear whether disulfides would survive the reductive conditions previously established for Fc-Sec conjugation.8 We found that in the absence of Fc-Sec protein, a model disulfide was unstable under anticipated coupling conditions (25 equiv DTT at pH 5.2, 80 min, data not shown). This indicated that undesired reductive cleavage may occur if a disulfide-containing linker were used to alkylate Fc-Sec. Therefore, an alternative approach to Fc-Sec modification was examined in which sensitive functionality could be introduced following Fc-Sec alkylation (Figure 1, Route B).
Chemistries used to attach drug cargos should be both mild and chemoselective in order to preserve the well-defined stoichiometry and residue specificity of Fc-Sec conjugates.8, 9 A number of bioorthogonal “click” reactions that meet these requirements have been reported recently.11 Among these, the most widely utilized click reactions employ Cu(I)-catalyzed azide-alkyne [3+2] cycloaddition (CuAAC) chemistries.12 However, because it can be difficult to remove cytotoxic Cu from the conjugated products,13, 14 CuAAC may not be appropriate for constructing Fc-Sec drug conjugates. Therefore, we evaluated strain-promoted azide-alkyne cycloaddition (SPAAC)15 and tetrazine ligation (TL)16 as metal-free alternatives to CuAAC.
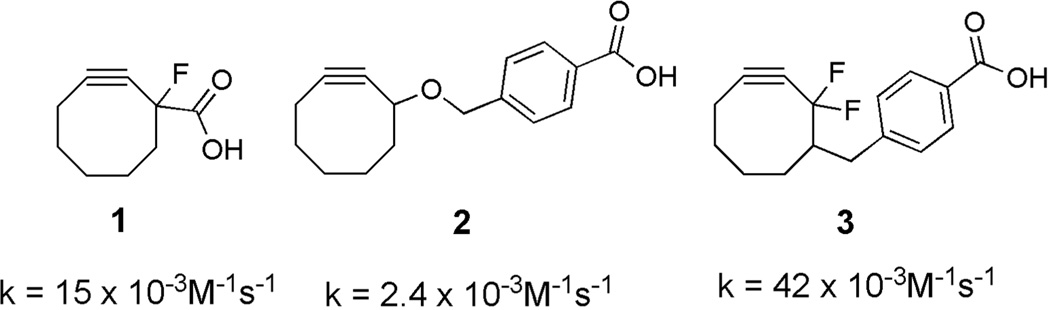
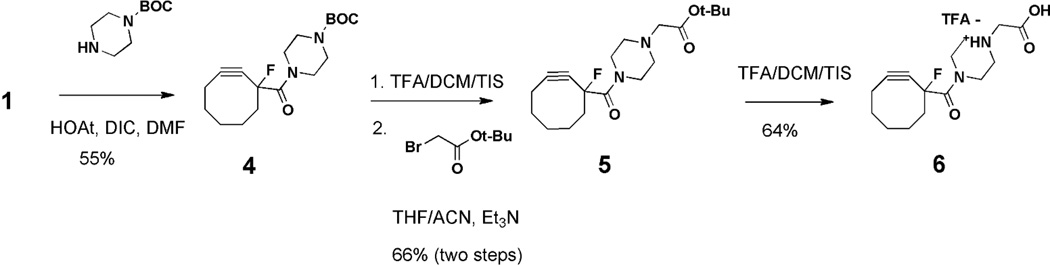
The synthetic complexity of many reactive cyclooctyne-containing reagents can be a major limitation to their use in SPAAC bioconjugations. This complexity often arises from the difficulty of introducing the required electron-withdrawing groups adjacent to the alkyne moiety.17 Although synthetic routes have improved in recent years, many of the most reactive cyclooctynes still require lengthy and low-yield procedures.15 For this reason, our attention was drawn to cyclooctyne 1,18 which affords a good balance between synthetic accessibility (3 steps, 47% overall yield for 1 versus 8 steps, ~25% overall yield for 319) and reactivity (relative reactivity compared to similar reagents: 3> 1 > 220, Figure 2). Cyclooctyne 1 was prepared according to literature procedures18 and converted to the water-soluble piperazine salt 6 in 4 - steps (Scheme 1). Although piperazine derivatives of 1 have not been reported, tertiary amines are well-known hydrophilic modifiers that can often improve the water solubility of liphophilic compounds.21, 22 Conversion to 6 improved the aqueous solubility of the cyclooctyne and rendered it as an easily handled solid.
Figure 2.
Examples of readily prepared cyclooctynes (1 and 2) and the synthetically more challenging 3. Rate constants refer to cycloaddition reactions with benzyl azide in CD3CN.18–20
Scheme 1.
Synthesis of the water-soluble cyclooctyne derivative 6.
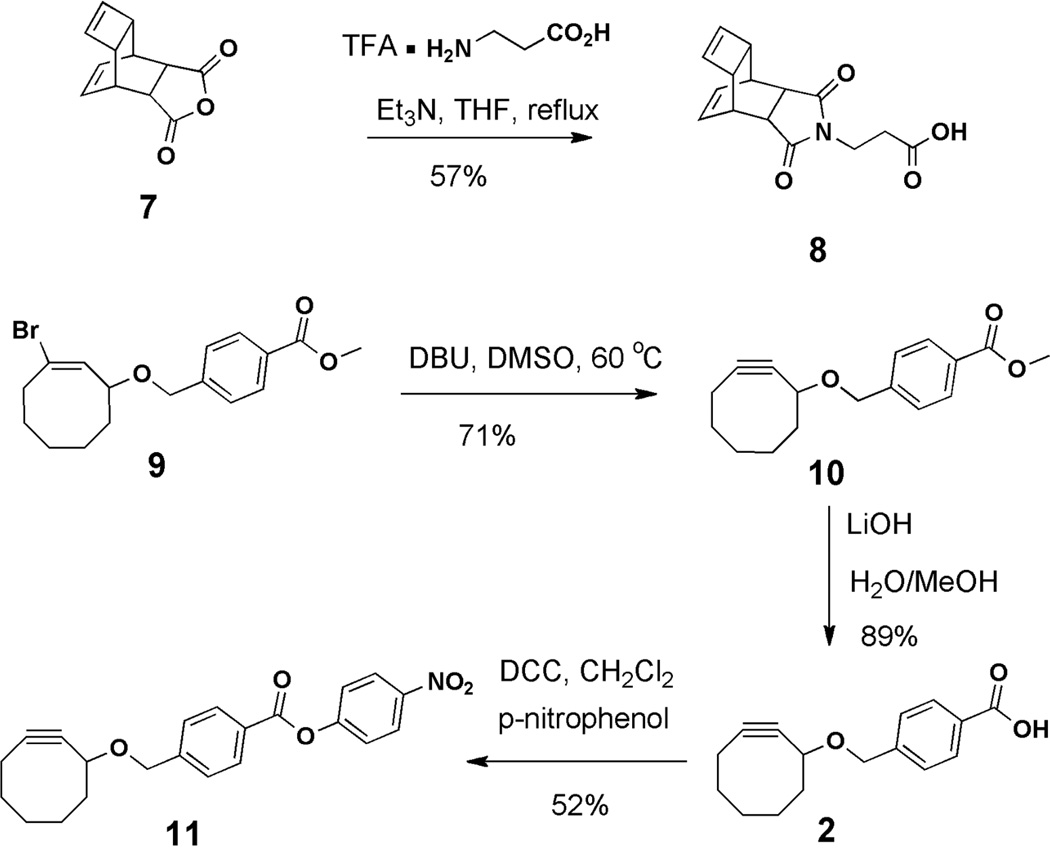
Although TL has appeared as a bioconjugation strategy only relatively recently,16 it is quickly becoming an attractive alternative to SPPAC.23 Tetrazine-based [4 + 2] cycloadditions are generally much faster than SPAAC reactions. They also utilize coupling partners (i.e. dienes and dienophiles) that are usually either commercially available or more synthetically accessible than cyclooctynes.24 This is exemplified by the dieneophile 8 used in the present work, which is both easily synthesized (Scheme 2) and an efficient TL coupling partner.25
Scheme 2.
Synthesis of TL dieneophiles 11 and 8.
Strained alkenes, such as norbornene, trans-cyclooctene, and cyclobutane have traditionally been the dienophiles of choice for TL strategies.15 Although other potentially suitable alkenes and alkynes, such as cyclooctyne, have been reported,26, 27 these have not yet been applied in a bioconjugation context. In order to evaluate the performance of cyclooctynes in side-by-side fashion with an established TL dieneophile such as 8, we included the known cyclooctyne 2 in the present study. The synthesis of 2 proceeded smoothly according to literature procedures,20 with the exception that the reported NaOMe-mediated bromide elimination in 9 to give 10 proved to be inefficient (Scheme 2). In contrast, the use of DBU at elevated temperature14 afforded 10 in good yield. Subsequent hydrolysis gave 2. Cyclooctyne 2 was converted to the corresponding p-nitrophenyl ester 11 prior to final incorporation into a folate-containing scaffold (Figure 3).
Figure 3.
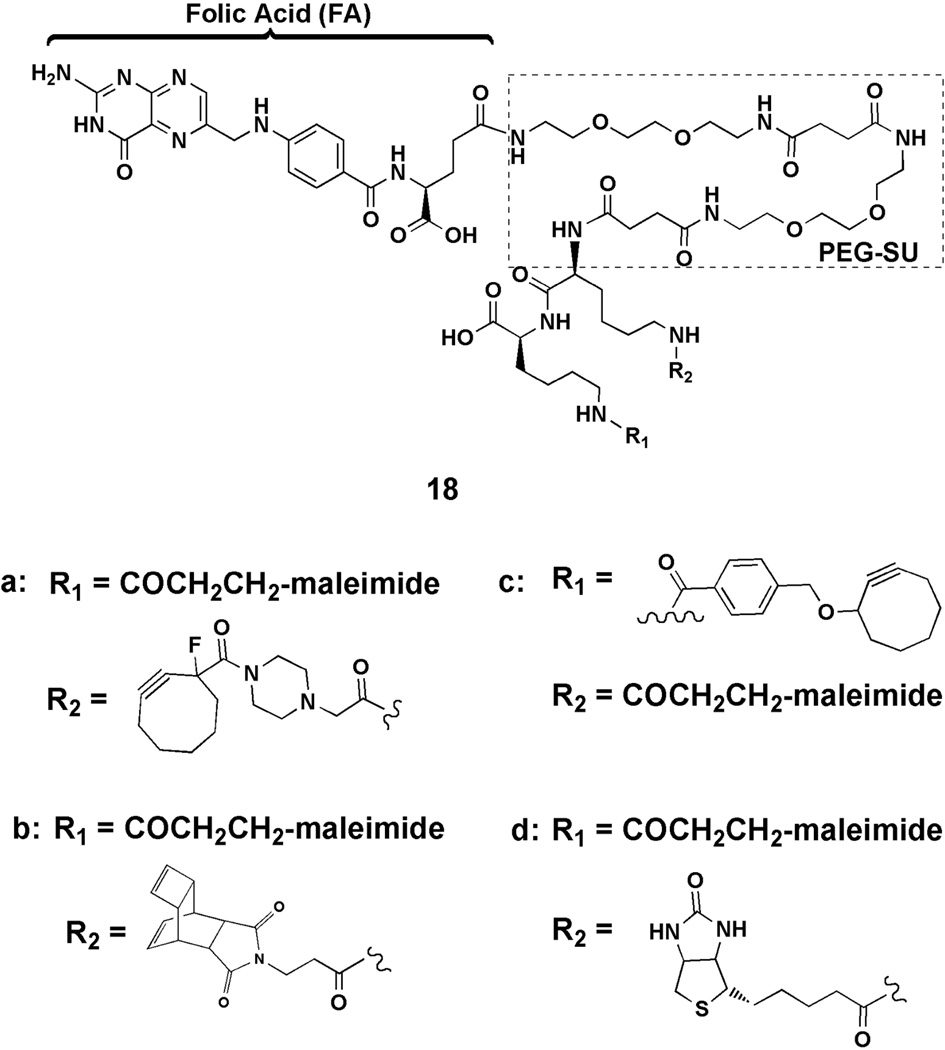
Bifunctional FA conjugates 18a–d for Fc-Sec programming.
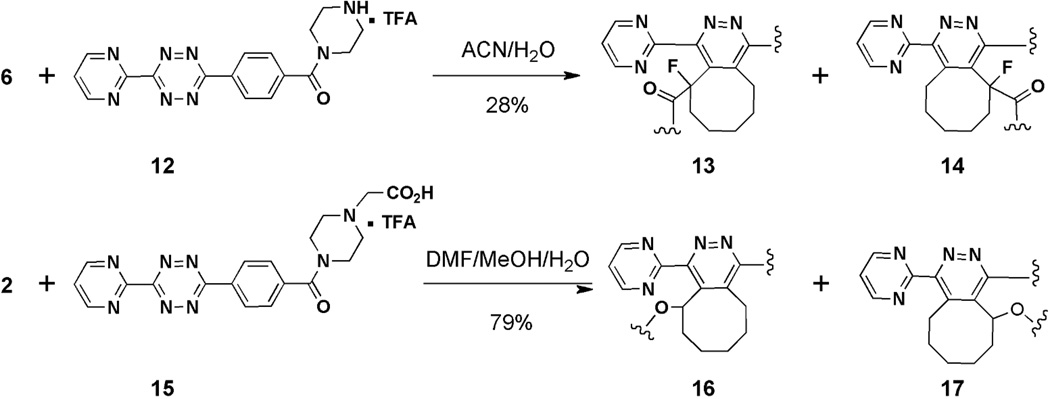
Before evaluating TL reactions with Fc-Sec constructs containing alkynes 2 and 6, their reactivities with tetrazines in free form were evaluated (Scheme 3). Reaction of 6 with tetrazine 12 resulted in a 28% combined yield of regioisomers 13 and 14, while the reaction between 2 and 15 proceeded smoothly to afford a mixture of 16 and 17 in 79% combined yield (Scheme 3). The ability of 2 and 6 to undergo [4 + 2] cycloaddition reactions with tetrazines in nonprotein-bound forms indicated that they would likely retain their reactivity once they were bound to Fc-Sec protein.
Scheme 3.
[4 + 2] Cycloaddition reactions of free 6 and 2 with tetrazines 12 and 15, respectively.
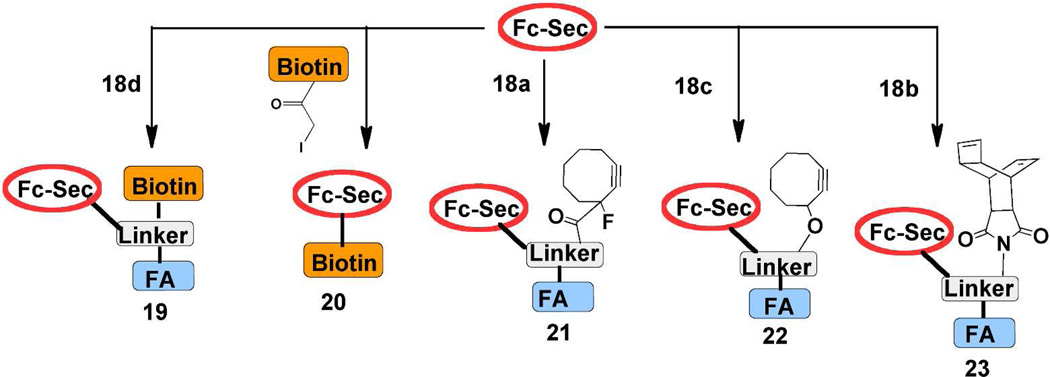
Bifunctional FA-containing constructs 18a–c, employing maleimide for Fc-Sec alkylation and clickable functionality for drug attachment, were prepared using a previously described PEG-SU-Lys-Lys-maleimide linker (Figure 3) (see Supporting Information).28 The biotin-containing derivative 18d was also prepared for use as a control in Fc-Sec alkylation and click experiments. In order to show that Fc-Sec programming was possible using 18a–d, Fc-Sec was first alkylated with 18d to afford 19 (Figure 4). Conjugate 19 was subsequently incubated with FA receptor-expressing (FR+) HeLa cells and analyzed by flow cytometry (Figure S1). For comparison, Fc-Sec was also alkylated using commercially available biotin-iodoacetamide to give the Fc-Sec-biotin adduct 20. It was found that 19 showed strong and selective binding to (FR+) cells (Figure S1A), whereas 20 failed to show binding (Figure S1B). Attempted alkylation of Fc-Stop8 (a nearly identical protein in which the C-terminal Sec residue is missing) with 18d resulted in only ~ 10% biotinylation relative to the same reaction with Fc-Sec (Figure S1C). These results demonstrate that alkylation of the Fc protein under our optimized conjugation conditions8 is very limited unless the protein harbors a Sec residue. This data also serves as indirect evidence that alkylation of Fc-Sec proteins occurs primarily at the C-terminal Sec residue and not at hinge cysteines.
Figure 4.
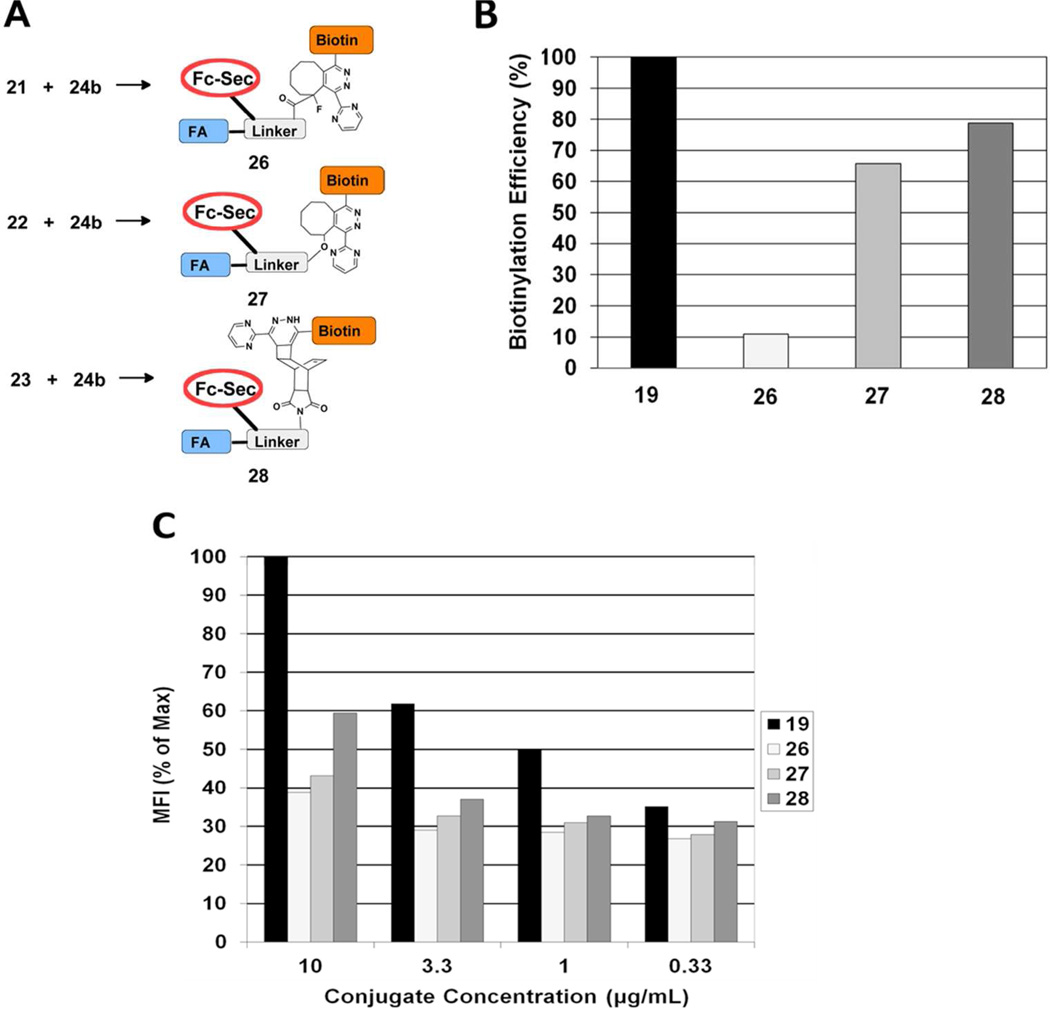
Fc-Sec was programmed with either 18d or biotin iodoacetamide to afford biotinylated constructs 19 and 20, respectively. Additional programming experiments were conducted with 18a, 18c, and 18b to afford “clickable” proteins 21–23.
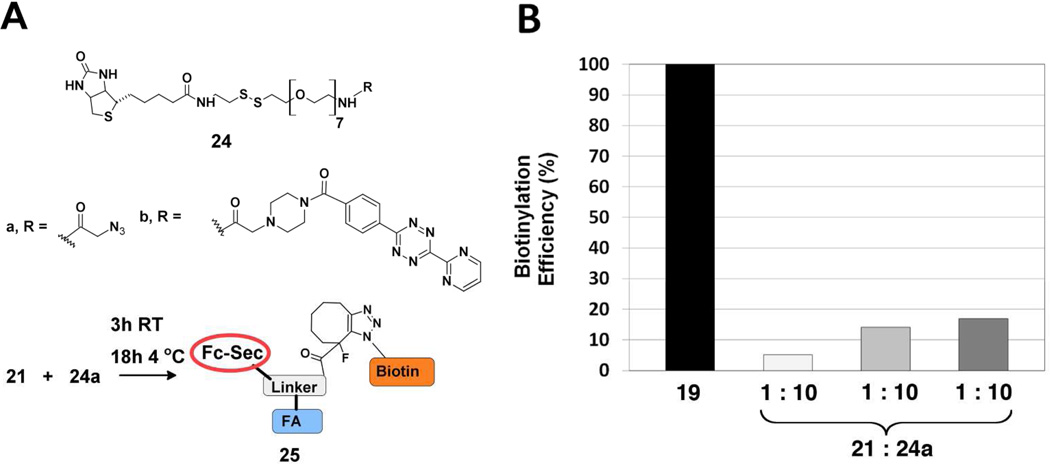
Having demonstrated successful programming with 18d, we next examined SPAAC and TL chemistries for “arming” Fc-Sec proteins (21–23) with either biotin-azide 24a29 or biotin-tetrazine 24b (Figure 5).29 The cycloaddition products (25–28) produced by these reactions were then analyzed by flow cytometry to determine the extent of biotinylation as described above for 19. Since conjugation occurs only at the single Sec residue, 19 consists of a 1:1:1 ratio of biotin:FA:Fc-Sec. Consequently, it represents the maximum level of biotinylation that can be achieved by the two-step approach shown in Figure 5 and could serve as a positive control for evaluating the efficiency of the SPAAC and TL reactions.
Figure 5.
Biotinylation reagents 24a and 24b used to “arm” Fc-Sec proteins 21–23. (A) SPAAC reaction of Fc-Sec protein 21 (5 µg/mL) with biotin azide 24a. Reactions were conducted at room temperature (3 h) then overnight (4 °C) in PBS, pH 7.4. (B) SPAAC reaction of 21 with 24a. Protein 21 was mixed with 10-, 50- and 100-fold excess 24a as described above and then incubated with FR(+) HeLa cells. The cells were stained with PE-coupled avidin and then analyzed by flow cytometry. The upper limit of biotinylation is defined as the mean fluorescence intensity (MFI) exhibited by 19. Biotinylation efficiency is expressed as 100 × (MFI25 / MFI19).
The SPAAC reaction of 21 with 24a was the least efficient method for arming Fc-Sec (Figure 5B). At the same reactant stoichiometry as used to prepare 19 (1:10 protein : small molecule modifier), the SPAAC reaction gave only 5% of the biotin load as was obtained for 19 (Figure 5B). At a 50-fold excess of 24a, the biotin load increased to approximately 15% of maximum. However, it did not improve substantially at higher concentrations of 24a (Figure 5B). The reaction of 22 with 24a was not examined. However, it would be expected to be even less efficient, based on the previously determined reaction rates of benzyl azide with 118 and 2.20
Of the three reagents tested with 24b in TL reactions, reagent 21 was the least reactive (Figure 6). Although this result was not unexpected given the electron deficient character of 1,26 it is noteworthy when compared to the analogous SPAAC reaction with 24a (Figure 5B). In this latter case, approximately the same levels of biotinylation were obtained with SPAAC as with TL, but at a 5-fold lower concentration of biotinylation reagent 24b (Figure 5). Protein 22 was much more effective reacting with 24b as compared with 21. Differences in steric crowding around the alkyne moieties in 6 and 2 may be partly responsible for the observed differences in reactivity between 21 and 22.26 Biotinylation efficiencies with the cyclobutane construct 23 approached 80% of the maximum, which made 23 the most reactive TL coupling partner tested. This is an important finding, since the dieneophile used in 23 (i.e. compound 8) was the simplest to prepare. It should also be noted that although fused cyclobutane-norborene compounds, such as 8, have been used previously in bioconjugation contexts,25, 30 this is the first example of their use in protein modification.
Figure 6.
(A) TL reactions of proteins 21–23 with biotin tetrazine 24b to generate 26–28 respectively. All reactions included 10-fold excess 24b and were conducted at room temperature (3 h) then overnight (4 °C) in PBS, pH 7.4. (B and C) Reaction products 26–28 were first incubated at the indicated concentrations (5 µg/mL data shown in B) with FR(+) HeLa cells and then analyzed by flow cytometry as described above. The upper limit of biotinylation defined as MFI exhibited by 19. (B) Biotinylation efficiency expressed as 100 × (MFI26, 27, or 28 / MFI19). (C) Maximum fluorescence signal defined as MFI of 19 at 10 µg/mL. All MFI data shown in C was normalized to MFI of 19 at 10 µg/mL.
The reactivity ranking observed in our initial experiments (23 > 22 > 21) was confirmed in subsequent titration binding experiments with 19, and 26–28 (Figure 6C). Among the three TL conjugates, 28 consistently gave higher MFI across the concentration range studied. These results suggest that the differences in biotin load observed in 26–28 accurately reflect differences in biotinylation efficiency of the various TL reactions and are not artifacts of the flow cytometry protocol used for their analysis.
In conclusion, reported herein is methodology whereby Fc-Sec proteins can be sequentially programmed to target cancer cells and then armed with cytotoxic payload using SPAAC or TL chemistries. This “program and arm” approach should be more compatible with cleaveable linkers, such as disulfides and hydrazones that may not otherwise survive the acidic, reducing conditions previously used to prepare Fc-Sec conjugates. Our work demonstrates for the first time that cyclooctynes can be effective dieneophiles in TL bioconjugation contexts. We are currently using these approaches to prepare Fc-Sec conjugates that are armed with cytotoxic drugs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the Intramural Research Program of the NIH, Center for Cancer Research, Frederick National Laboratory for Cancer Research and the National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Materials and methods including detailed synthetic procedures, Fc-Sec programming, arming of Fc-Sec-folate and flow cytometry are included. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 2.Ledford H. Toxic antibodies blitz tumours. Nature. 2011;476:380–381. doi: 10.1038/476380a. [DOI] [PubMed] [Google Scholar]
- 3.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 4.Gebauer M, Skerra A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr. Opin. Chem. Biol. 2009;13:245–255. doi: 10.1016/j.cbpa.2009.04.627. [DOI] [PubMed] [Google Scholar]
- 5.Carter PJ. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp. Cell Res. 2011;317:1261–1269. doi: 10.1016/j.yexcr.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C. Receptor-Fc fusion therapeutics, traps, and MIMETIBODY technology. Curr. Opin. Biotechnol. 2009;20:692–699. doi: 10.1016/j.copbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Hofer T, Thomas JD, Burke TR, Jr., Rader C. An engineered selenocysteine defines a unique class of antibody derivatives. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12451–12456. doi: 10.1073/pnas.0800800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofer T, Skeffington LR, Chapman CM, Rader C. Molecularly defined antibody conjugation through a selenocysteine interface. Biochemistry. 2009;48:12047–12057. doi: 10.1021/bi901744t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 11.Bertozzi CR. A decade of bioorthogonal chemistry. Acc. Chem. Res. 2011;44:651–653. doi: 10.1021/ar200193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Lallana E, Fernandez-Megia E, Riguera R. Surpassing the use of copper in the click functionalization of polymeric nanostructures: a strain-promoted approach. J. Am. Chem. Soc. 2009;131:5748–5750. doi: 10.1021/ja8100243. [DOI] [PubMed] [Google Scholar]
- 14.Ornelas C, Broichhagen J, Weck M. Strain-promoted alkyne azide cycloaddition for the functionalization of poly(amide)-based dendrons and dendrimers. J. Am. Chem. Soc. 2010;132:3923–3931. doi: 10.1021/ja910581d. [DOI] [PubMed] [Google Scholar]
- 15.Debets MF, van Berkel SS, Dommerholt J, Dirks AT, Rutjes FP, van Delft FL. Bioconjugation with strained alkenes and alkynes. Acc. Chem. Res. 2011;44:805–815. doi: 10.1021/ar200059z. [DOI] [PubMed] [Google Scholar]
- 16.Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz MK, Parameswarappa SG, Pigge FC. Synthesis of a DOTA--biotin conjugate for radionuclide chelation via Cu-free click chemistry. Org. Lett. 2010;12:2398–2401. doi: 10.1021/ol100774p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. Second-generation difluorinated cyclooctynes for copper-free click chemistry. J. Am. Chem. Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 21.Milosovich S, Hussain A, Dittert L, Aungst B, Hussain M. Testosteronyl-4-dimethylaminobutyrate-HCl: a prodrug with improved skin penetration rate. J. Pharm. Sci. 1993;82:227–228. doi: 10.1002/jps.2600820223. [DOI] [PubMed] [Google Scholar]
- 22.Rautio J, Nevalainen T, Taipale H, Vepsalainen J, Gynther J, Laine K, Jarvinen T. Synthesis and in vitro evaluation of novel morpholinyl- and methylpiperazinylacyloxyalkyl prodrugs of 2-(6-methoxy-2-naphthyl)propionic acid (Naproxen) for topical drug delivery. J. Med. Chem. 2000;43:1489–1494. doi: 10.1021/jm991149s. [DOI] [PubMed] [Google Scholar]
- 23.Devaraj NK, Weissleder R. Biomedical applications of tetrazine cycloadditions. Acc. Chem. Res. 2011;44:816–827. doi: 10.1021/ar200037t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewett JC, Bertozzi CR. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. Inverse-electron-demand Diels-Alder reaction as a highly efficient chemoselective ligation procedure: synthesis and function of a BioShuttle for temozolomide transport into prostate cancer cells. J. Pept. Sci. 2009;15:235–241. doi: 10.1002/psc.1108. [DOI] [PubMed] [Google Scholar]
- 26.Sauer J, Heldmann D, Hetzenegger J, Krauthan J, Sichert H, Schuster J. 1,2,4,5-Tetrazine: Synthesis and Reactivity in [4+2] Cycloadditions. Eur. J. Org. Chem. 1998:2885–2896. [Google Scholar]
- 27.Thalhammer F, Wallfahrer U, Sauer J. Reactivity of Simple Open-Chain and Cyclic Dienophiles in the Diels-Alder Reaction with Inverse Electron Demand. Tetrahedron Lett. 1990;31:6851–6854. [Google Scholar]
- 28.Thomas JD, Hofer T, Rader C, Burke TR., Jr. Application of a trifunctional reactive linker for the construction of antibody-drug hybrid conjugates. Bioorg. Med. Chem. Lett. 2008;18:5785–5788. doi: 10.1016/j.bmcl.2008.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas JD, Burke TR., Jr. Application of a water-soluble pyridyl disulfide amine linker for use in Cu-free click bioconjugation. Tetrahedron Lett. 2011;52:4316–4319. doi: 10.1016/j.tetlet.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiessler M, Waldeck W, Pipkorn R, Kliem C, Lorenz P, Fleischhacker H, Hafner M, Braun K. Extension of the PNA world by functionalized PNA monomers eligible candidates for inverse Diels Alder Click Chemistry. Int. J. Med. Sci. 2010;7:213–223. doi: 10.7150/ijms.7.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.