Abstract
Early vascular changes at the molecular level caused by adoption of a sedentary lifestyle are incompletely characterized. Herein, we employed the rodent wheel lock model to identify mRNAs in the arterial wall that are responsive to the acute transition from higher to lower levels of daily physical activity. Specifically, we evaluated whether short-term cessation of voluntary wheel running alters vascular mRNA levels in rat conduit arteries previously reported to have marked increases (i.e. iliac artery) versus marked decreases (i.e. renal artery) in blood flow during running. We used young female Wistar rats with free access to voluntary running wheels. Following 23-days of voluntary running (average distance of ~15-km/night; ~4.4-hrs/night), rats in one group were rapidly transitioned to a sedentary state by locking the wheels for seven days (n=9) or remained active (n=9) in a second group for an additional seven days. Real-time PCR was conducted on total RNA isolated from iliac and renal arteries to evaluate expression of 25 pro-atherogenic and anti-atherogenic genes. Compared to iliac arteries of wheel lock 0-day rats, iliac arteries of wheel lock 7-day rats exhibited increased expression of TNFR1 (+19%), ET1 (+59%), and LOX-1 (+31%) (p<0.05). Moreover, compared to renal arteries of wheel lock 0-day rats, renal arteries of wheel lock 7-day rats exhibited decreased expression of ETb (−23%), p47phox (−32%), and p67phox (−19%) (p<0.05). These data demonstrate that cessation of voluntary wheel running for seven days produces modest, but differential changes in mRNA levels between the iliac and renal arteries of healthy rats. This heterogeneous influence of short-term physical inactivity could be attributed to the distinct alteration in hemodynamic forces between arteries.
INTRODUCTION
The trends for physical inactivity continue to escalate in modern societies. It has been reported that more than 90% of Americans 12 years of age and older do not meet current guidelines for physical activity (Troiano et al., 2008). Cardiovascular diseases are among the chronic diseases most attributable to lack of physical activity (Nocon et al., 2008). While it is well accepted that long-term physical inactivity is associated with an increased cardiovascular risk (Szostak & Laurant, 2011; Booth et al., 2012), the early vascular changes at the molecular level caused by the adoption of a sedentary lifestyle have not been characterized.
Using the rodent wheel lock model, it has been demonstrated that cessation of physical activity increases visceral fat mass, hypothalamic leptin resistance, skeletal muscle insulin resistance, hepatic triglycerides, and causes impairments of fatty acid oxidation in skeletal muscle (Kump & Booth, 2005; Kump et al., 2006; Laye et al., 2007; Rector et al., 2008; Laye et al., 2009; Roberts et al. 2012). Undoubtedly, the wheel lock model is a valid translational tool to study the initiation of various metabolic maladies, as confirmed by paralleled human studies indicating that reducing normal daily physical activity (i.e., reducing step count from >10,000 to <1,500) causes impairment in insulin signaling (Krogh-Madsen et al., 2010) and expansion of intra-abdominal adipose tissue (Olsen et al., 2008). Nonetheless, none of our previous wheel lock studies examined the vascular consequences of removal of physical activity. Identification of vascular genes whose expression is altered in response to short-term physical inactivity may provide important information regarding the initial molecular mechanisms obligatory for a switch in vascular cell phenotype if inactivity persists. A recent American Heart Association Policy Statement advocates that, with primordial prevention (defined as prevention of risk factors in the first place), cardiovascular diseases are largely preventable (Weintraub et al., 2011). Thus, detection of early changes in vascular mRNAs induced by lack of physical activity and determination of whether effects of inactivity are artery-specific may provide clues for targets in future endeavors aimed at primordially preventing inactivity-associated cardiovascular risk.
In the present study, we employed the rodent wheel lock model to identify mRNAs in the arterial wall that are responsive to the acute transition from higher to lower levels of daily physical activity. Specifically, we evaluated whether cessation of voluntary wheel running for seven days alters specific vascular mRNA levels in rat conduit arteries previously reported to have marked increases (i.e. iliac artery) versus marked decreases (i.e. renal artery) in blood flow during running (Laughlin & Armstrong, 1982). Given that hemodynamic forces appear to be a key signal for regulation of vascular gene expression (Laughlin et al., 2008; Newcomer et al. 2011), we hypothesized that changes in the levels of vascular mRNAs with discontinuation of physical activity would be artery-specific.
METHODS
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. We used generation 4–5 female Wistar rats (n=18) that had been selectively bred to voluntarily run long distances. Our selective breeding procedures have been recently described (Roberts et al., 2011; Roberts et al.). We used rats selected to be highly active with the idea to mimic the high physical activity levels of our human ancestors as well as to produce the greatest downward shift in physical activity levels with our wheel lock model. Importantly, we used young healthy animals in order to study the vascular effects of inactivity in the absence of other comorbidities; that is, examining effects that are specific to inactivity before appearance of risk factors. At four weeks of age, rats were housed in cages equipped with a voluntary running wheel outfitted with a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA) for measuring daily running activity. Rats were maintained on a 12:12-h light-dark cycle (0700 to 1900) and all animals were provided food and water ad libitum. Following 23 days of access to voluntary running wheels, rats in one group were rapidly transitioned to a sedentary state by locking the wheels for seven days (n=9) or remained active (n=9) in a second group for an additional seven days. Thereafter, between 0800 and 0900, rats were anesthetized [ketamine (80 mg/kg), xylazine (10 mg/kg), and acepromazine (4mg/kg)] and whole body composition was measured via dual-energy X-ray absorptiometry (Hologic QDR-1000). Animals were then euthanized by removal of the heart in full compliance with the American Veterinary Medical Association Guidelines on Euthanasia.
Tissue sampling
Immediately following death, both renal and iliac arteries were harvested and rinsed with ice-cold Krebs saline. The left renal and iliac arteries were placed in a cold RNA-stabilizing agent (RNAlater; Ambion, Austin, TX); whereas the right renal and iliac arteries were placed in neutral-buffered 10% formalin for determination of vessel structure. On the same day as death, arteries were dissected clean of adipose tissue and excess adventitia in a petri dish containing either RNAlater or neutral-buffered 10% formalin. At sacrifice, omental fat was carefully removed and the weight was assessed.
RNA extraction and real-time PCR
We selected a panel of 25 mRNAs whose expression is frequently altered in association with increased cardiovascular risk. In particular, we chose genes related to control of vascular tone, antioxidant pathways, pro-oxidant pathways, inflammation, and thrombosis. Isolated arteries were kept in RNAlater for 24 hours at 4°C, then removed from the RNAlater solution and stored at −80°C until analysis. Arteries were homogenized in a lysing solution (Buffer RLT; Qiagen, Valencia, CA) containing 14.3 M β-mercaptoethanol (β-ME) using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA) following manufacture specifications and tissue lysates were then passed through the QIAshredder (Qiagen, Valencia, CA). Total RNA was isolated using the RNeasy fibrous tissue micro kit (Qiagen, Valencia, CA) and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration (Roseguini et al., 2010; Roseguini et al., 2011). First-strand cDNA was synthesized from total RNA by reverse transcription primed by a mixture of random hexamer and oligo(dT) primers (iScript cDNA synthesis kit; Bio-Rad, Hercules,CA). The reactions were incubated in a PCR Express Hybaid thermal cycler (Hybaid, Franklin, MA). Quantitative real-time PCR was performed as previously described (Roseguini et al., 2010; Roseguini et al., 2011) using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primer sequences (Table 1) were obtained from published literature or designed using the NCBI website. All primers were purchased from IDT (Coralville, IA). A 25-μl reaction mixture containing 20 μl of Power SYBR Green PCR Master Mix (Applied Biosystems) and the appropriate concentrations of gene-specific primers plus 5 μl of cDNA template was loaded in each well of a 96-well plate (duplicate samples). PCR was performed with thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A dissociation curve analysis was performed after each run to verify the specificity of the PCR products. β-actin primers were used to amplify the endogenous control product. Our group has established that β-actin is a suitable house-keeping gene for real-time PCR when examining vascular gene expression. In the present study, β-actin CTs were not different between groups or between arteries. mRNA expression values are presented as 2ΔCT whereby ΔCT = β-actin CT - gene of interest CT (Roberts et al., 2011; Roberts et al., 2012).
Table 1.
Forward and reverse primer sequences for quantitative real-time PCR
| Primer sequence (5′→3′) | Reference | ||
|---|---|---|---|
| Gene | Forward | Reverse | |
| βActin | CTGGCTCCTAGCACCATGAAG | GAGCCA CCAATCCACACAGA | (Park et al., 2008) |
| TNFα | CCCAGAAAAGCAAGCAACCA | CCTCGGGCCAGTGTATGAGA | |
| TNFR1 | TTGTAGGGATTCAGCTCCTGTC | CTCTTACAGGTGGCACGAAGTT | (Farid et al.) |
| TNFR2 | TGCAACAAGACTTCAGACACCGTG | AGGCATGTATGCAGATGGTTCCAG | (Farid et al.) |
| IL-6R | AAGCAGGTCCAGCCACAATGTAG | CCAACTGACTTTGAGCCAACGAG | (Farid et al. ) |
| ET1 | TTGCTCCTGCTCCTCCTTGAT | TAGACCTAGAAGGGCTTCCTAGT | (Maeda et al., 2002) |
| ETa | GGAATGGGAGCTTGCGG | TTTGCCACCTCTCGACGC | (Piuhola et al., 2003) |
| ETb | GATACGACAACTTCCGCTCCA | GTCCACGATGAGGACAATGAG | (Stenman et al., 2002) |
| LOX-1 | AGCTCGAGCACTGGCAGTTGG | GTCACAGCAGCAGGGCACCA | |
| OB-RB | GCAGCTATGGTCTCACTTCTTTTG | GGTTCCCTGGGTGCTCTGA | (Ryan et al., 2003) |
| NOX-2 | AAAGGAGTGCCCAGTACCAAAGT | TACAGGAACATGGGACCCACTAT | (Chabrashvili et al., 2003) |
| NOX-4 p22phox | AGAATGAGGATCCCAGAAAGCTT ACCTGACCGCTGTGGTGAA | ATGAGGAACAATACCACCACCAT GTGGAGGACAGCCCGGA | (Chabrashvili et al., 2003) |
| p47phox | ACGCTCACCGAGTACTTCAACA | TCATCGGGCCGCACTTT | (Chabrashvili et al., 2003) |
| p67phox | GCTTCGGAACATGGTGTCTAAGA | AGAGTCAGGCAGTAGTTTTTCACTTG | (Chabrashvili et al., 2003) |
| VCAM-1 | GAAGGAAACTGGAGAAGACAATCC | TGTACAAGTGGTCCACTTATTTCAAT | T (Park et al., 2008) |
| ICAM-1 | CACAAGGGCTGTCACTGTTCA | CCCTAGTCGGAAGATCGAAAGTC | (Park et al., 2008) |
| MCP-1 | CTGTCTCAGCCAGATGCAGTTAA | AGCCGACTCATTGGGATCAT | (Park et al., 2008) |
| E-Selectin | GCCATGTGGTTGAATGTAAAGC | GGATTTGAGGAACATTTCCTGACT | (Zhang et al., 2007) |
| PAI-1 | AGCTGGGCATGACTGACATCT | GCTGCTCTTGGTCGGAAAGA | (Wagenaar et al., 2004) |
| TM | CTGGTGTGCTCATTGGGATCT | GACAAAGAAGCGCCAAAAGC | (Wagenaar et al., 2004) |
| eNOS | AGGCATCACCAGGAAGAAGA | GGCCAGTCTCAGAGCCATAC | |
| KLF-2 | ACTTGCAGCTACACCAACTG | CTGTGACCCGTGTGCTTG | (Cullingford et al., 2008) |
| SOD-1 | TGTGTCCATTGAAGATCGTGTGA | TCTTGTTTCTCGTGGACCACC | (Chabrashvili et al., 2003) |
| SOD-2 | TTAACGCGCAGATCATGCA | CCTCGGTGACGTTCAGATTGT | (Chabrashvili et al., 2003) |
| SOD-3 | GGCCCAGCTCCAGACTTGA | CTCAGGTCCCCGAACTCATG | (Chabrashvili et al., 2003) |
Abbreviations: TNFα, tumor necrosis factor-alpha; TNFR, tumor necrosis factor receptor; IL-6R, interleukin-6 receptor; ET, endothelin; LOX-1, oxidized low-density lipoprotein receptor 1; OB-RB, leptin receptor; NOX, NADPH oxidase; VCAM-1, vascular cell adhesion molecule 1, ICAM-1, intercellular adhesion molecule 1; MCP-1, monocyte chemotactic protein; PAI-1, plasminogen activator inhibitor-1;TM, thrombomodulin; eNOS, endothelial nitric oxide synthase; SOD, superoxide dismutase
Vascular histology
Isolated arteries were kept in neutral-buffered 10% formalin for a minimum of 24 h and then processed to paraffin embedment as described previously (Roseguini et al., 2011; Arce-Esquivel et al., 2012). Four-micrometer sections were cut with an automated microtome (Microm, Thermo Fischer Scientific, Bellefonte, PA), floated onto positively charged slides (Thermo Fischer Scientific), baked for 20 min at 90°C and deparaffinized with xylene then hydrated with water. Histological assessment (i.e. structural changes) in isolated arteries was performed on sections stained with hematoxylin-eosin and Verhoeff’s method for elastin. For immunohistochemistry, sections were cut to assess expression of selected proteins (TNFR1, ET1, and LOX-1). Immunohistochemistry staining was performed on a Biocare Intellipath FLX automated slide stainer (Biocare Medical, Concord, CA) according to the following protocol. Sections were first treated with 3% hydrogen for 15 min peroxide to inhibit endogenous peroxidase. This was followed by a Tris buffer rinse. The LOX-1 and TNF-I antibodies received a protein kinase pretreatment (Dako 53020, DAKO, Carpenteria, CA) for 5 min, whereas no pretreatment was required for the ET1 antibody. Background Sniper (BS966M, Biocare Medical, Concord, CA) was applied for 10 min to inhibit nonspecific protein binding. All the primary antibodies were diluted using antibody diluent DaVinci Green (Biocare Medical) and the sections were incubated for 30 min at room temperature. After the appropriate washing steps were completed, sections were incubated with Biocare Mach 2 Universal HRP (M2U522L) for 30 min. Biocare Romulin Red AEC (RAEC810M) was applied for 10 min allowing visualization of primary antibody staining. For negative controls, histological sections were prepared as described, but incubation in primary antibody was replaced with mouse or rabbit IGg. Immunostaining was performed using rabbit anti-rat LOX-1 (1:200 dilution; Abcam Inc., Cambridge, MA), rabbit anti-rat TNFR1 (1:800 dilution, Abcam Inc., Cambridge, MA), or rabbit anti-rat ET1 (1:800 dilution; Peninsula Laboratories, Inc., San Carlos, CA). Immunostaining analysis was performed in the entire vessel wall (i.e. intima to external elastic lamina boundaries) following methods previously described (Arce-Esquivel et al., 2012). Sections were examined using an Olympus BX61 photomicroscope (Olympus, Melville, NY), and pictures that included the complete vessels’ cross-sectional area were captured at 20x magnification. In each sample, Image Pro Plus software (version 6.2.0.424; Media Cybernetics, Inc., Silver Spring, MD) was used to quantify the positive area of staining. Finally, in each sample stained with hematoxylin-eosin, the perimeter of the intimal layer and the perimeter of the outer medial layer (boundary between media and adventitia) were measured to determine lumen diameter and intima-media thickness. An investigator who was blinded to the experimental conditions performed the immunostaining procedures and measurements.
Statistical analysis
All data are presented as means ± SE. A 2 × 2 (group x artery) mixed design, repeated-measures ANOVA was used to evaluate the effects of wheel lock and vessel type on gene expression and all additional dependent variables. Between-group differences in body composition variables were determined using an independent t-test. The relationship between omental fat mass and vascular gene expression was evaluated using Pearson correlations. For all statistical tests, significance was set at 0.05. Statistical analyses were performed with SPSS 19.0. (SPSS, Chicago, IL).
RESULTS
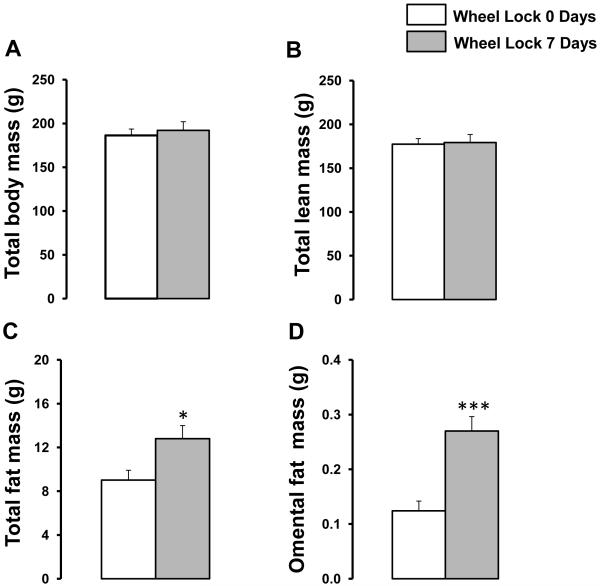
During the period of access to wheel running, rats averaged a running distance of approximately 15 km per night for days 17-23, and thereafter. There were no differences in running distances between the two groups prior to wheel lock. Both groups of rats averaged a calculated running speed of approximately 57 meter/min, an intensity that according to data from treadmill studies (Laughlin & Armstrong, 1982) is sufficient to significantly increase blood flow to the working skeletal muscles and decrease flow to the kidneys. The estimated time per night of intermittent running was ~4.4 hours distributed over the initial ~9 hours of the dark cycle. At sacrifice, both groups of rats had similar total body mass and total lean mass, however, wheel lock 7-day rats exhibited 42% and 117% greater total fat mass and omental fat mass, respectively, compared to wheel lock 0-day rats that ran 30 days (both p<0.05, Figure 1).
Figure 1.
Effects of 7 days of wheel lock on total body mass (A), total lean mass (B), total fat mass (C), and omental fat mass (D). *p<0.05, ***p<0.0001 vs. Wheel Lock 0 Days.
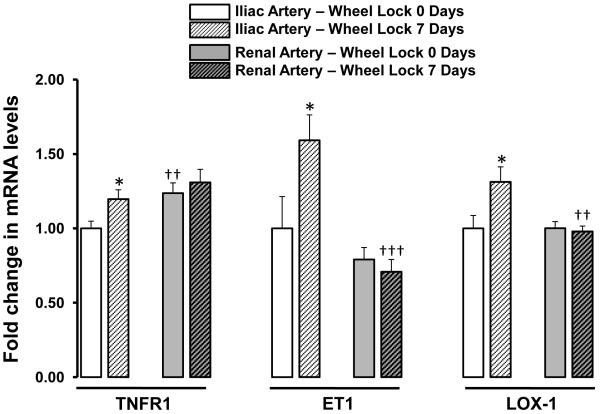
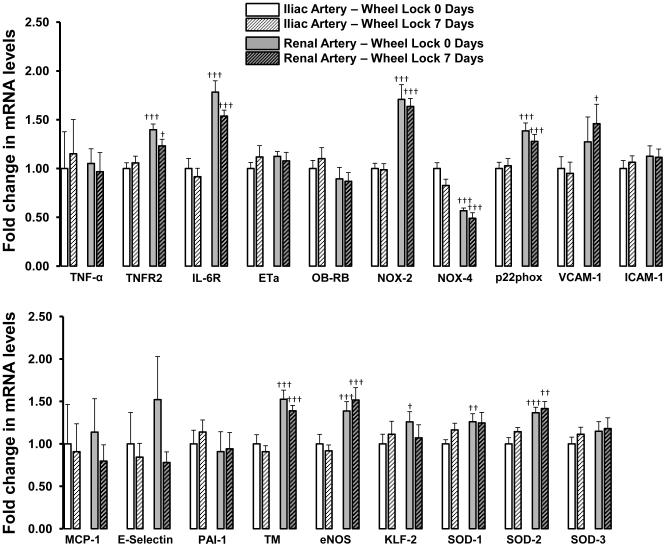
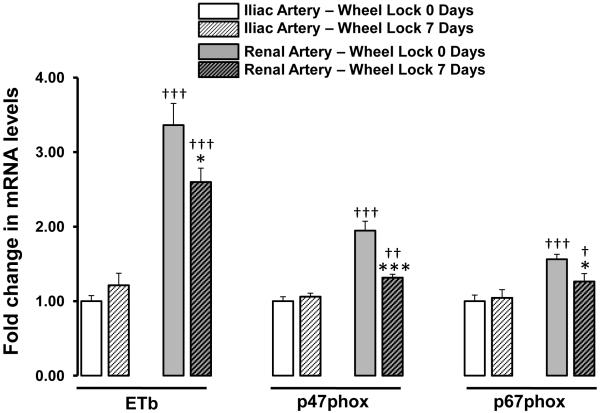
Figures 2 to 4 summarize the effects of wheel lock on iliac and renal artery gene expression. Compared to iliac arteries of wheel lock 0-day rats, iliac arteries of wheel lock 7-day rats exhibited increased expression of mRNA TNFR1 (+19%), ET1 (+59%), and LOX-1 (+31%) (all p<0.05) (Figure 2). In addition, compared to renal arteries of wheel lock 0-day rats, renal arteries of wheel lock 7-day rats exhibited decreased expression of mRNA ETb (−23%), p47phox (−32%), and p67phox (−19%) (all p<0.05) (Figure 3). Wheel lock did not produce significant changes in mRNA levels in the iliac and renal arteries for the reminder of the 19 genes that were evaluated (Figure 4). Additionally, there were a number of differences between iliac and renal arteries in mRNA levels (Figs 2-4).
Figure 2.
List of vascular genes whose mRNA levels are upregulated in the iliac, but not renal, artery in response to 7 days of wheel lock. *p<0.05 vs. Wheel Lock 0 Days; ††p<0.01, †††p<0.001 vs. Iliac Artery.
Figure 4.
List of vascular genes whose mRNA levels are unchanged in the iliac and renal arteries in response to 7 days of wheel lock. †p<0.05, ††p<0.01, †††p<0.001 vs. Iliac Artery.
Abbreviations: TNF-α, tumor necrosis factor-alpha; TNFR2, tumor necrosis factor receptor 2; IL-6R, interleukin-6 receptor; OB-RB, leptin receptor; NOX, NADPH oxidase; VCAM-1, vascular cell adhesion molecule 1, ICAM-1, intercellular adhesion molecule 1; MCP-1, monocyte chemotactic protein; PAI-1, plasminogen activator inhibitor-1; TM, thrombomodulin; eNOS, endothelial nitric oxide synthase; SOD, superoxide dismutase
Figure 3.
List of vascular genes whose mRNA levels are downregulated in the renal, but not iliac, artery in response to 7 days of wheel lock. *p<0.05, ***p<0.0001 vs. Wheel Lock 0 Days; †p<0.05, ††p<0.01, †††p<0.001 vs. Iliac Artery.
Abbreviations: ETb, endothelin b receptor.
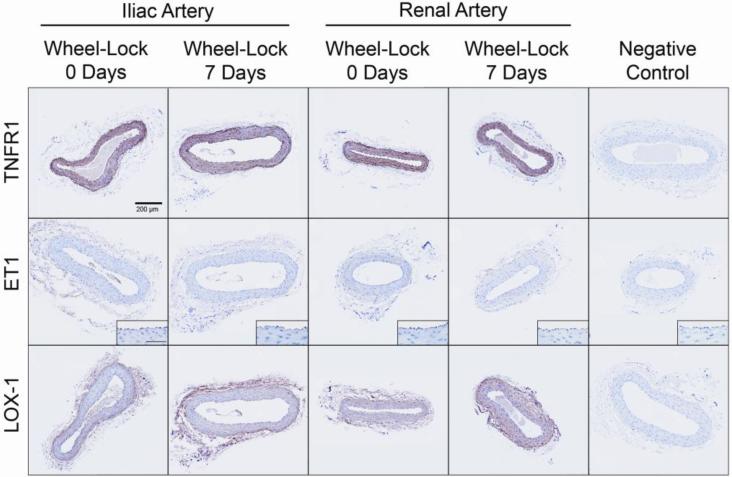
Figure 5 illustrates immunohistochemical localization of proteins encoded by the three genes (TNFR1, ET1, LOX-1) that demonstrated increased mRNA levels in the iliac artery with inactivity. Results indicate that 7 days of inactivity did not appear to induce changes in expression of these proteins as assessed by immunohistochemical staining. That is, no significant differences in protein expression were detected between wheel lock 0 days and 7 days for TNFR1 (34.8±2.3 vs. 39.0±2.1%; p=0.19), ET1 (0.6±0.1 vs. 0.5±0.1%; p=0.41) and LOX-1 (7.3±0.6 vs. 8.3±1.7%; p=0.57) in the iliac artery.
Figure 5.
Representative images of immunohistochemical staining (shown here in brown) for TNFR1, ET1, and LOX-1 in cross-sections of the iliac and renal arteries of wheel lock 0 days and 7 days rats. Pictures were captured at 20x magnification, whereas insets for ET1 were taken at 40x (scale bar = 50 microns). As illustrated, immunoreactivity of TNFR1 was abundant and it appeared homogenous across the arterial wall. In contrast, immunoreactivity of ET1was usually minimal and primarily localized in the endothelial cell layer. Immunoreactivity of LOX-1 generally appeared to be greater in the adventitia and endothelium, compared to the media. As summarized in text of the results section, mean data revealed no significant differences for any of the markers between groups.
No differences in lumen diameter and intima-media thickness were found between groups of rats. However, iliac arteries had greater lumen diameter (590±14 vs. 354±30 um; p<0.001) and intima-media thickness (77±6 vs. 61±6 um; p=0.07) compared to renal arteries.
Omental fat mass was inversely correlated with expression of LOX-1 (r=-0.50, p=0.03), p47phox (r=-0.50, p=0.03), p67phox (r=-0.59, p=0.01) in the renal artery and with expression of NOX-4 (r=-0.53, p=0.02) in the iliac artery; whereas a trend for a positive correlation was detected between omental fat mass and expression of SOD-3 (r=0.438, p=0.07) in the iliac artery.
DISCUSSION
The primary finding of the present study is that transition from high to low physical activity produced differential changes in vascular mRNA levels between rat iliac and renal arteries for 6 of the 25 genes interrogated. We speculate that the different influence of short-term physical inactivity on vascular mRNA levels between the iliac and renal arteries could be related to the reported differences in blood flow to the tissues perfused by these arteries during exercise (Laughlin & Armstrong, 1982). That is, when rats transition from daily wheel running this could remove episodic bouts of increased iliac artery blood flow occurring during running whereas, conversely, the renal arteries may experience the removal of corresponding bouts of decreased flow during running (Laughlin & Armstrong, 1982).
We employed the rodent wheel lock model to replicate a shift from high to low physical activity levels that is experienced in human life. The sudden lock of running wheels permits the study of the biological consequences of transitioning from a physically active to a sedentary life (Kump & Booth, 2005; Kump et al., 2006; Laye et al., 2007; Rector et al., 2008; Laye et al., 2009; Roberts et al. 2012). It should be noted that rats retain ambulatory cage activity after the wheel-lock and do not become fully immobile as occurs in other models of extreme inactivity such as hindlimb suspension in animals and bed rest in humans (Booth et al., 2008). Importantly, our objective was to identify mRNAs in the arterial wall that are responsive to the transient changes in physical activity rather than to characterize the long-term effects of inactivity on the vasculature. Identification of vascular genes whose expression is altered in response to short-term physical inactivity may provide important information regarding the initial molecular events underlying the first steps in the progression towards an impaired vascular cell phenotype if inactivity persists. In the current study, 25 target mRNAs (markers of endothelium-derived dilators, antioxidant pathways, pro-oxidant pathways, inflammation, and thrombosis) were selected for examination based on evidence that their expression is frequently altered in association with cardiovascular risk (Hulsmans et al.; Ross, 1999; Li et al., 2006; Tedgui & Mallat, 2006; Pacher et al., 2007; Atkins et al., 2008).
To gain new insights about the role of hemodynamic forces in signaling the early changes in vascular gene expression induced by physical inactivity, we studied two arteries perfusing tissues that presumably exhibit differential blood flow responses to physical activity. Indeed during treadmill running, blood flow is dramatically increased in the working skeletal muscle (i.e. hindlimb) and decreased in visceral organs (e.g. kidney, liver, spleen) of rats (Laughlin & Armstrong, 1982). As a result, it is reasonable to speculate that during wheel running at a similar speed (e.g. ~57 meter/min) to that during treadmill running, iliac and renal arteries may be exposed to increased and decreased levels of blood flow, respectively. It is also well-established that mean arterial pressure and pulse pressures are increased during exercise, but this has not been measured during wheel running; it is only logically presumed.
Results in this study indicate that cessation of physical activity for seven days evoked a statistically significant upregulation of mRNA for tumor necrosis factor receptor superfamily member 1A (gene name TNFRSF1A, also TNFR1, which can activate NFκB, mediate apoptosis and is a regulator of inflammation), endothelin 1 (EDN1, ET1, a potent vasoconstrictor produced by endothelial cells), and oxidized-low density lipoprotein (lectin-like) receptor 1 (OLR1, LOX-1, the receptor that mediates recognition, internalization and degradation of oxidized low-density lipoprotein) in the iliac artery. In contrast, in the renal artery, wheel lock induced a downregulation of mRNA for endothelin receptor type B (ENDRB, ETb, a G protein-coupled endothelial cell receptor which activates a phosphatidylinositol-calcium second messenger system upon binding with endothelin), neutrophil cytosolic factor 1 (NCF1, p47phox, a cytosolic subunit of the NADPH oxidase system), and neutrophil cytosolic factor 2 (NCF2, p67phox, also a cytosolic NADPH oxidase subunit). The observation that short-term inactivity stimulated the upregulation of expression of three pro-atherogenic genes in the iliac artery (Figure 2) and downregulation of three pro-atherogenic genes in the renal artery (Figure 3) is consistent with the premise that cessation of physical activity would produce distinct effects between arteries likely experiencing differential blood flow responses during activity bouts. Our immunohistochemical measures indicated that the amount of TNFR1, ET1, and LOX-1 protein in the iliac artery wall was not different between both groups of rats. The observation that increases in mRNA levels induced by 7 days of wheel lock were not associated with significant changes at the protein level, suggests that greater duration of inactivity may be required for changes in mRNA levels to lead to increased expression of these proteins. Nonetheless, the changes in mRNA establish alterations in pretranslational regulation indicating changes at either transcription and/or mRNA stability in response to inactivity.
The finding that ET1 mRNA levels are increased following one week of inactivity may be of particular relevance in view of evidence indicating that enhanced vascular tone of extremely inactive legs of long-term spinal cord injury patients is largely ET1 mediated (Thijssen et al., 2007). There is also evidence in humans that ET1 plays a role in the control of arterial compliance. For example, McEnery et al. (McEniery et al., 2003) experimentally demonstrated in humans that exogenous ET1 increases pulse wave velocity in large arteries (i.e., a non-invasive marker of arterial stiffness), a response that can be blunted by ETA receptor blockade. This observation may explain why conditions that exhibit upregulation of ET1 (e.g., inactivity) are also associated with arterial stiffening. Given that studies in endothelial cell culture provide direct evidence that a reduction of shear stress increases ET1 mRNA (Toda et al., 2008), it is likely that alterations in vascular expression of ET1 with inactivity are due to the removal of episodic bouts of increased blood flow.
While the present data support the hypothesis that changes in blood flow with physical inactivity might modulate vascular cell phenotype, the fact that the ‘responsive genes’ were different between the iliac and renal arteries allows the speculation that hemodynamic forces could interact with other physical inactivity-induced signals in the regulation of gene expression. In this regard, we (Company et al., 2010; Padilla et al., 2011a; Jenkins et al., 2012) and others (Szostak & Laurant, 2011) have recently proposed that physical inactivity may exert its effects on the vasculature by altering the normal phenotype of cells in adipose tissue resulting in changes in adipokine secretion into the systemic circulation. The crosstalk between adipose tissue and vascular cells is in fact well recognized in the literature (Ohman et al.; Esposito et al., 2002; Lyon et al., 2003; Hutley & Prins, 2005; Ritchie & Connell, 2007; Ouwens et al., 2010; Vykoukal & Davies, 2011). Herein, we observed that discontinuation of physical activity for seven days stimulated a remarkable (117%) expansion of omental fat mass (Figure 1), a finding that is consistent with other studies using the wheel lock model (Laye et al., 2007; Laye et al., 2009) and studies in humans assessing the impact of reducing the number of daily steps (Olsen et al., 2008). Interestingly, we observed significant inverse correlations between omental fat mass and the expression of four pro-oxidant mRNAs (LOX-1, p47phox, p67phox, NOX-4), and a trend for a positive correlation was detected between omental fat mass and the antioxidant gene SOD-3. While on one hand the direction of these relationships may be unexpected, these results could reflect a homeostatic mechanism whereby vascular cells decreased the expression of pro-oxidant genes and increased the expression of antioxidant genes in response to physical inactivity-induced visceral fat expansion. In addition to the possible alteration in adipokine secretion with physical inactivity, it is also conceivable that other circulating factors (e.g. myokines, inflammatory cells) may be responsible for the modulation of vascular mRNA levels after seven days of physical inactivity.
Potential limitations of the present investigation should be considered. First, while there is sufficient evidence from earlier microsphere studies that blood flow is increased in the rat iliac artery and decreased in the renal artery during treadmill running (Laughlin & Armstrong, 1982), shear stress levels in these arteries have not been quantified. We speculate that shear stress in the iliac and renal arteries parallels changes in blood flow during wheel running and during inactivity. Consistent with this presumption is the frequent observation that conduit artery diameter changes during exercise are relatively minor and therefore not sufficient to offset the positive relationship between blood flow and shear stress (Padilla et al., 2011a; Padilla et al., 2011b). It would have been advantageous to have measured iliac and renal blood flows in the present study, but microsphere studies during wheel running are particularly challenging. In fact, we are not aware of any studies in which regional blood flows have been measured during wheel-running activity. In addition, currently there are no available techniques to measure shear stress in peripheral arteries of rats during running. Second, because we studied mRNA levels from whole artery homogenates, it is unclear whether differences in gene expression reported in this study are originating from the endothelium, smooth muscle or adventia. In this context, Figure 5 illustrates immunohistochemical localization of TNFR1, ET1 and LOX-1 in cross-sections of the iliac and renal arteries. In addition, it should be noted that while iliac arteries had greater lumen diameter and intima-media thickness compared to renal arteries, seven days of wheel lock did not produce any structural changes in these arteries. Third, the present study was focused at identifying artery-specific changes in vascular mRNAs that would reflect initial transcriptional events underlying a shift in phenotype if inactivity were to persist. Thus, while our study achieved its primary aim, future studies are required to establish the time point at which changes in mRNA levels triggered by inactivity may result in changes at the protein level that can potentially lead to functional consequences. Fourth, while we advocate that maintenance of a physically active lifestyle, mimicked by access to voluntary running wheels, provides optimal vascular health and thus should represent the normal (i.e., control) state, the inclusion of a lifetime sedentary group into the study design would have facilitated better interpretation of the changes induced by 7 days of wheel lock.
In conclusion, the results of this study demonstrate that cessation of voluntary wheel running for seven days produces modest, but differential changes in vascular mRNA levels between the iliac and renal arteries of healthy rats. We speculate that the differing influence of short-term physical inactivity on vascular mRNA levels in these two arteries could be attributed to iliac arteries exhibiting removal of episodic bouts of increased blood flow during exercise, while renal arteries experiencing removal of episodic decreases in flow during exercise. We consider that the changes in mRNA levels reported here, although modest, may be of biological significance given the short term nature of the inactivity stimulus.
ACKNOWLEDGEMENTS
This work was supported by anonymous gifts (to FWB), AHA 11POST5080002 (to JP), NIH T32-AR048523 (to NTJ and JSM), and NIH RO1HL036088 (to MHL).
REFERENCES
- Arce-Esquivel AA, Kreutzer KV, Rush JW, Turk JR, Laughlin MH. Exercise does not attenuate early CAD progression in a pig model. Med Sci Sports Exerc. 2012;44:27–38. doi: 10.1249/MSS.0b013e318228879b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous Deficiency of Kruppel-Like Factor 2 Augments Experimental Atherosclerosis. Circulation Research. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP. Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol. 2008;102:381–390. doi: 10.1007/s00421-007-0606-5. [DOI] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic disease. Comprehensive Physiology. 2012 doi: 10.1002/cphy.c110025. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- Company JM, Booth FW, Laughlin MH, Arce-Esquivel AA, Sacks HS, Bahouth SW, Fain JN. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationships to visceral and subcutaneous fat. J Appl Physiol. 2010;109:1904–1912. doi: 10.1152/japplphysiol.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingford TE, Butler MJ, Marshall AK, Tham EL, Sugden PH, Clerk A. Differential regulation of KrÃ1/4ppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: Effects of endothelin-1, oxidative stress and cytokines. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2008;1783:1229–1236. doi: 10.1016/j.bbamcr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Nicoletti G, Giugliano D. Obesity, cytokines and endothelial dysfunction: a link for the raised cardiovascular risk associated with visceral obesity. J Endocrinol Invest. 2002;25:646–649. doi: 10.1007/BF03345092. [DOI] [PubMed] [Google Scholar]
- Farid AS, Mido S, Linh BK, Hayashi T, Horii Y. An atherogenic lipid profile with low serum paraoxonase-1 activity during nematode infection in rats. European Journal of Clinical Investigation. 40:984–993. doi: 10.1111/j.1365-2362.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. The FASEB Journal. 25:2515–2527. doi: 10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci. 2005;330:280–289. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Jenkins NT, Martin JS, Laughlin MH, Padilla J. Exercise-induced signals for vascular endothelial adaptations: implications for cardiovascular disease. Curr Cardiovasc Risk Rep. 2012 doi: 10.1007/s12170-012-0241-5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, Hall GV, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol. 2010;108:1034–1040. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol. 2005;565:911–925. doi: 10.1113/jphysiol.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kump DS, Laye MJ, Booth FW. Increased mitochondrial glycerol-3-phosphate acyltransferase protein and enzyme activity in rat epididymal fat upon cessation of wheel running. Am J Physiol Endocrinol Metab. 2006;290:E480–E489. doi: 10.1152/ajpendo.00321.2005. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. American Journal of Physiology - Heart and Circulatory Physiology. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. Journal of Applied Physiology. 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol. 2009;106:161–168. doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol. 2007;102:1341–1347. doi: 10.1152/japplphysiol.01018.2006. [DOI] [PubMed] [Google Scholar]
- Li YH, Shi GY, Wu HL. The role of thrombomodulin in atherosclerosis: from bench to bedside. Cardiovasc Hematol Agents Med Chem. 2006;4:183–187. doi: 10.2174/187152506776369953. [DOI] [PubMed] [Google Scholar]
- Lyon CJ, Law RE, Hsueh WA. Minireview: Adiposity, Inflammation, and Atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- Maeda S, Miyauchi T, Iemitsu M, Tanabe T, Yokota T, Goto K, Yamaguchi I, Matsuda M. Effects of exercise training on expression of endothelin-1 mRNA in the aorta of aged rats. Clin Sci. 2002;103(Suppl 48):118S–123S. doi: 10.1042/CS103S118S. [DOI] [PubMed] [Google Scholar]
- McEniery C, Qasem A, Schmitt M, Avolio A, Cockcroft J, Wilkinson I. Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42:1975–1981. doi: 10.1016/j.jacc.2003.06.016. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Thijssen DHJ, Green DJ. Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise-induced hemodynamics. Journal of Applied Physiology. 2011;111:311–320. doi: 10.1152/japplphysiol.00033.2011. [DOI] [PubMed] [Google Scholar]
- Nocon M, Hiemann T, MÃ1/4ller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. European Journal of Cardiovascular Prevention & Rehabilitation. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA. 2008;299:1261–1263. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. Journal of Cellular and Molecular Medicine. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher Pl, Beckman JS, Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiological Reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology. 2011a;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilation during prolonged lower limb exercise: role of shear rate. Experimental Physiology. 2011b;96:1019–1027. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Hirose R, Dang K, Xu F, Behrends M, Tan V, Roberts JP, Niemann CU. Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery. 2008;143:243–251. doi: 10.1016/j.surg.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Piuhola J, Szokodi In, Kinnunen P, Ilves M, deChÃc̷tel R, Vuolteenaho O, Ruskoaho H. Endothelin-1 Contributes to the Frank-Starling Response in Hypertrophic Rat Hearts. Hypertension. 2003;41:93–98. doi: 10.1161/01.hyp.0000050929.96979.ec. [DOI] [PubMed] [Google Scholar]
- Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Appl Physiol. 2008;586:4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA, Connell JMC. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Childs TE, Brown JD, Davis JW, Booth FW. Early depression of Ankrd2 and Csrp3 mRNAs in the polyribosomal and whole-tissue fractions in skeletal muscle with decreased voluntary running. Journal of Applied Physiology. 2012;112:1291–1299. doi: 10.1152/japplphysiol.01419.2011. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, Booth FW. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol Behav. 2011;105:661–668. doi: 10.1016/j.physbeh.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Roseguini BT, Arce-Esquivel AA, Newcomer SC, Laughlin MH. Impact of a single session of intermittent pneumatic leg compressions on skeletal muscle and isolated artery gene expression in rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1658–1668. doi: 10.1152/ajpregu.00457.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseguini BT, Soylu M, Whyte JJ, Yang HT, Newcomer SC, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol. 2010;298:H1991–H2000. doi: 10.1152/ajpheart.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis. An inflammatory disease. New England Journal of Medicine. 1999;340:115–128. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Ryan NK, Van der Hoek KH, Robertson SA, Norman RJ. Leptin and Leptin Receptor Expression in the Rat Ovary. Endocrinology. 2003;144:5006–5013. doi: 10.1210/en.2003-0584. [DOI] [PubMed] [Google Scholar]
- Stenman E, Malmsjo M, Uddman E, Gido G, Wieloch T, Edvinsson L. Cerebral Ischemia Upregulates Vascular Endothelin ETB Receptors in Rat. Stroke; a journal of cerebral circulation. 2002;33:2311–2316. doi: 10.1161/01.str.0000028183.04277.32. [DOI] [PubMed] [Google Scholar]
- Szostak J, Laurant P. The forgotten face of regular physical exercise: a ‘natural’ anti-atherogenic activity. Clin Sci. 2011;121:91–106. doi: 10.1042/CS20100520. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiological Reviews. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Ellenkamp R, Kooijman M, Pickkers P, Rongen GA, Hopman MTE, Smits P. A Causal Role for Endothelin-1 in the Vascular Adaptation to Skeletal Muscle Deconditioning in Spinal Cord injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:325–331. doi: 10.1161/01.ATV.0000253502.83167.31. [DOI] [PubMed] [Google Scholar]
- Toda M, Yamamoto K, Shimizu N, Syotaro O, Kumagaya S, Igarashi T, Kamiya A, Ando J. Differential gene responses in endothelial cells exposed to a combination of shear stress and cyclic stretch. Journal of Biotechnology. 2008;133:239–244. doi: 10.1016/j.jbiotec.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J Vasc Surg. 2011;54:819–831. doi: 10.1016/j.jvs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med. 2004;36:782–801. doi: 10.1016/j.freeradbiomed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Weintraub WS, Daniels SR, Burke LE, Franklin BA, Golf DCJ, Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- Zhang X-Y, Hayasaka S, Hayasaka Y, Cui H-S, Chi Z-L. Effect of N-acetylcysteine on Lipopolysaccharide-Induced Uveitis in Rats. Japanese Journal of Ophthalmology. 2007;51:14–20. doi: 10.1007/s10384-006-0382-5. [DOI] [PubMed] [Google Scholar]