Abstract
Objective
To determine which baseline socio-demographic, lifestyle, anthropometric, clinical, and ocular risk factors predict the development of open-angle glaucoma (OAG) in an adult population.
Design
A population-based, prospective cohort study.
Participants
A total of 3,772 self-identified Latinos aged 40 years and older from Los Angeles, California who were free of OAG at baseline.
Methods
Participants from the Los Angeles Latino Eye Study had standardized study visits at baseline and 4-year follow-up with structured interviews and a comprehensive ophthalmologic examination. OAG was defined as the presence of an open angle and a glaucomatous visual field abnormality and/or evidence of glaucomatous optic nerve damage in at least one eye. Multivariate logistic regression with stepwise selection was performed to determine which potential baseline risk factors independently predict the development of OAG.
Main Outcome Measure
Odds ratios for various risk factors.
Results
Over the 4-year follow-up, 87 participants developed OAG. The baseline risk factors that predict the development of OAG include: older age (odds ratio [OR] per decade, 2.19; 95% confidence intervals [CI], 1.74-2.75; P<0.001), higher intraocular pressure (OR per mmHg, 1.18; 95% CI, 1.10-1.26; P<0.001), longer axial length (OR per mm, 1.48; 95% CI, 1.22-1.80; P<0.001), thinner central cornea (OR per 40 μm thinner, 1.30; 95% CI, 1.00-1.70; P=0.050) higher waist to hip ratio (OR per 0.05 higher, 1.21; 95% CI, 1.05-1.39; P=0.007) and lack of vision insurance (OR, 2.08; 95% CI, 1.26-3.41; P=0.004).
Conclusions
Despite a mean baseline IOP of 14 mmHg in Latinos, higher intraocular pressure is an important risk factor for developing OAG. Biometric measures suggestive of less structural support such as longer axial length and thin CCT were identified as important risk factors. Lack of health insurance reduces access to eye care and increases the burden of OAG by reducing the likelihood of early detection and treatment of OAG.
Primary open-angle glaucoma (OAG) is a progressive optic neuropathy that leads to irreversible vision loss. The disease is usually asymptomatic in its early stages. Consequently, under-diagnosis of OAG is common, with more than three-quarters of the persons with OAG being undiagnosed.1 Because medical and surgical treatments are effective in delaying or preventing the development of OAG, identifying high-risk populations for targeted surveillance and early intervention is essential to reducing vision loss from OAG.
Older age, high intraocular pressure (IOP), family history of glaucoma, and African ancestry are well-established risk factors for developing OAG.2 Factors that are likely to increase the impact of IOP on the lamina cribrosa, i.e., thinner central corneal thickness (CCT), have also been associated with a higher risk of developing OAG.3 Other potential, but inconsistently associated risk factors include longer axial length (myopia), systemic hypertension, cardiovascular diseases, obesity, and various lifestyle factors.4 Most of these associations were determined using cross-sectional studies, which are limited in estimating relative risks directly and establishing the temporal relationship between exposure and disease development. To our knowledge, only three population-based studies5-12 have rigorously investigated baseline risk factors that predict the development of OAG based on standardized ophthalmic examinations (Table 1). However, none of these studies have been conducted in the United States (U.S.) or have studied Latinos. As the fastest growing minority group in the U.S.,13 Latinos have a high age-specific prevalence of OAG; therefore, it is important to identify risk factors that predict the development of OAG in a population-based sample of Latinos. Furthermore, identifying modifiable risk factors can help inform the development of screening and intervention programs and potentially reduce the burden of vision loss from OAG.
TABLE 1.
Prior Population-Based Studies of Risk Factors for Incident Open-Angle Glaucoma Diagnosed by Standardized Ocular Examinations.
| Study | Population | Follow-up period | Number of cases with incident OAG | Baseline risk factors identified in each study |
|---|---|---|---|---|
| The Barbados Eye Study5 | 3,222 residents of Barbados, West Indies; aged 40-84 years old; 93% African-descent | 9 years from baseline (1987–1992) to follow-up (1992-1997) and 1997–2002). | 125 | Older age,5 family history of glaucoma,5 higher IOP,6 lower systolic blood pressure,5 lower ocular perfusion pressures,5 and thinner CCT.5 |
| The Rotterdam Study10 | 3,939 residents of Rotterdam, Netherlands; aged 55 years and older; 95% Caucasian. | 9.7 years from baseline (1990-1993) to follow-up (1997-1999 and 2002-2006). | 108 | Older age,7 higher baseline IOP,7 lower BMI for women,10 and use of calcium channel antagonists.9 |
| The Visual Impairment Project11 | 3,271 residents of Melbourne, Victoria, Australia; aged 40 years and older; 100% Caucasian. | 5 years from baseline (1992-1994) to follow-up (1997-1999). | 6612 | Older age,11 higher IOP,12 family history of glaucoma,12 presence of age-related macular degeneration,12 presence of pseudoexfoliation,12 a cup-disc ratio greater than 0.7,12 and use of a-blockers.12 |
OAG=Open-angle glaucoma; IOP=intraocular pressure; CCT=central corneal thickness; BMI=body mass index; US=the United States.
The objective of the present investigation was to identify baseline factors that may predict the development of OAG in a population-based, prospective cohort study of Latinos.
METHODS
Study Design and Population
The Los Angeles Latino Eye Study (LALES) is a population-based cohort study of eye disease in self-identified Latinos aged 40 years and older living in 6 census tracts in the city of La Puente, Los Angeles County, California. Details of the study design, methods, and baseline data have been reported elsewhere.14 Briefly, baseline examination was performed from 2000 to 2003 with 4-year follow-up examination from 2004 to 2008. All eligible participants of the baseline LALES examination were invited to return for a home interview and a clinical examination. Similar questionnaire and examination procedures were used for both baseline and follow-up studies. Trained ophthalmologists and technicians performed a comprehensive ocular examination using standardized protocols, which included visual field testing, Goldmann applanation IOP measurement, and simultaneous stereoscopic fundus photography of the optic disc. In addition, an interviewer-administered questionnaire was used to assess ocular and medical histories, and laboratory testing was performed to obtain objective diagnostic criteria. Among the 5,907 living eligible participants who had completed an in-home questionnaire and a clinical ophthalmology examination at baseline, 4,538 (77%) completed the 4-year follow-up clinical exam.
The study protocol was approved by the Institutional Review Board/Ethics Committee at the University of Southern California and adhered to the recommendations of the Declaration of Helsinki. Written, informed consent was obtained from all participants.
Diagnosis of OAG and definitions of incident of OAG
Detailed descriptions of all OAG diagnosis-related tests and definitions have been previously reported.1 In short, participants’ peripheral vision was tested using the Humphrey Automated Field Analyzer II (Carl Zeiss Meditech, Dublin, CA). Visual field (VF) was evaluated using a Swedish Interactive Threshold Algorithm (SITA) Standard C24. Optic nerve findings were evaluated from the simultaneous stereoscopic optic disc photographs using a stereoscopic viewer (Asahi viewer, Pentax, Englewood, CO).
A three-step process was used to determine the OAG diagnosis. First, two glaucoma specialists evaluated all clinical history and examination data, including visual acuity (VA), Van Herrick test results, gonioscopy results, evaluation of the anterior and posterior ocular segments, clinical optic disc and fundus evaluation, optic disc photographs, and VF results. Second, the two specialists determined the presence or absence of OAG using specific guidelines. The two specialists graded both optic disc photographs and VFs independently and masked to each other. In determining the diagnosis of glaucoma, the specialists classified each eye of each person with particular consideration to the optic disc photographs and VFs. The diagnosis was assigned to each eye if both graders agreed. In the event of disagreement, a third glaucoma specialist assessed the data. An agreement between two of the three specialists was used to assign the eye. Additionally, the principal investigator (RV) performed a confirmatory review of all cases diagnosed as OAG. Based on the presence or absence of optic disc damage, VF defect, or both, as well as the degree of compatibility of these changes with glaucoma, the diagnoses were classified into definite glaucoma/probable glaucoma, and ocular hypertension. Specifically, OAG was defined in the following ways:
The primary definition required the presence of an open-angle, congruent, characteristic, or compatible glaucomatous VF abnormality, and evidence of characteristic/compatible glaucomatous optic disc damage in at least one eye after ophthalmologic exclusion of other possible causes. Specifically, OAG was diagnosed if an open angle; at least two reliable, congruent VF tests (Humphrey C24 SITA Standard and/or full threshold C24-2); and optic disc damage characteristic of glaucoma were present, or if an open angle, at least one abnormal VF test, and optic disc damage characteristic or compatible with glaucoma were present.
Open-angle glaucoma was also diagnosed if there was an open angle and one of the following four criteria: (1) end-stage disease with VA of ≤20/200 and a cup–disc ratio of 1.0, an open angle, and absence of VF data; (2) at least one abnormal VF test with characteristic/compatible glaucomatous VF defects and no evidence of optic disc damage; (3) characteristic/compatible glaucomatous optic disc damage with no evidence of VF abnormality; and (4) other combinations of VF (lack of perfect congruence between the two or three VFs) and optic disc abnormalities that are both compatible with glaucoma. IOP level was not considered in establishing the diagnosis of OAG.
incident OAG was defined as the development of definite or probable OAG in either eye or both eyes during the follow-up period among participants who were free of OAG in both eyes at baseline.
Baseline Risk Factors
Details of risk factor measurements have been reported previously.15-19 Socio-demographic and lifestyle factors, medical histories, physiological measurements, and ocular factors were all evaluated as potential risk factors. The socio-demographic factors evaluated included age, gender, country of birth (U.S. vs. other), acculturation (high vs. low), native American ancestry, level of education (<6 years, 6-11 years, ≥12 years of education), employment status (employed, retired, other unemployed), income level (<$15k, $15k-<$30k, ≥$30k), marital status (married/with partner vs. other), possession of vision insurance in the 12 months before the baseline interview, and family history of glaucoma. Lifestyle factors analyzed included cigarette smoking status at baseline, age at first smoke, number of pack-years of smoking, number of years since cessation of smoking, history of alcohol use, and type of alcohol consumed. Medical history included history of type 2 diabetes mellitus (T2DM), duration of T2DM, levels of random blood glucose and HbA1c, and histories of hypertension, cardiovascular diseases, nuclear opacity, macular degeneration, and eye trauma. Physiological measurements included body mass index (BMI), waist-to-hip ratio (WHR), waist circumference, systemic blood pressures (systolic, diastolic, mean arterial, pulse pressure), and corresponding ocular perfusion pressures. Ocular factors included IOP at baseline, iris color, axial length, and CCT. Myopic refractive error was not included because of its high correlation with axial length. Corneal power was not included in the model selection because it was measured only among 41% of the participants.
Statistical Analyses
The current analysis aimed to examine potential risk factors that predict the development of OAG during a 4 year follow-up period among participants who were free of OAG in both eyes at baseline. Therefore, only participants who completed an in-home questionnaire at baseline and had reliable glaucoma data at both baseline and 4-year follow-up were included.
The development (incidence) of OAG was dichotomized into yes/no categories. One eye of each participant was selected based on the following criteria. If the participant had only one eye diagnosed with OAG, then that eye was selected. If both eyes were glaucomatous or non-glaucomatous, the eye with the worse mean deviation on Humphrey VF testing was selected.
Baseline characteristics of participants with and without incident OAG were compared using t tests for comparison of means and χ2 tests for comparison of proportions. Baseline risk factors were initially analyzed univariately. Continuous variables such as age, BMI, WHR, systemic blood pressure, ocular perfusion pressure, IOP, and other ocular measurements were included into the model as continuous variables as well as in clinically meaningful categorical groups. Multivariable logistic regression analyses with forward stepwise selection were used to identify baseline risk factors that predict the development of OAG (P ≤0.20 for entry into the model and P ≤0.10 for retention in the model). The variables selected in the final model were the same when we ran a full model which included the variables that best discriminate risk levels for each exposure in the univariate analysis, and when corrected Akaike information criterion 20, 21 was used as the criterion for selecting variables (data not shown).
To further describe the relationship between baseline factors and the risk of developing OAG, locally weighted scatter-plot smoothing (LOWESS) plots22 were generated, adjusting for other covariates from the final logistic regression model, using Stata software version 11 (StataCorp, College Station, TX) with a bandwidth of 0.80 and Cleveland's tricube weighting function. The LOWESS technique uses an iterative, locally weighted, least-squares method to produce a smooth fit line to the data and reduce the influence of outliers. LOWESS plot was analyzed in the following manner to determine at which level the incidence of OAG increased. First, the tangent slope to the LOWESS plot at each data point was calculated using the equation: slope=(Yi+1-Yi)/(Xi+1-Xi). Then, changes in the slope values were compared for each pair of consecutive points by measuring the ratio and difference between their slope values. The data point at which these 2 values were higher compared to previous slopes was considered to be the turning point in the increasing incidence of OAG.
Possible interactions between independent risk factors were tested by including proper cross-product terms in the regression models, and likelihood ratio tests comparing models with and without the interaction term were used to estimate the significance of the interaction. Category-specific attributable fraction was estimated using the formula Pdi×(ORi-1)/ORi described in Rockhill et al.23
SAS 9.2 (SAS Institute Inc., Cary, NC) was used for all statistical analyses, with a significance level set at P≤0.05. All reported P values are two-sided.
RESULTS
Among the 4,538 participants with an ophthalmic examination at both baseline and follow-up, 599 (13%) participants did not have complete data for glaucoma diagnosis: 398 had ungradable fundus photographs at follow-up, 118 underwent in-home examination, 48 refused dilation, 2 were physically unable to comply with the examination, and 33 with unknown reasons for incomplete data. Among the remaining 3,939 participants with complete reliable glaucoma data at both visits, 167 had been diagnosed with OAG at baseline; therefore 3,772 participants were at risk for developing OAG and thus included in the current analyses. Compared with non-participants, participants were older (54.6 vs. 53.7 years old, P=0.002) and more likely to be married (73.5% vs. 70.7%, P=0.03), have health insurance (67.2% vs. 56.8%, P<0.001), systemic co-morbidities (41.7% vs. 35.2%, P<0.001), and a history of systemic hypertension (30.1% vs. 26.8%, P=0.01), but were not significantly different in gender, birth place, acculturation, employment status, income level, education level, self-reported health status, and history of diabetes and ocular diseases.
Over a 4-year follow-up, 87 of the 3,772 eligible participants developed OAG, representing a 4-year incidence rate of 2.3%. Compared with participants who did not develop OAG during the 4-year follow-up (Table 2), those who did were significantly older, had higher systolic blood pressure, higher mean arterial blood pressure, higher pulse pressure, higher systolic perfusion pressure, and more likely to have a myopic refractive error, longer axial length, and nuclear opacity (all P values ≤0.004). Among participants with OAG, the mean IOP at baseline was 17 mmHg, with 15% having an IOP of >21 mmHg and 7% having received IOP-lowering treatment. In participants who did not develop OAG, the mean IOP at baseline was 14 mmHg, with 2% having an IOP of >21 mmHg and 1% having received IOP-lowering treatment. A more detailed univariate analysis of the association between baseline risk factors and risk of developing OAG in four years is presented in Table 3, available at http://aaojournal.org.
TABLE 2.
Baseline Characteristics of Participants With and Without Incident Open-angle Glaucoma in the Los Angeles Latino Eye Study
| Characteristics | Incident OAG (N=87) | No OAG (N=3,685) | P ** |
|---|---|---|---|
| Age, yrs* | 63±12 | 54±10 | <0.001 |
| Male gender | 47% | 40% | 0.18 |
| Married/with partner | 69% | 74% | 0.26 |
| History of T2DM | 27% | 19% | 0.072 |
| Family history of glaucoma | 10% | 9% | 0.70 |
| Blood pressure, mmHg* | |||
| Systolic | 131±21 | 123±18 | <0.001 |
| Diastolic | 77±13 | 76±11 | 0.32 |
| Mean arterial | 95±13 | 91±12 | 0.004 |
| Pulse | 54±19 | 47±14 | <0.001 |
| Perfusion pressure, mmHg* | |||
| Systolic | 115±20 | 108±18 | 0.001 |
| Diastolic | 61±13 | 61±11 | 0.61 |
| Mean arterial | 79±13 | 77±12 | 0.21 |
| IOP at baseline, mmHg* | 16.5±5.0 | 14.4±2.9 | <0.001 |
| IOP >21 mmHg at baseline | 15% | 2% | <0.001 |
| IOP lowering treatment at baseline | 7% | 1% | <0.001 |
| Refractive error, diopter | <0.001 | ||
| >-1 | 75% | 86% | |
| ≤-1 to >-3 | 10% | 9% | |
| ≤-3 | 15% | 4% | |
| Axial length, mm* | 23.7±1.0 | 23.3±1.1 | 0.003 |
| CCT, μm* | 547±44 | 551±35 | 0.43 |
| Corneal power, diopter*† | 43.5±1.7 | 43.8±1.7 | 0.34 |
| Presence of any nuclear opacity | 18% | 6% | <0.001 |
OAG=open angle glaucoma; IOP=intraocular pressure; CCT=central corneal thickness; T2DM=type 2 diabetes mellitus.
Mean ± Standard deviation.
P values were calculated using X2 test for categorical variables and t test for continuous variables.
Data on corneal power were available only for 1,369 participants.
TABLE 3.
Univariate Analysis of Baseline Factors Predicting the Development of OAG in the Los Angeles Latino Eye Study
| No. of participants | No. with incident OAG |
Univariate analysis*
|
||
|---|---|---|---|---|
| Risk factors | OR (95% CI) | P value | ||
| Age (yrs) | ||||
| 40–49 | 1459 | 14 | 1.00 (reference) | <0.001 |
| 50–59 | 1198 | 17 | 1.49 (0.73-3.03) | |
| 60–69 | 743 | 27 | 3.89 (2.03-7.47) | |
| 70–79 | 291 | 16 | 6.00 (2.90-12.44) | |
| ≥80 | 47 | 7 | 18.06 (6.91-47.16) | |
| Per 10 years older | - | - | 2.07 (1.71-2.52) | <0.001 |
| Gender | ||||
| Male | 1495 | 39 | 1.00 (reference) | 0.13 |
| Female | 2243 | 42 | 1.40 (0.90-2.18) | |
| Country of birth | ||||
| Other | 2864 | 58 | 1.00 (reference) | 0.28 |
| U.S. | 870 | 23 | 1.31 (0.81-2.14) | |
| Acculturation | ||||
| High (≥1.9) | 2513 | 53 | 1.00 (reference) | 0.72 |
| Low (<1.9) | 1221 | 28 | 0.92 (0.58-1.46) | |
| Native American ancestry | ||||
| No | 3554 | 78 | 1.00 (reference) | 0.62 |
| Yes | 180 | 3 | 0.76 (0.24-2.42) | |
| Education | ||||
| 0-5 yrs | 979 | 25 | 1.00 (reference) | 0.24 |
| 6-11 yrs | 1467 | 35 | 0.93 (0.55-1.57) | |
| High school graduate or more | 1285 | 21 | 0.63 (0.35-1.14) | |
| Employment | ||||
| Employed | 1903 | 30 | 1.00 (reference) | <0.001 |
| Unemployed | 1310 | 22 | 0.94 (0.54-1.63) | |
| Retired | 513 | 29 | 3.51 (2.00-6.17) | |
| Income | ||||
| <$15k | 1079 | 35 | 1.00 (reference) | 0.004 |
| $15k-<$30k | 1278 | 30 | 0.72 (0.44-1.18) | |
| ≥$30k | 911 | 10 | 0.33 (0.16-0.67) | |
| Refused/don't know | 470 | 6 | 0.39 (0.16-0.92) | |
| Marital status | ||||
| Married/with partner | 2774 | 57 | 1.00 (reference) | 0.41 |
| Other | 956 | 24 | 1.23 (0.76-1.99) | |
| Health insurance | ||||
| Yes | 2488 | 55 | 1.00 (reference) | 0.82 |
| No | 1242 | 26 | 0.94 (0.59-1.52) | |
| Vision insurance | ||||
| Yes | 1952 | 39 | 1.00 (reference) | 0.46 |
| No | 1744 | 41 | 1.18 (0.76-1.84) | |
| Smoking status | ||||
| Nonsmoker | 2304 | 47 | 1.00 (reference) | 0.68 |
| Ex-smoker | 912 | 23 | 1.24 (0.75-2.06) | |
| Current smoker | 504 | 10 | 0.97 (0.49-1.94) | |
| Pack-years among smokers | ||||
| >0–4 | 573 | 9 | 0.77 (0.37-1.57) | 0.23 |
| 5–19 | 461 | 11 | 1.17 (0.60-2.28) | |
| ≥20 | 289 | 11 | 1.90 (0.97-3.71) | |
| Age at first smoke | ||||
| <16 years old | 262 | 8 | 1.51 (0.71-3.24) | 0.79 |
| 16-20 years old | 611 | 13 | 1.04 (0.56-1.94) | |
| ≥21 years old | 541 | 12 | 1.09 (0.57-2.07) | |
| Years since cessation of smoking | ||||
| ≥20 years | 372 | 12 | 1.60 (0.84-3.05) | 0.58 |
| 10-19 years | 266 | 6 | 1.11 (0.47-2.62) | |
| 1–9 years | 255 | 5 | 0.96 (0.38-2.44) | |
| Alcohol drinking status | ||||
| None | 1449 | 25 | 1.00 (reference) | 0.028 |
| Ex-/partial drinker | 1782 | 50 | 1.64 (1.01-2.67) | |
| Current/heavy drinker | 496 | 6 | 0.70 (0.28-1.71) | |
| Alcohol type | ||||
| Wine | ||||
| Other/no alcohol | 3268 | 68 | 1.00 (reference) | 0.32 |
| Yes | 459 | 13 | 1.37 (0.75-2.50) | |
| Beer | ||||
| Other/no alcohol | 2288 | 47 | 1.00 (reference) | 0.53 |
| Yes | 1439 | 34 | 1.15 (0.74-1.80) | |
| Liquor | ||||
| Other/no alcohol | 3010 | 69 | 1.00 (reference) | 0.29 |
| Yes | 717 | 12 | 0.73 (0.39-1.35) | |
| Wine and beer | ||||
| Other/no alcohol | 3431 | 72 | 1.00 (reference) | 0.31 |
| Yes | 296 | 9 | 1.46 (0.72-2.96) | |
| Wine and liquor | ||||
| Other/no alcohol | 3479 | 77 | 1.00 (reference) | 0.51 |
| Yes | 248 | 4 | 0.72 (0.26-2.00) | |
| Beer and liquor | ||||
| Other/no alcohol | 3204 | 70 | 1.00 (reference) | 0.91 |
| Yes | 523 | 11 | 0.96 (0.51-1.83) | |
| History of glaucoma among 1st-degree relatives | ||||
| No | 3326 | 71 | 1.00 (reference) | 0.96 |
| Yes | 239 | 5 | 0.98 (0.39-2.45) | |
| Presence of Type 2 diabetes | ||||
| No** | 3009 | 60 | 1.00 (reference) | 0.12 |
| Yes | 701 | 21 | 1.52 (0.92-2.51) | |
| Duration of Type 2 diabetes | ||||
| New case | 134 | 3 | 1.13 (0.35-3.64) | 0.28 |
| <10 years | 345 | 13 | 1.92 (1.05-3.54) | |
| ≥10 years | 218 | 5 | 1.15 (0.46-2.90) | |
| Hemoglobin A1c | ||||
| <7% | 3173 | 67 | 1.00 (reference) | 0.49 |
| ≥7% | 541 | 14 | 1.23 (0.69-2.21) | |
| Random blood glucose | ||||
| <200 ml/dl | 3392 | 75 | 1.00 (reference) | 0.71 |
| ≥ 200 ml/dl | 316 | 6 | 0.86 (0.37-1.98) | |
| Waist circumference | ||||
| ≤median (99.5cm) | 1878 | 35 | 1.00 (reference) | 0.21 |
| >median | 1826 | 45 | 1.33 (0.85-2.08) | |
| Per cm larger | 1.01 (1.00-1.03) | 0.16 | ||
| Waist-hip ratio | ||||
| Q1 ( ≤0.925) | 931 | 12 | 1.00 (reference) | 0.001 |
| Q2 (>0.925-0.963) | 928 | 12 | 1.00 (0.45-2.24) | |
| Q3 ( >0.963-1.005) | 922 | 21 | 1.78 (0.87-3.65) | |
| Q4 (>1.005) | 920 | 35 | 3.03 (1.56-5.87) | |
| Per 0.05 higher | - | - | 1.33 (1.17-1.5) | <0.001 |
| Body mass index, kg/m2 | ||||
| <25 | 395 | 9 | 1.00 (reference) | 0.68 |
| 25-<30 | 1388 | 33 | 1.04 (0.50-2.20) | |
| ≥30 | 1908 | 37 | 0.85 (0.41-1.77) | |
| Per 1.0 higher | - | - | 0.99 (0.95-1.03) | 0.49 |
| History of age-related macular degeneration | ||||
| No | 3300 | 69 | 1.00 (reference) | 0.71 |
| Yes | 332 | 8 | 1.16 (0.55-2.43) | |
| Nuclear opacity | ||||
| No | 3415 | 63 | 1.00 (reference) | 0.0 01 |
| Yes | 223 | 13 | 3.29 (1.78-6.08) | |
| History of eye trauma | ||||
| No | 2996 | 65 | 1.00 (reference) | 1.00 |
| Yes | 738 | 16 | 1.00 (0.57-1.74) | |
| Cardiovascular disease | ||||
| No | 2532 | 48 | 1.00 (reference) | 0.10 |
| Yes | 1202 | 33 | 1.46 (0.93-2.29) | |
| History of elevated blood pressure (including history of medical treatment) | ||||
| No | 2632 | 52 | 1.00 (reference) | 0.21 |
| Yes | 1094 | 29 | 1.35 (0.85-2.14) | |
| Systolic blood pressure | ||||
| ≤120 mm Hg | 1880 | 26 | 1.00 (reference) | 0.003 |
| 121–140 mm Hg | 1240 | 35 | 2.07 (1.24-3.46) | |
| >140 mm Hg | 606 | 20 | 2.43 (1.35-4.39) | |
| Per 10 mmHg higher | - | - | 1.24 (1.12-1.38) | <0.001 |
| Diastolic blood pressure | ||||
| ≤80 mm Hg | 2566 | 47 | 1.00 (reference) | 0.0 84 |
| 81–90 mm Hg | 855 | 23 | 1.48 (0.89-2.45) | |
| >90 mm Hg | 305 | 11 | 2.01 (1.03-3.91) | |
| Per 10 mmHg higher | - | - | 1.14 (0.93-1.40) | 0.20 |
| Mean arterial blood pressure | ||||
| ≤80 mm Hg | 603 | 10 | 1.00 (reference) | 0.029 |
| 81-90 mm Hg | 1173 | 17 | 0.87 (0.40-1.92) | |
| >90 mm Hg | 1950 | 54 | 1.69 (0.85-3.34) | |
| Per 10 mmHg higher | - | - | 1.29 (1.09-1.52) | 0.005 |
| Pulse Pressure | ||||
| ≤54 mm Hg | 2791 | 48 | 1.00 (reference) | 0.007 |
| 55-64 mm Hg | 513 | 14 | 1.60 (0.88-2.93) | |
| 65-80 mm Hg | 314 | 13 | 2.47 (1.32-4.61) | |
| >80 mm Hg | 108 | 6 | 3.36 (1.41-8.04) | |
| Per 10 mmHg higher | - | - | 1.32 (1.16-1.49) | <0.001 |
| Intraocular pressure | ||||
| <15 mm Hg | 2350 | 35 | 1.00 (reference) | <0.001 |
| 15 to 20 mm Hg | 1288 | 34 | 1.79 (1.11-2.89) | |
| >21 mm Hg | 81 | 12 | 11.50 (5.72-23.12) | |
| Per mm Hg higher | - | - | 1.19 (1.12-1.26) | <0.001 |
| Systolic perfusion pressure | ||||
| ≤120 mm Hg | 1296 | 14 | 1.00 (reference) | 0.0 02 |
| 121–140 mm Hg | 1580 | 42 | 2.50 (1.36-4.60) | |
| >140 mm Hg | 843 | 25 | 2.80 (1.45-5.41) | |
| Per 10 mmHg higher | - | - | 1.19 (1.07-1.33) | 0.0 02 |
| Diastolic perfusion pressure | ||||
| ≤60 mm Hg | 1712 | 35 | 1.00 (reference) | 0.83 |
| 61–70 mm Hg | 1312 | 29 | 1.08 (0.66-1.78) | |
| >70 mm Hg | 695 | 17 | 1.20 (0.67-2.16) | |
| Per 10 mmHg higher | - | - | 0.96 (0.79-1.18) | 0.72 |
| Mean arterial perfusion pressure | ||||
| ≤70 mm Hg | 1020 | 20 | 1.00 (reference) | 0.69 |
| 71–80 mm Hg | 1305 | 27 | 1.06 (0.59-1.89) | |
| >80 mm Hg | 1394 | 34 | 1.25 (0.72-2.18) | |
| Per 10 mmHg higher | 1.29 (1.09-1.52) | 0.005 | ||
| Iris color | ||||
| Blue/Green/Hazel | 1285 | 32 | 1.00 (reference) | 0.40 |
| Light brown | 1961 | 42 | 0.86 (0.54-1.36) | |
| Dark brown | 477 | 7 | 0.58 (0.26-1.33) | |
| Central corneal thickness | ||||
| Per 40 pm thinner | - | - | 1.16 (0.90-1.50) | 0.25 |
| Refractive error | ||||
| >-1 D | 3204 | 61 | 1.00 (reference) | |
| ≤-1 D and >-3 D | 341 | 7 | 1.08 (0.49-2.38) | 0.85 |
| ≤ -3 D | 171 | 11 | 3.54 (1.83-6.86) | <0.001 |
| Axial Length | ||||
| Per mm longer | - | - | 1.35 (1.14-1.61) | 0.002 |
OR=odds ratio; CI=confidence interval; OAG=open-angle glaucoma; U.S.=United States; D=diopter.
Based on univariate logistic regression models. P values were estimated from likelihood ratio tests. Data from 34 persons (<1%) reporting intraocular pressure-lowering treatment at baseline were excluded.
Subjects diagnosed with type 1 diabetes were excluded from the analysis
In the multivariate analysis, the following baseline risk factors predicted the development of OAG over a 4-year follow-up period: older age, higher IOP, thinner central cornea, longer axial length, greater WHR, and lack of vision insurance in the past 12 months (Table 4).
TABLE 4.
Independent Baseline Predictors and the Associated Risk of Developing Open-Angle Glaucoma in the Los Angeles Latino Eye Study
| Baseline Risk factors | OR (95% CI)* | P* |
|---|---|---|
| Socio-demographic factors | ||
| Age (per decade older) | 2.19 (1.74-2.75) | <0.001 |
| Lack of vision insurance | 2.08 (1.26-3.41) | 0.004 |
| WHR (per 0.05 higher) | 1.21 (1.05-1.39) | 0.007 |
| Ocular factors: | ||
| IOP (per mmHg higher) | 1.18 (1.10-1.26) | <0.001 |
| Axial length (per mm longer) | 1.48 (1.22-1.80) | <0.001 |
| CCT (per 40 μm thinner) | 1.30 (1.00-1.70) | 0.050 |
OR=odds ratio; CI=confidence interval; IOP=intraocular pressure; WHR= waist to hip ratio; CCT=central corneal thickness; OAG=open-angle glaucoma.
Based on logistic regression models adjusting for age, IOP, axial length, lack of vision insurance, WHR, and CCT at baseline. P values were estimated from likelihood ratio tests. Among the 3,772 participants at risk for incident OAG, data from 34 persons (<1%) reporting IOP-lowering treatment at baseline and 82 persons (<3%) with missing data for at least one of the risk factors were excluded; the remaining 3,656 subjects including 78 incident cases were included in this analysis.
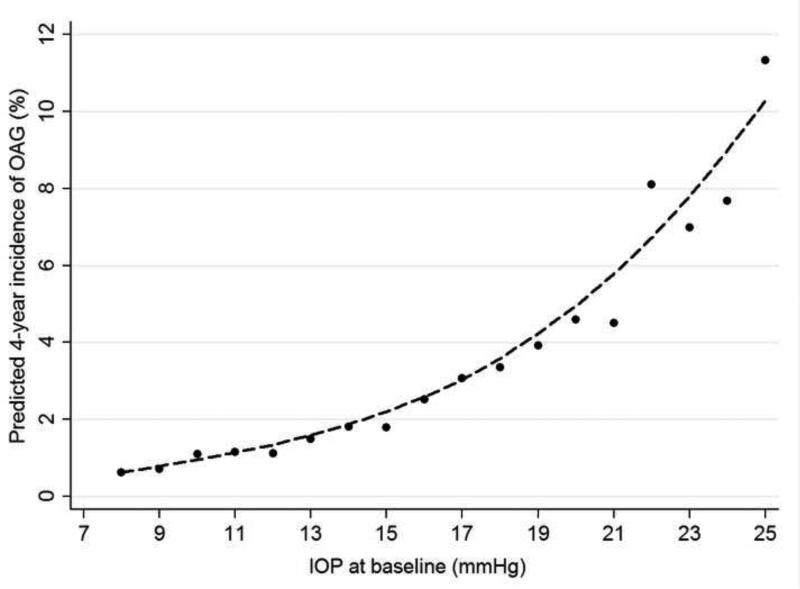
A nonlinear dose-response relationship was present between baseline IOP and the 4-year incidence of OAG (Figure 1). The 4-year incidence rate of OAG was 0.7% among individuals with a baseline IOP of <10 mm Hg, increasing in a non-linear manner to 20.0% among those with a baseline IOP of ≥25 mmHg (Table 5). The adjusted odds ratios also increased in a dose response manner over the entire range of IOPs, although only IOPs over 22mmHg are statistically significant due to the relatively smaller number of cases.
FIGURE 1.
LOWESS plot demonstrating the independent relationship between baseline intraocular pressure and the four-year incidence of open-angle glaucoma in the Los Angeles Latino Eye Study. The predicted four-year incidence of open-angle glaucoma was estimated using a locally weighted regression after adjusting for other covariates found to be significant in the final logistic regression model. LOWESS=locally weighted scatterplot smoothing; OAG=open-angle glaucoma; IOP=intraocular pressure.
TABLE 5.
Four-year Risk of Developing Open-angle Glaucoma Stratified by Level of Intraocular Pressure at Baseline
| Baseline Intraocular Pressure range (mm Hg) | No. at Risk | 4-year incidence rate, % (95% confidence interval) | Adjusted odds ratio (95% confidence interval)* | P* |
|---|---|---|---|---|
| <10 | 148 | 0.7 (0.0-3.7) | 1.00 (reference) | - |
| 10 to <13 | 984 | 1.6 (0.9-2.6) | 2.35 (0.31-18.12) | 0.41 |
| 13 to <16 | 1521 | 1.4 (0.9-2.2) | 2.06 (0.27-15.64) | 0.48 |
| 16 to <19 | 795 | 3.1 (2.1-4.6) | 3.72 (0.49-28.32) | 0.20 |
| 19 to <22 | 214 | 4.2 (1.9-7.8) | 5.79 (0.71-47.22) | 0.10 |
| 22 to <25 | 47 | 12.8 (4.8-25.7) | 19.82 (2.21-177.71) | 0.008 |
| ≥25 | 10 | 20.0 (2.5-55.6) | 30.85 (2.26-421.51) | 0.010 |
Based on logistic regression models adjusting for age, axial length, lack of vision insurance, wait to hip ratio, and central corneal thickness at baseline.
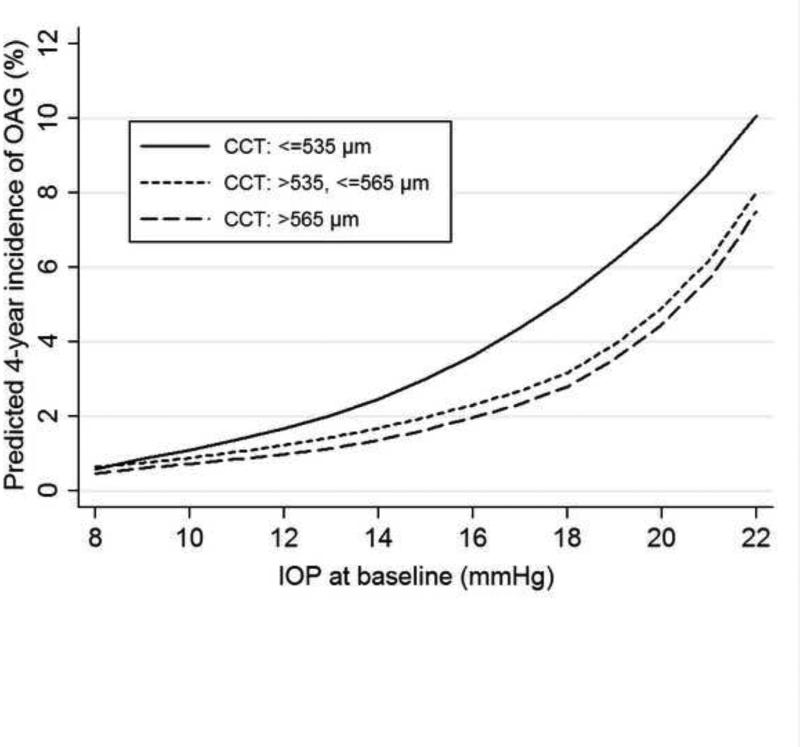
Baseline CCT independently predicted the development of OAG after adjusting for baseline IOP and other covariates (Table 4). We further explored if the relationship between baseline IOP and the development of OAG was modified by CCT. Figure 2 shows the LOWESS plot for the baseline IOP-OAG incidence relationship stratified by the tertiles of CCT distribution (≤535, >535-565, >565 μm). With higher baseline IOPs, the thinner CCT group (≤535 μm) exhibited the greatest increase in OAG incidence, while those with normal or thicker CCT had similar increases (Table 6). This difference between individuals with CCT≤535μm and those with CCT>535 μm was statistically significant (P=0.043).
FIGURE 2.
LOWESS plots illustrating the independent relationship between baseline intraocular pressure and the four-year incidence of open-angle glaucoma stratified by central corneal thickness in the Los Angeles Latino Eye Study. The predicted four-year incidence of open-angle glaucoma was estimated using a locally weighted regression after adjusting for other covariates found to be significant in the final logistic regression model. LOWESS=locally weighted scatterplot smoothing; OAG=open-angle glaucoma; IOP=intraocular pressure; CCT=central corneal thickness.
TABLE 6.
Relationship Between Baseline Intraocular Pressure and Risk of Developing Open-Angle Glaucoma Stratified by Central Corneal Thickness in the Los Angeles Latino Eye Study
| IOP at baseline (mmHg) | CCT: >565 μm |
CCT: >535-565 μm |
CCT: <535 Mm |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Incidence rate | OR (95% CI)* | N | Incidence rate | OR (95% CI)* | N | Incidence rate | OR (95% CI)* | |
| <13 | 283 | 1.4% | 1.00 (reference) | 357 | 1.4% | 1.00 (reference) | 484 | 1.7% | 1.00 (reference) |
| 13 to <19 | 783 | 1.3% | 0.86 (0.26-2.81) | 811 | 2.3% | 1.68 (0.61-4.62) | 671 | 2.2% | 1.13 (0.46-2.74) |
| ≥19 | 142 | 4.2% | 2.22 (0.56-8.59) | 67 | 3.0% | 1.83 (0.33-10.05) | 58 | 15.5% | 8.22 (2.87-23.52) |
| Interaction between IOP (continuous) and CCT (≤535 vs. >535 μm ): P=0.043** | |||||||||
OR=odds ratio; CI=confidence interval; IOP=intraocular pressure; CCT=central corneal thickness; WHR=waist to hip ratio.
Based on logistic regression models adjusting for age, IOP, axial length, lack of vision insurance, WHR, and CCT at baseline.
Estimated from likelihood ratio test comparing models with and without the interaction term.
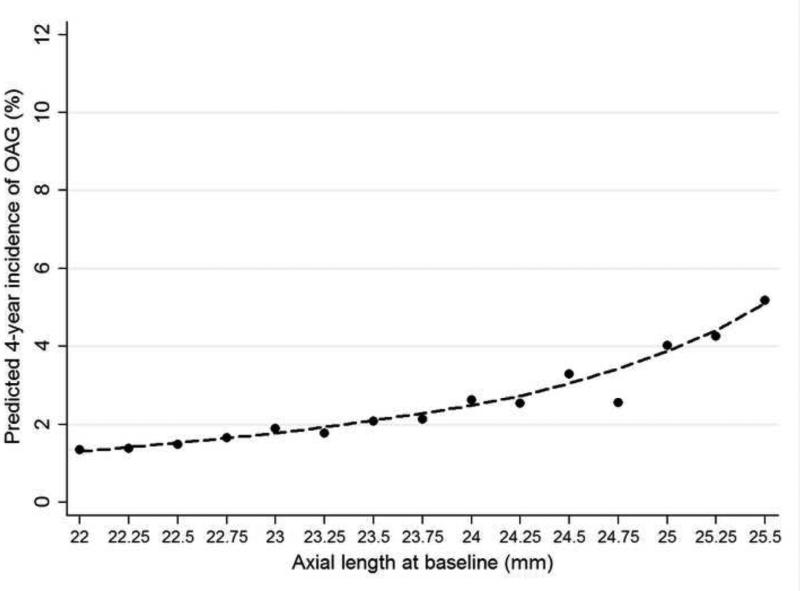
A longer axial length at baseline predicted a higher incidence of OAG (Table 4, Figure 3). We also explored whether the relationship between baseline IOP and the risk of developing OAG was modified by axial length and no interactions were found. Therefore, persons with higher levels of axial myopia were at highest risk of developing glaucoma particularly at higher IOPs. Furthermore, at the same IOP level, persons with longer axial lengths were more likely to develop OAG than those with relatively shorter axial lengths. Baseline nuclear opacities did not predict the development of OAG, and adjusting for nuclear opacity did not substantially change the predictive relationship between baseline axial length and the development of OAG. Corneal power was also examined as a risk factor among the 41% of participants with the data, and no relationship was identified between baseline corneal power and the development of OAG.
FIGURE 3.
LOWESS plots illustrating the independent relationship between baseline axial length and the four-year incidence of open-angle glaucoma in the Los Angeles Latino Eye Study. The predicted four-year incidence of open-angle glaucoma was estimated using a locally weighted regression after adjusting for other covariates found to be significant in the final logistic regression model. LOWESS=locally weighted scatterplot smoothing; OAG=open-angle glaucoma.
Abdominal obesity, as defined by the World Health Organization (WHO)24 (WHR>0.90 for men and >0.85 for women), was observed among 87% of men and 66% of women at baseline (data not shown). Each 0.05 higher baseline WHR predicted a 21% higher risk of developing OAG (OR, 1.21; 95% CI, 1.05-1.39). The effect of WHR was present among men (per 0.05 greater baseline WHR: OR, 1.34; 95% CI, 1.05-1.73) but not among women (OR, 1.14; 95% CI, 0.94-1.37); however, this gender difference was not statistically significant (P=0.38). Another measure of adiposity, baseline BMI, was also examined to determine if it predicted the development of OAG. There were more obese, especially morbidly obese (BMI≥35) women (23%) than men (12%) in the LALES population. BMI did not predict the development of OAG (data not shown). Waist circumference, another adiposity measure, also did not predict the development of OAG.
After adjustment for age, IOP, axial length, lack of vision insurance, WHR, and CCT level, other baseline variables including cigarette smoking, alcohol intake, systemic blood pressures, ocular perfusion pressures, history of T2DM (OR, 1.07; 95% CI, 0.62-1.82), history of cataract surgery(OR, 1.24; 95% CI, 0.45-3.43), and family history of glaucoma (OR, 1.00; 95% CI, 0.39-2.57) did not predict the development of OAG. Specifically, the ORs (95% CI) were 0.99 (0.58-1.70) for former smokers and 1.31 (0.64-2.67) for current smokers when compared to nonsmokers, 1.59 (0.95-2.64) for former alcohol drinkers and 0.76 (0.28-2.06) for current alcohol drinkers when compared to non-drinkers. For each mm Hg higher systolic and diastolic blood pressure, and pulse pressure, the associated ORs (95% CI) were 1.02 (0.91-1.15), 1.04 (0.86-1.25), and 1.01 (0.87-1.18) respectively.
DISCUSSION
We report the first prospective study of baseline risk factors that predict the development of OAG in a large population-based sample in the United States. This is also the first report of such risk factors in Latinos. Older age, higher IOP level, longer axial length, thinner central cornea, greater WHR, and lack of vision insurance at baseline predicted the development of OAG over a 4-year period. Our study also highlights that the impact of higher baseline IOP on the risk of developing OAG was the highest among participants with thin CCT.
Although elevated IOP is no longer a defining criterion for the diagnosis of glaucoma, it remains an important risk factor for OAG in different studies.6, 7, 12 The exact mechanism by which elevated IOP causes optic nerve damage and vision loss has not been completely elucidated. However, evidence suggests that elevated IOP can damage the optic nerve head and retina progressively by producing structural damage at the optic nerve head, reducing retinal blood flow, and increasing the expression of cytokines.25 In the LALES, most (85%) individuals who developed OAG had a baseline IOP ≤21 mmHg. Nonetheless, similar to what was observed in our cross-sectional examination,19 the risk of developing OAG over a 4-year period increased with higher baseline IOP in a non-linear fashion. Thus, the risk attributable to a 1 mmHg higher IOP at baseline is not the same across the IOP spectrum. The risk associated with 1 mmHg is higher at higher levels of IOP compared to lower levels of IOP. Hence, it is not accurate to compare the risk associated with each 1 mmHg higher IOP both within and across studies as the risk varies depending on where in the IOP spectrum that 1 mm Hg resides (Table 5).
Although higher IOPs are associated with higher incidence of OAG, a majority of Latinos in the LALES had an IOP in the 10-19 mmHg range, providing a larger contribution to the burden of incident OAG in the LALES than individuals with IOPs in the ocular hypertension range – 21mmHg or higher. The attributable risk fractions associated with a baseline IOP of 10-<13, 13-<16, 16-<19, 19-<22, 22-<25, and ≥25 mmHg were 12%, 15%, 20%, 9%, 8%, and 3% respectively. These data emphasize the fact that from a public health perspective, when conducting screening programs IOP should not be considered as a primary screening measure for detecting patients who have or are likely to develop OAG.
It has been well documented that CCT can affect the accuracy of tonometric readings, leading to a potential underestimation of true IOP among individuals with thinner corneas and overestimation of true IOP among those with thicker corneas.26, 27 In keeping with these observations and the findings from our previous cross-sectional investigation of OAG prevalence,18 we found that the same level of baseline IOP measured by Goldmann applanation tonometry predicted a higher rate of developing OAG among individuals with thinner CCT than among those with thicker CCT. This further emphasizes the suggestion that thinner CCT is a risk factor for the development of OAG independent of its impact on measured IOP. In addition to CCT, corneal curvature and other biomechanical properties of the cornea, such as Young's modulus, have also been shown to affect the accuracy of IOP measurement with Goldmann applanation tonometry.28, 29 Furthermore, corneal resistance factor, a new corneal viscoelastic parameter measured by the Ocular Response Analyzer (ORA; Reichert Ophthalmic Instruments, Buffalo, NY) ,30 has been shown to explain more of the inter-individual variation in IOP readings from Goldmann applanation tonometer than CCT.31
Similar to the Barbados Eye Study,5 we found that thinner CCT at baseline independently predicted the development of OAG. This observation is in concert with the fact that OAG is more common among people of African descent, the population that tends to have a lower mean CCT than others.32 We also found that the CCT-OAG association was stronger or perhaps limited to individuals with baseline IOP over 21 mmHg. This agrees with the Ocular Hypertension Treatment Study,33 which found a significant association between thinner central corneal and the development of OAG among ocular hypertensives. Our observation is also consistent with the findings from the Early Manifest Glaucoma Trial (EMGT) that thinner CCT was a significant risk factor of OAG progression in patients with higher baseline IOP (≥21 mmHg), but not in those with lower baseline IOP.3 In addition to its influence on tonometric readings, CCT may be correlated with other ocular biomechanical and structural characteristics that can affect the integrity of the lamina cribrosa and consequently influence inter-individual susceptibility to glaucomatous damage caused by excessive IOP. One example of such characteristics is corneal hysteresis (CH), a measure of viscous dampening in the corneal tissue. It has been shown that in multivariate analysis, CH was significantly associated with rapid glaucomatous visual field progression.34
Baseline WHR predicted the development of OAG in our study, while BMI did not. WHR, which measures abdominal obesity normalized by body size, has been shown to be a better predictor of diabetes,35 cardiovascular diseases,36 and all-cause mortality37 than BMI. WHR may be more relevant for age-related diseases because muscle loss and changes in regional adipose tissue distribution are common with aging.38 To our knowledge, the role of obesity in the development of OAG was studied only in four other longitudinal studies, and results were inconsistent.5, 10, 12, 39 All four studies examined the role of general obesity measured by BMI. The Barbados Eye Study5 and the Visual Impairment Project12 found no association between baseline BMI and the risk of developing OAG. The Rotterdam Study10 found a clear gender difference between baseline BMI and the risk of developing OAG with this relationship being statistically significant only among women. In the combined analysis of the Nurses’ Health Study and Health Professionals Follow-up Study,39 BMI was not associated with self-reported incident OAG overall; however, higher BMI was associated with a lower risk of developing normal-tension OAG (IOP≤ 21 mmHg at diagnosis) among women. Only these last two studies10, 39 examined abdominal obesity measured by waist circumference or WHR, and neither measure was associated with the development of OAG. It is unclear how obesity may affect the development of OAG. One proposed mechanism is the alteration in IOP level. Many previous epidemiologic studies40 as well as the LALES have found that higher BMI was associated with an elevated IOP; however, this effect is opposite to what would be expected if higher BMI was associated with a lower risk of OAG as shown in the prior studies.10, 39 Less is known about the effect of greater abdominal obesity on IOP. In the LALES, higher WHR was also associated with an elevated IOP; however, this association with IOP was statistically significant only among women (data not shown). The fact that greater baseline WHR was predictive of developing OAG after adjustment for IOP in our study indicates that the relationship between WHR-OAG is probably not, at least not entirely, mediated by IOP. Further studies, particularly longitudinal studies, are needed to confirm our observations in other populations and to explore possible explanations.
Many cross-sectional epidemiologic and clinical studies reported a higher risk of having OAG among individuals with myopia.41 We have previously reported in our cross-sectional study that longer axial length was an important independent risk factor associated with a higher prevalence of OAG.19 No previous prospective study has shown a relationship between axial myopia and the risk of developing OAG. The current study is the first prospective population-based study to provide evidence that longer baseline axial length independently predicts the development of OAG. It has been postulated that at a given IOP level, scleral stress may be greater in axially longer eyes than in shorter eyes. 19 We did observe that the risk of developing OAG was higher in those with higher IOPs and longer axial lengths, confirming the higher susceptibility of longer eyes to developing OAG that may be mediated through higher sclera stress. Other abnormalities associated with axially longer eyes, such as alterations in connective tissues and scleral rigidity, may also play an important role in the development of OAG.
Lack of insurance coverage, a major reason for lower eye care utilization rates,42 has been associated with a higher prevalence of visual impairment in the United States.43 Consistently, our study found that lack of vision insurance in the past 12 months at baseline predicted the development of OAG, a major contributing factor of visual impairment among Americans. Since most regular health insurance policies do not routinely cover vision care, we examined the independent roles of general health insurance and vision care insurance on the development of OAG (data not shown), and found that a lack of vision care coverage remained a significant risk factor for the development of OAG among individuals with health insurance, underlining the importance of providing vision care coverage in addition to health insurance.
Our study has several strengths, including its population-based design, prospective follow-up with risk factors measured at the time of enrollment before disease diagnosis, high participation rate, and standardized data collection and disease diagnosis. Nevertheless, the study is limited in a number of aspects. First, participants who were receiving medical treatment at enrollment or had a history of laser or surgical therapy for ocular hypertension were excluded from the current analysis. This exclusion may have led to an underestimation of the impact of baseline IOP on the development of OAG. However, such exclusion is unlikely to have impacted our results substantially, because our results were essentially unchanged when data from these participants were included in the analysis with adjustment for IOP-lowering treatment (data not shown). Second, results from the study may be not applicable to other Latino populations because the study population consists of mostly Mexican Americans. In addition, the risk factors examined in this analysis were assessed at the baseline of the study, so the contribution of the same risk factor during different life stages or cumulative exposures over the life course remains unknown. Third, while the stepwise selection approach can be associated with issues such as biases in parameter estimation and multiple comparisons,44 we believe such automatic model selection is still a valuable tool for empirical model building when our knowledge of risk factors for the development of OAG is limited. Another limitation of the study is the low number of cases that developed OAG, which may have limited our ability to detect associations with factors such as diabetes and hypertension. A longer follow-up with a larger number of persons developing OAG may provide additional data to further validate our findings.
In summary, we report the first population-based prospective investigation of baseline risk factors for developing OAG in the United States. Results from our study have important public health implications. It is likely that a considerable proportion of OAG could have been prevented if IOP were further reduced particularly among individuals without ocular hypertension. In addition, our study identifies that axial length is an important contributing factor to the development of OAG and suggests that structural properties of the eye such as long axial length (and its associated thinner scleral wall) and thin CCT also play a role in the pathogenesis of OAG. Finally we highlight the need of targeting intervention strategies towards individuals who lack vision insurance or have limited access to care.
Supplementary Material
Acknowledgments
Financial Support: National Institutes of Health Grants NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, New York, and Pfizer Inc. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflict of interest exists for any author.
REFERENCES
- 1.Varma R, Ying-Lai M, Francis BA, et al. Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–48. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53:S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, et al. EMGT Group Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R. Lifestyle exposures and eye diseases in adults. Am J Ophthalmol. 2007;144:961–9. doi: 10.1016/j.ajo.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leske MC, Wu SY, Hennis A, et al. BESs Study Group Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Nemesure B, Honkanen R, Hennis A, et al. Barbados Eye Studies Group. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007;114:1810–5. doi: 10.1016/j.ophtha.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 7.de Voogd S, Ikram MK, Wolfs RC, et al. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam Study. Ophthalmology. 2005;112:1487–93. doi: 10.1016/j.ophtha.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 8.de Voogd S, Ikram MK, Wolfs RC, et al. Is diabetes mellitus a risk factor for open-angle glaucoma? The Rotterdam Study. Ophthalmology. 2006;113:1827–31. doi: 10.1016/j.ophtha.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 9.Müskens RP, de Voogd S, Wolfs RC, et al. Systemic antihypertensive medication and incident open-angle glaucoma. Ophthalmology. 2007;114:2221–6. doi: 10.1016/j.ophtha.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 10.Ramdas WD, Wolfs RCW, Hofman A, et al. Lifestyle and risk of developing open-angle glaucoma: the Rotterdam Study. Arch Ophthalmol. 2011;129:767–72. doi: 10.1001/archophthalmol.2010.373. [DOI] [PubMed] [Google Scholar]
- 11.Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002;109:1047–51. doi: 10.1016/s0161-6420(02)01040-0. [DOI] [PubMed] [Google Scholar]
- 12.Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44:3783–9. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Census Bureau [August 10, 2011];(NP-T5-F) Projections of the resident population by race, Hispanic origin, and nativity: middle series, 2025 to 2045. Available at: http://www.census.gov/population/projections/nation/summary/np-t5-f.pdf. January 13, 2000.
- 14.Varma R, Paz SH, Azen SP, et al. Los Angeles Latino Eye Study Group The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–31. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Doshi V, Ying-Lai M, Azen SP, Varma R, Los Angeles Latino Eye Study Group Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:639–47. doi: 10.1016/j.ophtha.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Memarzadeh F, Ying-Lai M, Chung J, et al. Los Angeles Latino Eye Study Group Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2010;51:2872–7. doi: 10.1167/iovs.08-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopra V, Varma R, Francis BA, et al. Los Angeles Latino Eye Study Group Type 2 diabetes mellitus and the risk of open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–32. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis BA, Varma R, Chopra V, et al. Los Angeles Latino Eye Study Group Intraocular pressure, central corneal thickness, and prevalence of open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;146:741–6. doi: 10.1016/j.ajo.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzin AA, Varma R, Reddy HS, et al. Los Angeles Latino Eye Study Group Ocular biometry and open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2010;117:1713–9. doi: 10.1016/j.ophtha.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33:261–304. [Google Scholar]
- 21.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. 2nd ed. Springer; New York: 2009. pp. 219–57. [Google Scholar]
- 22.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Statist Assoc. 1979;74:829–36. [Google Scholar]
- 23.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Morrison JC, Johnson EC, Cepurna W, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog Retin Eye Res. 2005;24:217–40. doi: 10.1016/j.preteyeres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol. 1993;115:592–6. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 27.Manni G, Oddone F, Parisi V, et al. Intraocular pressure and central corneal thickness. Prog Brain Res. 2008;173:25–30. doi: 10.1016/S0079-6123(08)01103-5. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–55. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Francis BA, Hsieh A, Lai MY, et al. Los Angeles Latino Eye Study Group Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology. 2007;114:20–6. doi: 10.1016/j.ophtha.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 30.Narayanaswamy A, Chung RS, Wu RY, et al. Determinants of corneal biomechanical properties in an adult Chinese population. Ophthalmology. 2011;118:1253–9. doi: 10.1016/j.ophtha.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Kotecha A, Elsheikh A, Roberts CR, et al. Corneal thickness- and age-related biomechanical properties of the cornea measured with the Ocular Response Analyzer. Invest Ophthalmol Vis Sci. 2006;47:5337–47. doi: 10.1167/iovs.06-0557. [DOI] [PubMed] [Google Scholar]
- 32.Dimasi DP, Burdon KP, Craig JE. The genetics of central corneal thickness. Br J Ophthalmol. 2010;94:971–6. doi: 10.1136/bjo.2009.162735. [DOI] [PubMed] [Google Scholar]
- 33.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 34.De Moraes CV, Hill V, Tello C, et al. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012;21:209–13. doi: 10.1097/IJG.0b013e3182071b92. [DOI] [PubMed] [Google Scholar]
- 35.Schulze MB, Heidemann C, Schienkiewitz A, et al. Comparison of anthropometric characteristics in predicting the incidence of type 2 diabetes in the EPIC-Potsdam Study. Diabetes Care. 2006;29:1921–3. doi: 10.2337/dc06-0895. [DOI] [PubMed] [Google Scholar]
- 36.Welborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–5. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 37.Simpson JA, MacInnis RJ, Peeters A, et al. A comparison of adiposity measures as predictors of all-cause mortality: the Melbourne Collaborative Cohort Study. Obesity (Silver Spring) 2007;15:994–1003. doi: 10.1038/oby.2007.622. [DOI] [PubMed] [Google Scholar]
- 38.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol. 2009;19:724–31. doi: 10.1016/j.annepidem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasquale LR, Willett WC, Rosner BA, Kang JH. Anthropometric measures and their relation to incident primary open-angle glaucoma. Ophthalmology. 2010;117:1521–9. doi: 10.1016/j.ophtha.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung N, Wong TY. Obesity and eye diseases. Surv Ophthalmol. 2007;52:180–95. doi: 10.1016/j.survophthal.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcus MW, de Vries MM, Montolio FGJ, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–94. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Lee DJ, Lam BL, Arora S, et al. Reported eye care utilization and health insurance status among US Adults. Arch Ophthalmol. 2009;127:303–10. doi: 10.1001/archophthalmol.2008.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitale S, Cotch MF, Sperduto RD. Prevalence of visual impairment in the United States. JAMA. 2006;295:2158–63. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 44.Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP. Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol. 2006;75:1182–9. doi: 10.1111/j.1365-2656.2006.01141.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.