Abstract
Introduction
P-selectin is a cell adhesion molecule shown to play a role in venous thromboembolism. We evaluated whether higher P-selectin is associated with chronic venous insufficiency (CVI).
Materials and Methods
In a cohort of 2408 participants, the San Diego Population Study, peripheral venous disease was established by symptoms, clinical examination, and ultrasound. We measured P-selectin in a subsample of 352 CVI cases frequency matched to controls. Cases included four hierarchical groups of increasing severity of CVI.
Results
The association of P-selectin with CVI considering all cases was weak, with an age, race and sex-adjusted odds ratio (OR) of 1.3 (95% CI 1.0-2.2) for values in the 3rd versus 1st tertile. The OR for cases in the two most severe groups was 2.3 (95% CI 1.2-4.2). Addition of body mass index to the model reduced this OR to 1.9 (95% CI 1.0-3.6).
Conclusions
Higher circulating P-selectin was associated with more severe CVI, but not CVI overall. Results support that platelet and endothelial cell activation may be involved in the pathogenesis of CVI.
Keywords: P-selectin, chronic venous insufficiency, risk factor, blood coagulation
Chronic venous insufficiency (CVI) includes a spectrum of findings characterized by impaired venous return. CVI is associated with disability and significant adverse impact on quality of life. Manifestations range from varicose veins to pain, skin breakdown, and ulcerations. Although CVI is common and has substantial morbidity, there are few estimates of its prevalence worldwide [1]. In the United States, an estimated 25 million have varicose veins, two to six million have advanced CVI, and 500,000 have venous ulcerations [2]. The disease presents a substantial financial burden as well, contributing to 92 of every 100,000 hospitalizations in the United States. Risk factors for CVI include age, family history, obesity, sedentary lifestyle, and smoking [3]. The best established etiology of CVI is deep vein thrombosis (DVT), when it is complicated by post thrombotic syndrome (PTS) [4]. PTS occurs in up to 50% of patients with DVT and is often caused by ongoing venous obstruction and valvular reflux [5, 6]. PTS can also occur after asymptomatic DVT, thus explaining some fraction of CVI in the general population [7]. Prior research in our lab revealed an association between hemostatic factors and CVI including higher factor VIII, von Willebrand factor, and D-dimer, supporting this concept [8].
P-selectin is a cell adhesion molecule found in the α granules of platelets and the Weibel-Palade bodies of endothelial cells. Once expressed, P-selectin is released in soluble form and interacts with the P-selectin glycoprotein ligand-1 (PSGL-1) present on leukocytes. Several mouse models have demonstrated the importance of P-selectin and PSGL-1 in leukocyte recruitment to thrombi and fibrin generation [9]. Elevations in P-selectin are implicated in stroke, acute myocardial infarction, coronary artery disease, and peripheral artery disease [10]. High plasma P-selectin is also associated with risk of first and recurrent venous thromboembolism (VTE) and with VTE in cancer patients [11-14]. Pharmacologic P-selectin inhibitors are being developed for use in reducing the risk of thrombosis, including VTE [15]. Importantly, P-selectin may play a role in vein wall fibrosis following clot formation, thus contributing to venous insufficiency [16].
Given the above findings, we studied the association of soluble P-selectin level with risk of CVI. We hypothesized that higher P-selectin is a risk factor for CVI.
Materials and Methods
Participants
The reported analysis is a cross-sectional study using data from the baseline visit of an existing cohort, the San Diego Population Study. Participants were 2,408 men and women selected as a stratified random sample of employees and retirees of the University of California, San Diego [17]. Spouses or significant others of participants were included and 199 volunteers who asked to participate were enrolled. Stratification factors included age, sex and ethnicity. Participants were enrolled from 1994 to 1998. Participants self-defined race/ethnicity from a list and informed consent was provided according to institutional review boards of participating institutions.
At the baseline visit personal and family health history, demographic information, blood samples, physical examination and vascular examination were performed. Thrombosis history was defined via interview as positive answers to standardized questions on whether participants had previous DVT or PE treated with anticoagulants. History of superficial vein thrombosis was defined similarly but did not require treatment with anticoagulants. Prior history of trauma, burns, fractures, and prolonged surgery or vascular procedures were recorded. Participants answered standardized questions to assess leg symptoms previously or currently [18].
Vascular Examination
At baseline, duplex ultrasound was used with standardized methods to quantify functional disease, reflux, and obstruction with Acuson128 duplex ultrasonograph (Siemens Corporation, Mountain View CA) with a 5-MHz transducer. A pressure monitoring system was used for Valsalva reflux testing and valvular insufficiency was defined as Valsalva reflux > 0.5 seconds. Partial and complete venous obstruction was assessed by degree of compressibility of the venous walls, with normal defined as complete compressibility. Presence of superficial venous functional disease (SFD) and deep venous functional disease (DFD) were defined by ultrasound as reflux or abnormal compression in superficial and deep veins, respectively. Visible venous disease corresponded to the Clinical Etiologic Anatomic Pathologic (CEAP) classification categories: C0 (none), C1 (telangectasias or reticular veins), C2 (varicose veins), C3 (edema); C4 (pigmentation, eczema, lipodermatosclerosis, or atrophie blanche), C5 (healed venous ulcer); C6 (active venous ulcer) [19]. We classified trophic changes of skin (TCS) as C4-C6. Lower extremity edema was considered independently.
Case Control Study Design
A cross-sectional case-control study was conducted within the cohort to analyze the association of P-selectin with risk and severity of CVI. Of the 2,404 participants, 725 participants did not meet criteria for being a case or control and were excluded from the analysis. We selected four hierarchal cases groups with increasing severity of CVI based on clinical and imaging characteristics. Severity of disease was based on the patient's most affected lower extremity. The lowest severity group consisted of 125 patients with DFD and no TCS or symptoms. The second group included 137 patients with SFD and TCS or edema, as well as those with a normal ultrasound and TCS. The third group included 59 participants with DFD, symptoms including aching or edema, and no TCS. The final and most severe group consisted of 49 patients with DFD and TCS, regardless of symptoms. The total case group included 370 patients however 18 had insufficient blood samples thereby leaving 352 participants. The control group was selected from 1313 participants without subjective or objective findings for CVI including no SFD, DFD, edema, VV, TCS, and leg aching. From among the 1270 potential controls with blood samples, we selected 352 participants frequency matched to cases by 10-year age group, race, and sex.
In 2010, after we defined case groups for our study, an Institutes of Medicine subcommittee revised their definition for CVI to be consistent with the updated CEAP classification of 2004 from the American Venous Forum [20]. Using the new definition, participants in case group one and those without edema in group three, no longer had chronic venous insufficiency. We repeated our analysis excluding these participants, which left 199 cases and 352 controls.
Laboratory Analysis
Blood was drawn at the study visit and centrifuged within 3 hours with serum or plasma stored at -70°C. Samples were retrieved from the 352 cases and 352 controls and soluble P-selectin was assayed in EDTA plasma using a commercially available ELISA kit (R&D Systems, Minneapolis, MN) by technicians blind to case control status. The coefficients of variation across four samples of various concentrations were 5.4-10.3% and the range of the assay was 18.6-911 ng/ml.
Statistical Analysis
Participant characteristics were reported and compared using t-tests or Chi-squared tests comparing all case groups combined and the control group. For modeling, the distribution of P-selectin was divided into tertiles based on the control group distributions. Logistic regression models were used to calculate the odds ratio and 95% confidence interval of CVI with increasing P-selectin, by comparing participants in the second and third tertiles to those in the first tertile as the reference group. Control for potential confounders was completed using multivariable modeling with serial addition of potential confounders thought to be related to both CVI and P-selectin including BMI, prior venous thrombosis, smoking history, family history of venous ulceration, and history of cancer. P values were calculated with a test for trend across tertiles of P-selectin. We repeated analyses considering only cases in the two most severe CVI groups, compared to controls. To determine if there was an even higher risk of CVI with more extreme values of P-selectin, sensitivity analysis was performed assessing the odds ratio of CVI for those with P-selectin above compared to below the 90th percentile of the distribution in the control group. Lastly, to further assess the relationship of P-selectin with severity of CVI we conducted ordinal regression analysis using the ordered case groups as an ordinal outcome.
Results
Table 1 compares characteristics of the 352 controls and 352 cases. The two groups were similar in age, sex, and race due to the frequency matching. Body mass index (BMI) was higher in the case group than controls. The case group had a higher prevalence of prior venous thrombosis, history of venous surgery, family history of venous thrombosis, and family history of venous ulcers. There were no significant differences between the groups in prevalence of hypertension, diabetes, smoking, or history of cancer.
Table 1.
Characteristics of Cases and Controls
| Characteristic | Controls (n=352) | Cases (n=352) | p |
|---|---|---|---|
| Age - mean (sd) | 62.3 (11.2) | 62.7 (11.6) | 0.61 |
| Sex, Male | 41% | 41% | 0.97 |
| Race, White | 68% | 68% | 0.96 |
| African American | 10% | 10% | 0.99 |
| Hispanic | 11% | 11% | 0.99 |
| Asian | 11% | 11% | 0.92 |
| BMI, kg/m2 - mean (sd) | 26.4 (4.6) | 28.0 (5.3) | <0.001 |
| History of Smoking | 55% | 49% | 0.16 |
| Hypertension | 28% | 32% | 0.26 |
| Diabetes | 6% | 7% | 0.54 |
| Prior Venous Thrombosis | 1% | 13% | <0.001 |
| Venous Surgery | 1% | 15% | <0.001 |
| FH of Venous Thrombosis | 4% | 9% | 0.01 |
| FH of Venous Ulcer | 1% | 4% | 0.007 |
| History of Cancer | 14% | 10% | 0.10 |
| P-selectin, ng/ml - mean (sd) | 34.1 (13.2) | 35.2 (13.7) | 0.30 |
Body Mass Index (BMI) Family History (FH)
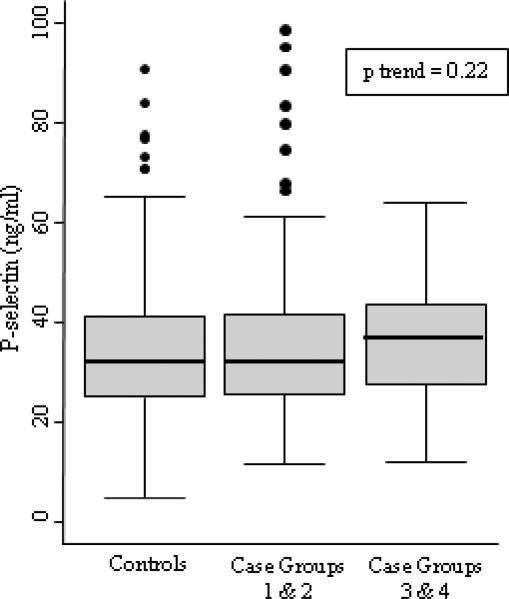
The distribution of P-Selectin concentration in controls ranged from 18.6 to 911ng/ml. The mean value was slightly higher but not significantly different in cases compared to controls (35.2 versus 34.1 ng/ml, p=0.30). The mean for combined case groups 1 and 2 was 34.9 ng/ml, and for combined case groups 3 and 4 was 36.2 ng/ml (Figure 1). Rrisk factor levels by P-selectin tertiles in controls are shown in Table 2. Of the risk factors evaluated, only higher BMI was associated with higher P-selectin. While those in the top tertile of P-selectin were more likely to be hypertensive than those with lower levels, this relationship was not statistically significant (p=0.08). The correlation coefficients of P-selectin with age and BMI were 0.06 (p = 0.25) and 0.16 (p=0.004), respectively.
Figure 1.
Box plots showing the distribution of P-selectin levels for controls and cases. The center line of the box is the median, the outside edges are the 25th and 75th percentile, and the whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles. Observations outside the whiskers are represented by dots. The test for trend p value is included for the three categories represented in the figure.
Table 2.
Risk Factor levels by P-selectin Tertiles in Controls
| Characteristic | Tertile 1 | Tertile 2 | Tertile 3 | p* |
|---|---|---|---|---|
| P-selectin, ng/ml | 4.9-26.8 | 26.8-37.0 | 37.0-90.6 | |
| Age - mean (sd) | 61 (11) | 62 (11) | 63 (11) | 0.26 |
| Sex, Male | 39% | 41% | 42% | 0.63 |
| Race, White | 75% | 63% | 66% | 0.13 |
| African American | 10% | 10% | 8% | |
| Hispanic | 7% | 14% | 14% | |
| Asian | 7% | 14% | 13% | |
| BMI, kg/m2 - mean (sd) | 25.7 (4.3) | 26.1 (4.1) | 27.0 (5.0) | 0.03 |
| History of Smoking | 55% | 53% | 54% | 0.84 |
| Hypertension | 23% | 27% | 33% | 0.08 |
| Diabetes | 3% | 6% | 6% | 0.29 |
| Prior Venous Thrombosis | 1% | 0% | 3% | 0.37 |
| Venous Surgery | 1% | 2% | 2% | 0.79 |
| FH of Venous Thrombosis | 5% | 5% | 3% | 0.34 |
| FH of Venous Ulcer | 1% | 1% | 1% | 1.00 |
| History of Cancer | 13% | 18% | 12% | 0.85 |
Body Mass Index (BMI) Family History (FH)
Test for trend except for race, where a p value for overall difference by race from ANOVA for mean P-selectin by race is provided.
In Table 3, odds ratios of CVI by tertiles of P-selectin are shown using tertile 1 as the referent. Comparing all case groups (n=352) with controls (n=352), the odds ratios of CVI in tertiles 2 and 3 were 1.2 (95% CI 0.8-1.9) and 1.5 (95% CI 1.0-2.2), respectively. The only potential confounder which attenuated this association was BMI, which resulted in an odds ratio for tertile 3 of 1.3 (95% CI 1.0-2.2). The odds ratios were unchanged with further addition of other potential confounders listed in the table. In evaluation of the two highest CVI case groups (n=80) compared to all controls (n=352), the odds ratios of CVI were 1.4 (95% CI 0.7-2.7) for P-selectin in the 2nd tertile and 2.3 (95% CI 1.2-4.2) in the 3rd tertile. Addition of BMI to the model again modestly attenuated this association to an odds ratio of 1.9 (95% CI 1.0-3.6) in tertile 3. The odds ratio remained unchanged with further addition of the other potentially confounders. Results did not differ substantively with exclusion of participants with previous venous thrombosis. In sensitivity analyses including all CVI cases there was no increased risk of CVI for P-selectin above versus below the 90th percentile; odds ratio 1.0 (95% CI 0.6-1.7). For P-selectin defined as a continuous variable, the odds ratio of CVI for a 1-SD increase in P-selection was 1.1 (95% CI 0.9-1.3).
Table 3.
Odds Ratios of CVI by Tertiles of P-selectin (Tertile 1 as Referent)
| All case groups (n=352) vs controls (n=352) |
2 highest case groups (n=80) vs controls (n=352) |
|||||
|---|---|---|---|---|---|---|
| Model Covariates | Tertile 2 | Tertile 3 | p* | Tertile 2 | Tertile 3 | p* |
| Age, Sex, Race | 1.2 (0.8-1.9) | 1.5 (1.0-2.2) | 0.04 | 1.4 (0.7-2.7) | 2.3 (1.2-4.2) | 0.01 |
| + BMI | 1.2 (0.8-1.8) | 1.3 (0.9-2.0) | 0.17 | 1.4 (0.7-2.7) | 1.9 (1.0-3.6) | 0.06 |
| + Prior VT | 1.2 (0.8-1.8) | 1.3 (0.8-1.9) | 0.25 | 1.4 (0.7-3.0) | 1.8 (0.9-3.7) | 0.09 |
| + Smoking History | 1.2 (0.8-1.8) | 1.3 (0.8-1.9) | 0.25 | 1.5 (0.7-3.0) | 1.9 (0.9-3.8) | 0.08 |
| + FH of VU | 1.2 (0.8-1.8) | 1.3 (0.8-1.9) | 0.29 | 1.5 (0.7-3.1) | 1.9 (0.9-3.8) | 0.08 |
| + History of Cancer | 1.2 (0.8-1.8) | 1.2 (0.8-1.9) | 0.31 | 1.5 (0.7-3.2) | 1.9 (0.9-3.7) | 0.09 |
Body Mass Index (BMI), Venous Thrombosis (VT), Family History (FH), Venous Ulcer (VU)
Test for trend across tertiles
The results from ordinal regression analysis showed a relationship of P-selectin in the top tertile with severity of CVI across the 4 case groups. The odds ratio of being in one higher severity group of CVI was 1.6 (95% CI 1.1-2.4). This attenuated to 1.4 (0.9-2.0) after accounting for BMI and was not influenced by the other potential confounders (data not shown).
Using the new Institutes of Medicine case definition of CVI, in a post hoc analysis the odds ratios of CVI in tertiles 2 and 3 of P-selectin were 1.3 (95% CI 0.8-2.1) and 1.6 (95% CI 1.0-2.6), respectively. Addition of BMI to the model modestly attenuated this association to an odds ratio of 1.4 (95% CI 0.9-2.3) in tertile 3. The odds ratios were unchanged with further addition of other potential confounders.
Discussion
We demonstrated that higher levels of P-selectin were associated with CVI, but only for more severe disease as defined in this study. Findings suggest that platelet and endothelial cell activation and release of P-selectin may be involved in the pathogenesis of CVI. The association of P-selectin with CVI was partially attenuated when adjusting for BMI, but not other potential confounding variables.
We are not aware of other studies evaluating the relationship of P-selectin with CVI. However, previous studies reported associations of higher P-selectin levels with increased incidence of DVT and with risk of recurrent VTE [12-14]. Higher levels of P-selectin might relate to CVI by virtue of being a marker of thrombosis risk. This would be consistent with knowledge that P-selectin is involved in vein fibrosis after thrombosis [16]. In addition, prior studies have reported associations of other circulating proteins involved in thrombosis and inflammation with CVI or post-thrombotic syndrome [8, 21]. Our study expands the association of P-selectin with VTE to the complications following thrombotic events and to CVI in general.
The observed association of P-selectin and CVI here was partially accounted for by BMI. Cytokines and adipokines released from adipose tissue play both a direct and indirect role in haemostasis and coagulation [22]. Adipokines produced by the adipose tissue including tumor necrosis factor-alpha, resistin, and leptin were associated with CVI in the cohort studied here [23]. Higher BMI is associated with increased inflammatory markers and these in turn are associated with PTS. In a study of inflammatory markers and risk of PTS, BMI was also a confounder [21]. Conversely, the study by Ay, et al. showed an association of higher P-selectin with VTE risk in cancer patients, where BMI was not a confounder [24]. It is possible that obesity is the primary risk factor for CVI and that P-selectin is elevated as a consequence of obesity and has no role in the pathogenesis of CVI. Production of P-selectin by adipose tissue has not been reported but our findings and others suggest further study is indicated.
Our definition of CVI severity was created for this study and does not represent a validated classification of disease. The purpose was not to propose a new definition of CVI severity for clinical use, but to address associations of biomarkers with our definition of severity. Indeed in this study and previous reports biomarkers were more strongly associated with more severe CVI, lending credence to the definition we used [8, 23]. Nonetheless, findings require replication.
Our study has limitations that deserve discussion. The cross sectional design renders findings subject to reverse causality, meaning that the CVI could have caused an increase in P-selectin. We had relatively few cases of CVI in the more severe CVI groups. Applying a newer definition of CVI from the Institutes of Medicine did not substantially change interpretation of our results, but led to a smaller sample size and limited power to detect the modest observed association. The strengths of our study include a well characterized population with thorough evaluation of severity of CVI based on symptoms, clinical examination and standardized ultrasound imaging. Additionally, the analysis included all well established risk factors for VTE and CVI to assess for potential confounding. Of note, the same San Diego population is currently being examined to determine incidence of CVI. This will provide prospective confirmation of the current findings.
In conclusion, these findings suggest an association of higher P-selectin with risk of CVI. More study is needed including the assessment of the interrelationship of adipose tissue and P-selectin, and consideration of the utility of P-selectin in prediction of PTS in patients with DVT.
Acknowledgements
None
Sources of Funding
Funding for the project was provided by ROI HL083926 (M. Cushman) and R01 HL53487 (M.H. Criqui) from the National Institutes of Health and an American Society of Hematology Trainee Award (L. Bryan).
Abbreviations
- CVI
Chronic venous insufficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
Contributor Information
Locke J Bryan, University of Vermont, Department of Medicine..
Peter W Callas, University of Vermont, Department of Biometry..
Michael H Criqui, University of California San Diego, Department of Family and Preventive Medicine..
Mary Cushman, University of Vermont, Department of Medicine; 208 South Park Drive, Colchester, VT 05446; phone: (802) 656-8968; fax: (802) 656-8965; mary.cushman@uvm.edu..
References
- 1.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175–84. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 2.White JV, Ryjewski C. Chronic venous insufficiency. Perspect Vasc Surg Endovasc Ther. 2005;17:319–27. doi: 10.1177/153100350501700406. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Denenberg JO, Bergan J, Langer RD, Fronek A. Risk factors for chronic venous disease: the San Diego Population Study. J Vasc Surg. 2007;46:331–7. doi: 10.1016/j.jvs.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alguire PC, Mathes BM. Chronic venous insufficiency and venous ulceration. J Gen Intern Med. 1997;12:374–83. doi: 10.1046/j.1525-1497.1997.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004;164:17–26. doi: 10.1001/archinte.164.1.17. [DOI] [PubMed] [Google Scholar]
- 6.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Wille-Jørgensen P, Jorgensen LN, Crawford M. Asymptomatic postoperative deep vein thrombosis and the development of postthrombotic syndrome. A systematic review and meta-analysis. Thromb Haemost. 2005;93:236–41. doi: 10.1160/TH04-09-0570. [DOI] [PubMed] [Google Scholar]
- 8.Cushman M, Callas PW, Denenberg JO, Bovill EG, Criqui MH. Risk factors for peripheral venous disease resemble those for venous thrombosis: the San Diego Population Study. J Thromb Haemost. 2010;8:1730–5. doi: 10.1111/j.1538-7836.2010.03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandendries ER, Furie BC, Furie B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thromb Haemost. 2004;92:459–66. doi: 10.1160/TH04-05-0306. [DOI] [PubMed] [Google Scholar]
- 10.Merten M, Thiagarajan P. P-selectin in arterial thrombosis. Z Kardiol. 2004;93:855–63. doi: 10.1007/s00392-004-0146-5. [DOI] [PubMed] [Google Scholar]
- 11.Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008;112:2703–8. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 12.Blann AD, Noteboom WM, Rosendaal FR. Increased soluble P-selectin levels following deep venous thrombosis: cause or effect? Br J Haematol. 2000;108:191–3. doi: 10.1046/j.1365-2141.2000.01813.x. [DOI] [PubMed] [Google Scholar]
- 13.Kyrle PA, Hron G, Eichinger S, Wagner O. Circulating P-selectin and the risk of recurrent venous thromboembolism. Thromb Haemost. 2007;97:880–3. [PubMed] [Google Scholar]
- 14.Rectenwald JE, Myers DD, Hawley AE, Longo C, Henke PK, Guire KE, et al. D-dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost. 2005;94:1312–7. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- 15.Ramacciotti E, Myers DD, Wrobleski SK, Deatrick KB, Londy FJ, Rectenwald JE, et al. P-selectin/PSGL-1 inhibitors versus enoxaparin in the resolution of venous thrombosis: a meta-analysis. Thromb Res. 2010;125:e138–42. doi: 10.1016/j.thromres.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers DD, Henke PK, Wrobleski SK, Hawley AE, Farris DM, Chapman AM, et al. P-selectin inhibition enhances thrombus resolution and decreases vein wall fibrosis in a rat model. J Vasc Surg. 2002;36:928–38. doi: 10.1067/mva.2002.128636. [DOI] [PubMed] [Google Scholar]
- 17.Criqui MH, Jamosmos M, Fronek A, Denenberg JO, Langer RD, Bergan J, et al. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003;158:448–56. doi: 10.1093/aje/kwg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langer RD, Ho E, Denenberg JO, Fronek A, Allison M, Criqui MH. Relationships between symptoms and venous disease: the San Diego population study. Arch Intern Med. 2005;165:1420–4. doi: 10.1001/archinte.165.12.1420. [DOI] [PubMed] [Google Scholar]
- 19.Beebe HG, Bergan JJ, Bergqvist D, Eklof B, Eriksson I, Goldman MP, et al. Classification and grading of chronic venous disease in the lower limbs. A consensus statement. Eur J Vasc Endovasc Surg. 1996;12:487–91. doi: 10.1016/s1078-5884(96)80019-0. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 20.Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–52. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Shbaklo H, Holcroft CA, Kahn SR. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb Haemost. 2009;101:505–12. [PubMed] [Google Scholar]
- 22.Faber DR, de Groot PG, Visseren FL. Role of adipose tissue in haemostasis, coagulation and fibrinolysis. Obes Rev. 2009;10:554–63. doi: 10.1111/j.1467-789X.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 23.Allison MA, Cushman M, Callas PW, Denenberg JO, Jensky NE, Criqui MH. Adipokines are associated with lower extremity venous disease: the San Diego population study. J Thromb Haemost. 2010;8:1912–8. doi: 10.1111/j.1538-7836.2010.03941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ay C, Jungbauer LV, Sailer T, Tengler T, Koder S, Kaider A, et al. High concentrations of soluble P-selectin are associated with risk of venous thromboembolism and the P-selectin Thr715 variant. Clin Chem. 2007;53:1235–43. doi: 10.1373/clinchem.2006.085068. [DOI] [PubMed] [Google Scholar]