SUMMARY
Background
The pathophysiology of sickle cell disease (SCD) is complex, with increasing evidence of a pronounced prothrombotic state.
Objective
We investigated thrombin generation in SCD utilizing calibrated automated thrombography (CAT) and Ddimer, with subsequent correlation to clinical disease.
Patient/Methods
The study included 51 patients homozygous for hemoglobin S, either admitted for vaso-occlusive crisis (VOC) (n=34) or while in steady state and being seen in outpatient clinic (n=37). Twenty patients had blood drawn during both VOC and steady state. Mean values for CAT and D-dimer were compared between groups. Mean values for patients with and without clinical complications such as avascular necrosis and stroke were also compared. Linear regression was used to evaluate correlation to number of hospitalizations and for all pediatric patients, transcranial doppler (TCD) velocities.
Results
The mean D-dimer during VOC (2743 ± 3118 ng/ml) was significantly higher than during steady state (1151 ± 802, p<0.0001). Comparison of crisis and steady state by CAT also revealed a significant difference in all phases of thrombin generation, including mean endogenous thrombin potential (1381 ± 295 nM vs 923 ± 316, p<0.0001) and peak thrombin generated (284 ± 9 vs 223 ± 18, p=0.0002). There were no significant differences in mean values for the clinical outcomes examined in adults. In pediatric patients, however, increased TCD velocities correlated with steady state Ddimer (r2=0.32, p=0.02) and thrombin-antithrombin complex (r2=0.28, p=0.04.
Conclusion
Hypercoagulable markers distinguish between patients with SCD during and between VOC, but do not correlate with specific clinical phenotypes.
Keywords: sickle cell disease, thrombin, hypercoagulable state
INTRODUCTION
Sickle cell disease (SCD) is one of the most common inherited diseases worldwide and the most common inherited blood disorder within the United States. Patients with SCD experience both large and small vessel occlusions leading to end-organ damage and complications such as avascular necrosis (AVN), cerebral vascular accidents, and acute chest syndrome[1]. These vaso-occlusive events are likely multifactorial and result from various processes, including: reduced red cell deformability[2]; abnormal red cell adhesive properties[3, 4]; intimal proliferation[5]; and a chronically activated coagulation pathway.
Numerous studies provide laboratory evidence of a hypercoagulable state in patients with SCD[6–9]. This hypercoagulable state has been documented by various abnormalities of cytokines, coagulation markers, and increased phosphatidylserine exposure[10]. An important component of the hypercoagulable state is increased thrombin production, and recent evidence also reveals that procoagulant microparticles play an important role in thrombin production[11]. This accentuated thrombin production is supported by findings of an elevated D-dimer, thrombin-antithrombin complex (TAT) and prothrombin fragment 1.2 in steady state[12]. Subsequent studies have also determined that this hypercoagulable state increases further during sickle cell crisis[8]. An increased plasma D-dimer, prothrombin fragment 1.2, and TAT complexes during an acute vaso-occlusive crisis (VOC)[10] are also suggestive of thrombin generation as a potential role in the pathophysiology of VOC. Global assays, such as calibrated automated thrombography (CAT), have recently become available to evaluate time dependent changes in the hemostatic system during clotting, and give a unique perspective to thrombin generation in diseases such as SCD. CAT allows an improved ability to determine thrombin generation and thrombin kinetics as well as potentially act as an indicator of the microparticle environment[13].
We postulated that we could use global assays such as the calibrated automated thrombography (CAT) to document more specific changes in thrombin generation that occur in patients with SCD during crisis and steady state. Furthermore, we sought to identify age related changes in thrombin generation as seen in other chronic disease[14, 15] and evaluate potential correlations with clinical disease outcomes such as AVN, stroke, number of hospitalizations, and transcranial doppler (TCD) velocities.
DESIGN AND METHODS
Patients
Adult and pediatric patients greater than 4 years of age with SCD (type SS) were approached either during a hospitalization for pain crisis or during outpatient visits for steady state. Hospitalized patients were enrolled and provided blood samples within 36 hours of admission. Patients enrolled during outpatient visits were confirmed to be at baseline pain scores and were at least 2 weeks from their last admission. Exclusion criteria included the following: red blood cell transfusions within the last 4 weeks; use of anticoagulation; chronic or daily use of steroids or non-steroidal anti-inflammatory medication; and diagnosis of end stage renal disease or creatinine >2.0mg/dL. All patients gave written informed consent, children provided assent when applicable, and the study was approved by the institutional review board. Patient records were reviewed to determine: hydroxyurea use; number of admissions within the past year; history of avascular necrosis; and history of cerebral vascular accidents. Standard evaluation for pediatric patients up to age 16 years of age include an annual TCD to determine risk of stroke, therefore all pediatric patients had mean values of left and right middle cerebral artery velocities recorded. All pediatric patients had TCDs performed on day of blood draw or within the past year.
Collection of blood samples
Blood was drawn using a large bore needle with a BD Vacutainer system and standard tourniquet collection. Adult patients with ports or central access devices had at least 10ml of waste blood drawn prior to collection. Difficult blood draws or blood draws requiring significant manipulation were discarded. Blood was collected into two adult sodium citrate tubes (0.1M) and centrifuged within 30 minutes of collection. Platelet poor plasma (PPP) was prepared by a first centrifugation at 3500g for 15 minutes followed by a second centrifugation of the plasma at 9500g for 10 minutes. The resulting PPP samples were then separated into 1ml aliquots and snap-frozen in liquid nitrogen at −80C. The median time samples were stored prior to testing was 3 months (range 1.5 to 4). All samples were thawed in water (room temperature) for 5 minutes and testing was subsequently performed within 10 minutes following pre-heating of the instrument for 10 minutes. Inter-assay coefficient of variation (CV) for normal samples was less than 10% (performed over 6 months time).
Reagents and assays
For each sample, we tested coagulation using a D-dimer ELISA (Diagnostica Stago) and Thrombin Generation Assay (Stago). The PPP Low (1 pM tissue factor, 4uM phospholipids) was used as an activator. The CAT was run in a Fluoroskan Ascent plate reader with Thrombinoscope software (Stago) against standard Thrombin Calibrator (Stago) with all samples performed in triplicate. Thrombin-antithrombin (TAT) complex ELISA (Diagnostica Stago) tests was performed for all pediatric patients.
Statistics
The mean values of D-dimer, each phase of the TGA (lag phase, slope, peak thrombin, and endogenous thrombin potential), and TAT (pediatric patients only) were compared between crisis and steady state. Comparison was performed with all patient samples using unpaired t-tests. Comparison was performed for patients with paired data with paired t-tests. Paired t-test was also used to compare mean values of D-dimer and TGA phases for the presence or absence of avascular necrosis, hydroxyurea use, and stroke. Finally, a linear regression model was used to compare D-dimer, TAT and thrombin generation with number of hospitalizations and TCD velocities. All statistical analyses were done using Graphpad Prism 5 software. No adjustment was made for multiple comparisons; p<0.05 considered statistically significant.
RESULTS
Fifty-one patients were enrolled in the study providing 34 samples during crisis and 37 samples during steady state. There were 20 patients who provided samples during both crisis and steady state (paired samples). The median age of patients used for analysis was 17 years old (range 4 – 41); 59% were female (see table 1a and 1b). There were 20 pediatric patients and 31 adult patients. There were 45 patients (81%) being treated with hydroxyurea (HU) at time of enrollment.
Table 1.
| a. Unpaired Patient Characteristics (n=51) | ||
|---|---|---|
| Pediatric | Adult | |
| Number of patients | 20 | 31 |
| Mean Age - years | 9.75 | 24.9 |
| Female (%) | 10 (50) | 20 (65) |
| CVA (%) | 2 (10) | 5 (16) |
| AVN (%) | 1 (5) | 4 (13) |
| Hydroxyurea use (%) | 18 (90) | 23 (74) |
| b. Paired Patient Characteristics (n=20) | ||
|---|---|---|
| Pediatric | Adult | |
| Number of patients | 9 | 11 |
| Mean Age - years | 11.6 | 24.3 |
| Female (%) | 2 (22) | 4 (36) |
| CVA (%) | 1 (11) | 3 (27) |
| AVN (%) | 1 (5) | 2 (18) |
| Hydroxyurea use (%) | 5 (55) | 10 (90) |
CVA, cerebral vascular accident; AVN, avascular necrosis
Hypercoagulable markers
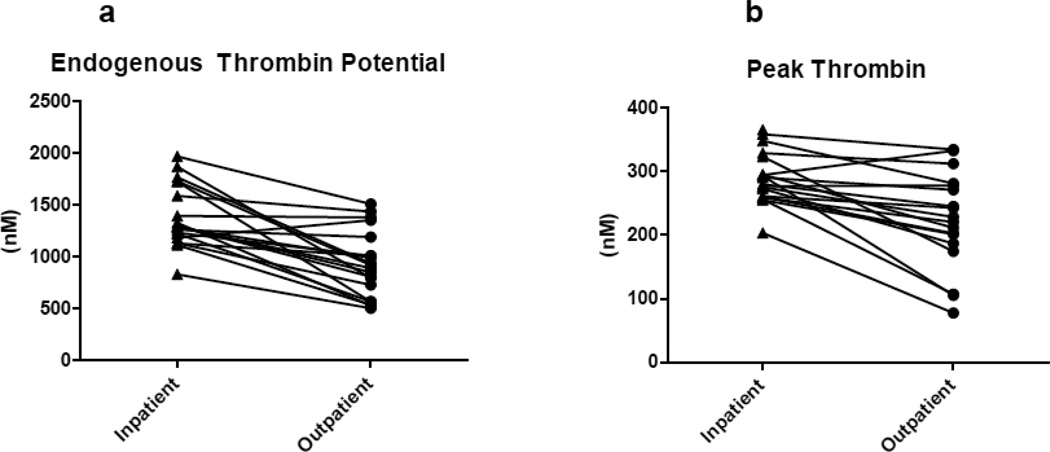
Unpaired analysis of the mean D-dimer during inpatient crisis was significantly higher than during outpatient steady state. Comparison of crisis and steady state by the CAT using unpaired analysis found a significant decrease in crisis for lag phase and slope, as well as an increased endogenous thrombin potential (ETP). Peak thrombin production, however, was non-significantly increased. Comparison of mean values hypercoaguable markers between crisis and non crisis states is shown in Table 2a and 2b. Paired comparisons of ETP and peak thrombin between crisis and non crisis states are shown in Figure 1a and 1b.
Table 2.
| a. Unpaired comparison (n=51) of mean values for hypercoagulable markers in crisis and non crisis | |||
|---|---|---|---|
| Crisis | Non crisis | ||
| D-dimer (ng/ml) | 2743 ± 3118 | 1151 ± 802** | |
| TGA | Lag phase (min) | 3.6 ± 1.5 | 4.7 ± 1.7** |
| Slope (nM/min) | 5.05 ± 1.3 | 7.99 ± 1.6** | |
| Peak (nM) | 273 ± 60 | 244 ± 73 | |
| ETP (nM) | 1355 ± 321 | 1143 ± 470* | |
| b. Paired comparison (n=20) of mean values for hypercoagulable markers in crisis and non crisis | |||
|---|---|---|---|
| Crisis | Non crisis | ||
| D-dimer (ng/ml) | 3077 ± 3655 | 1041±619* | |
| TGA | Lag phase (min) | 3.2 ± 1.2 | 4.6 ± 1.99** |
| Slope (nM/min) | 4.99 ± 1.1 | 7.99 ± 1.6** | |
| Peak (nM) | 289 ±41 | 223 ± 75** | |
| ETP (nM) | 1381 ± 295 | 923 ± 316** | |
TGA, thrombin generation assay; ETP, endogenous thrombin potential;
p<0.05;
p<0.01
Figure 1.
Thrombin generation assay: paired analysis (n=20) of patients with SCD during VOC (inpatient) as compared to steady state (outpatient).
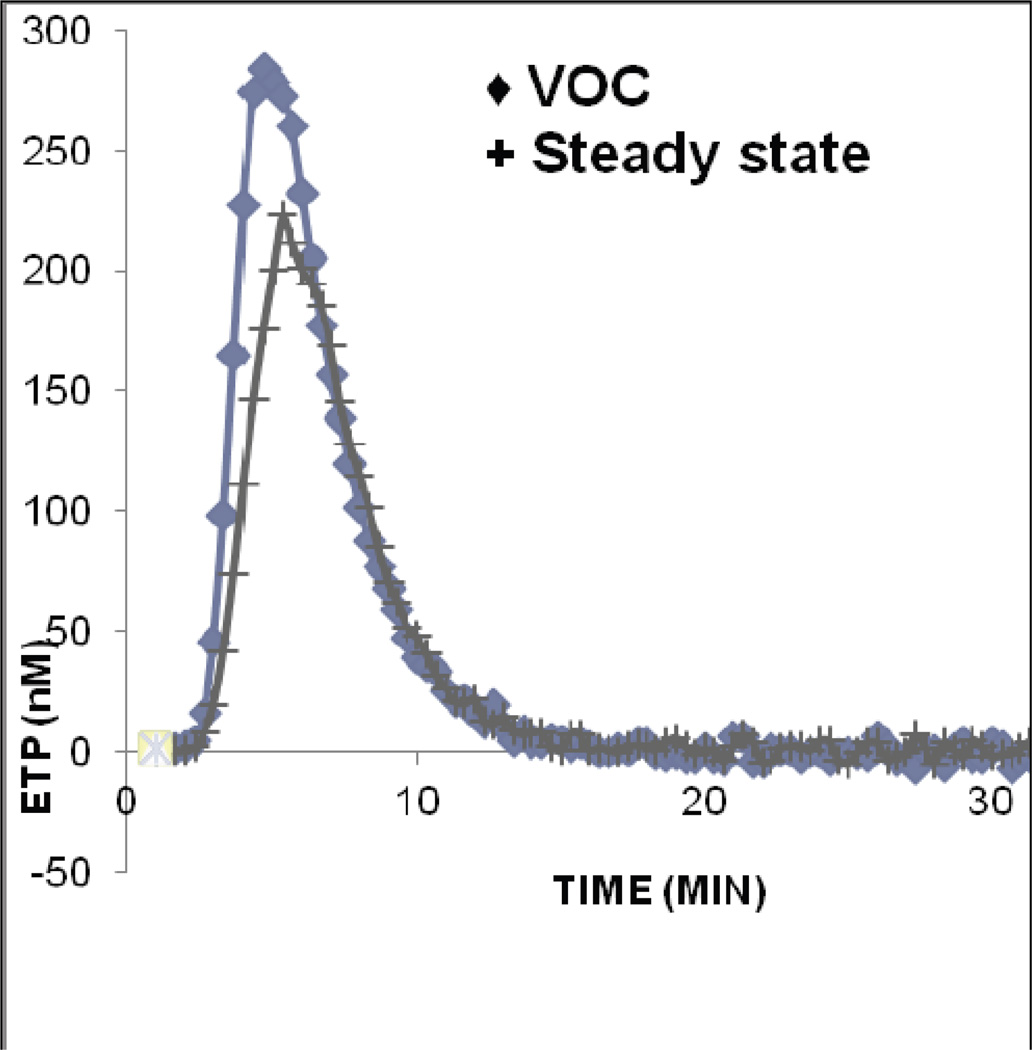
Paired analysis revealed the mean D-dimer during VOC was significantly higher than steady state. Paired comparison of crisis and steady state by CAT revealed a significant difference in all phases of thrombin generation (Figure 2).
Figure 2.
Thrombin generation assay: Results of paired analysis (n=20) of patients with SCD during VOC as compared to steady state
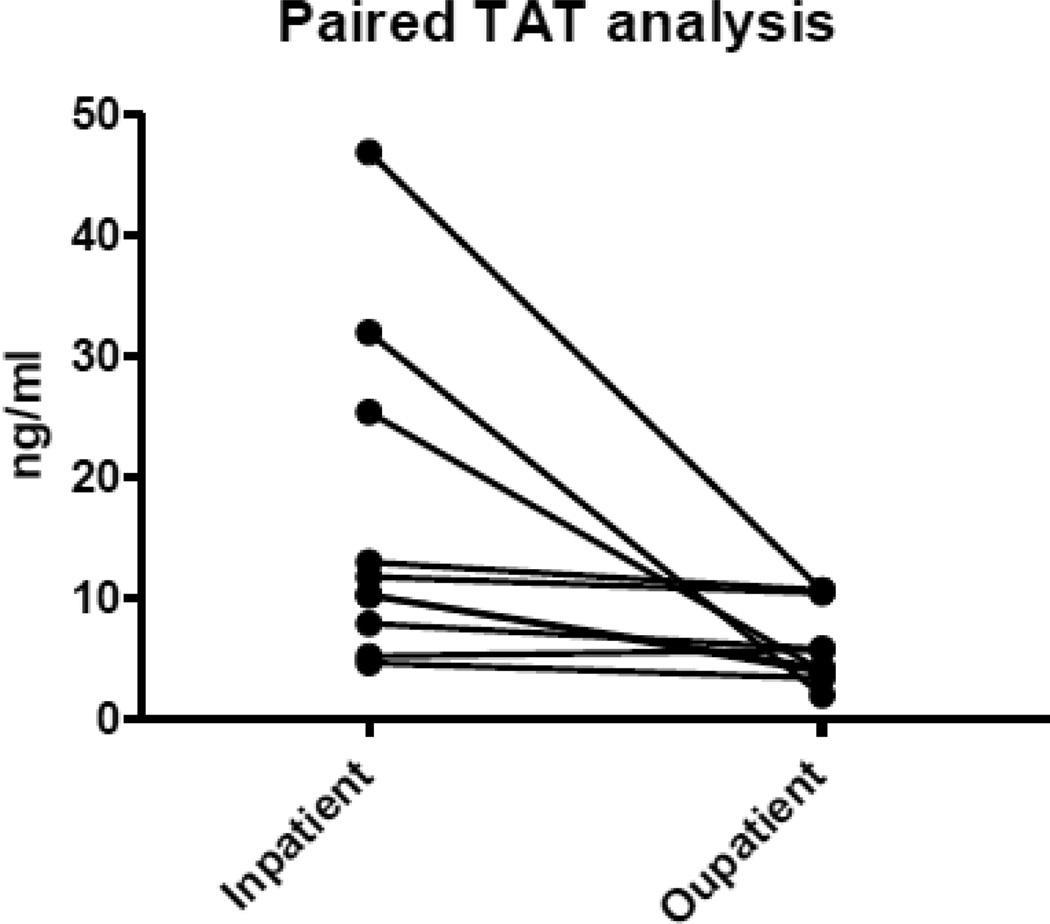
All pediatric patients (n=20) had TAT additionally performed, with 10 paired samples. There was a significant increase in crisis when compared to steady state during paired analysis (16.76 ± 13.74 vs. 6.12 ± 3.3, p=0.02) and unpaired analysis (18.82 ± 14.42 vs. 8.67 ± 4.3, p=-0.02) (Figure 3).
Figure 3.
Thrombin-antithrombin: paired analysis (n=10) of pediatric patients with SCD during VOC (inpatient) as compared to steady state (outpatient).
Clinical Outcomes (hospitalizations, stroke, avascular necrosis, TCD velocities)
Over the one year prior to enrollment the mean number of admissions was 2.6 (range 0–16). No correlation was found with D-dimer or each phase of CAT with the number of hospitalizations by linear regression. Mean values of D-dimer and CAT also did not differ between patients with and without AVN or stroke.
Standard annual TCDs were performed on all pediatric patients (n=20). By linear regression, there was no correlation between phases of thrombin generation by CAT during steady state and TCD velocities. However, there was a correlation between increased TCD velocities and an increased steady state D-dimer (r2=0.32, p=0.02) and TAT (r2=0.28, p=0.04). During VOC, D-dimer, TAT, and all phases of TGA, in contrast, did not show correlation with increased TCD velocities.
Discussion
Our results reveal an increase in thrombin production in patients with SCD during crisis. Although the use of global assays has been useful in documenting both hypocoagulable as well as hypercoagulable states in other diseases[16], its use in SCD has revealed conflicting results[9, 17, 18]. We now report our use of TGA in patients in SCD and document an increase in crisis beyond steady state levels. Additionally, we found a significant increase in D-dimer and TAT during crisis, both of which have been documented to be elevated in steady state and correlate with the results obtained by TGA.
Chaari et al compared thrombin generation in adult patients with SCD during steady state and healthy controls. They found no significant difference in any phase of thrombin generation between the two groups. They used 5pM tissue factor (TF) and 4uM phospholipids to trigger thrombin generation in PPP, which is a higher trigger than used in our study[17]. Betal et al also performed studies on 23 patients with SCD in steady state with comparison to 6 controls using both 5pM and 1pM TF concentrations with 4uM phospholipid and corn trypsin inhibitor for contact phase inhibition. They found a reduced ETP and slope during steady state for both concentrations of TF, in addition to a reduced lag time with the 5pM TF[18]. More recently, Noubouossie et al performed TGA testing in pediatric patients with SCD at steady state using 1pM TF and 4uM phospholipid and found significantly shorter lag time and a higher peak, but no difference in ETP. They hypothesized there may be an accentuated amplification phase of thrombin generation in patients with SCD that might account for their finding. Interestingly, they also evaluated 13 patients during crisis and had blood drawn within 48 hours of admission. Comparison of patients during crisis with patients at steady state found no significant difference in thrombin generation[9].
In contrast to these previous reports, we found differences in all phases of thrombin generation when comparing crisis to steady state. Importantly, our paired analysis of 20 patients revealed that lag time, slope, peak and ETP were all significantly different in crisis as compared to steady state. We used used Stago low PPP activator which contained 1 pM tissue factor and 4uM phospholipids as activators based on findings of an endogenous elevation of whole blood procoagulant tissue factor (TF) activity at baseline in patients with SCD[19]. Corn trypsin inhibitor (CTI) was also not used to inhibit FXII activation or contact activation and believed to be of minimal influence on the contact pathway at higher tissue factor concentrations[20]. Spronk et al examined thrombin generation at various tissue factor concentrations and recommended the addition of CTI for activation with tissue factor concentrations below 0.5 pM. No studies, however, have been performed attempting in patients with SCD to determine the value of CTI.
There are many factors leading to variability between institutions and laboratories which perform global assays such as TGA. Although there has been increasing interest in using global assays to evaluate hypercoagulable states, there has been much debate as to the proper testing technique for performing these assays[21, 22]. Importantly, efforts have been underway to attempt to standardize sample processing and testing techniques[21], however there are no current standard recommendations. These testing and sample differences may account for some of the differences seen in previous studies. Chaari et al used a higher TF activator which may have led to no significant difference between patients with SCD and controls. The results of Betal et al actually found a reduced ETP, which is in contrast to our study; however, corn trypsin inhibitor was used to inhibit contact activation. As described earlier, although the use of CTI has been recommended at low TF triggers[20], the findings of higher TF in SCD[23] led to our decision to not use CTI in our study, therefore leading to difficulty in comparing the two results. Finally, the results of Nouboussie et al also reveal increased thrombin generation, specifically the ‘thrombin burst’ or amplification phase, in patients with SCD at steady state. Interestingly, they did not find a significant difference in patients with VOC from patients at steady state. They utilized a low 1pM TF activator and double spin of samples, which were similar to our study. However, due to samples being drawn up to 48 hours following hospitalization, patients may have already had partial resolution of their VOC. This may account for differences from our study for which samples were drawn within 36 hours (median 10 hours, range 2 to 32).
We also attempted to correlate thrombin generation results to clinical disease. Due to the heterogeneity of SCD, few studies have correlated clinical disease complications to hypercoagulability. D-dimer has been shown to be not only elevated in crisis, but also with complications of SCD such as leg ulcers, chronic cholecystitis, AVN, joint pain and infection[24]. More recently, Dar et al showed that abnormal D-dimer results during VOC correlated with chest x-ray abnormalities[25]. Our results revealed no correlation of D-dimer or thrombin generation with clinical disease complications. The small number of patients in our study with stroke (n=7) and avascular necrosis (n=5) likely contributed to the lack of significant difference.
Interestingly, our findings did reveal a correlation of increased D-dimer and TAT levels to increased TCD velocities in pediatric patients. Although no past studies have attempted to correlate TCD velocities to hypercoagulable markers, Mourad et al examined markers in crisis, steady state as well as in patients with stroke. Patients with SCD and silent or clinically overt stroke had significantly higher thrombotic markers when compared to patients without stroke[26]. Further study is warranted to determine if hypercoaguable markers can be used, in addition to TCD, to predict cerebral vasculopathy and/or stroke.
Limitations of this study include the heterogeneity of SCD and our number of patients included in our study. We included paired analysis and the inclusion of only type SS disease to address these potential limitations. In addition, we collected all samples within 36 hours to avoid potential resolution of pain and obtain samples most representative of their VOC. Finally, an important limitation, as discussed earlier, is the discrepancy in results seen between laboratories and institutions. With no current standardization of sample preparation and testing techniques, institutional standards and techniques were used to perform our assays. The continued development of standards for global assays such as the TGA will be important to making more broad clinical inferences as well to assist in comparisons and reproducibility between studies.
In summary, we found thrombin generation is increased in VOC compared to steady state, as measured by TGA and surrogate markers such as D-dimer and TAT. In addition, increased D-dimer and TAT were associated with higher TCD velocities and should be studied prospectively. The use of global assays such as TGA are promising in evaluating patients with SCD and with the development of testing standards, will hopefully lead to an alternative strategy to determining risk and response to therapies.
Acknowledgements
The authors would like to thank Diagnostica Stago for providing calibrated automated thrombography (CAT) reagents and D-dimer ELISA kits, Sharon Hall for sample collection and CAT testing, Keith Klemp for ELISA testing, and Sheila Lambert-Adams for enrolling patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Tomer A, Harker LA, Kasey S, Eckman JR. Thrombogenesis in sickle cell disease. J Lab Clin Med. 2001 Jun;137(6):398–407. doi: 10.1067/mlc.2001.115450. [DOI] [PubMed] [Google Scholar]
- 2.Ballas SK, Larner J, Smith ED, Surrey S, Schwartz E, Rappaport EF. Rheologic predictors of the severity of the painful sickle cell crisis. Blood. 1988 Oct;72(4):1216–1223. [PubMed] [Google Scholar]
- 3.Hebbel RP. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991 Jan 15;77(2):214–237. [PubMed] [Google Scholar]
- 4.Kaul DK, Fabry ME, Nagel RL. Erythrocytic and vascular factors influencing the microcirculatory behavior of blood in sickle cell anemia. Ann N Y Acad Sci. 1989;565:316–326. doi: 10.1111/j.1749-6632.1989.tb24179.x. [DOI] [PubMed] [Google Scholar]
- 5.Rothman SM, Fulling KH, Nelson JS. Sickle cell anemia and central nervous system infarction: a neuropathological study. Ann Neurol. 1986 Dec;20(6):684–690. doi: 10.1002/ana.410200606. [DOI] [PubMed] [Google Scholar]
- 6.Ataga KI, Cappellini MD, Rachmilewitz EA. Beta-thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. Br J Haematol. 2007 Oct;139(1):3–13. doi: 10.1111/j.1365-2141.2007.06740.x. [DOI] [PubMed] [Google Scholar]
- 7.Francis RB., Jr Elevated fibrin D-dimer fragment in sickle cell anemia: evidence for activation of coagulation during the steady state as well as in painful crisis. Haemostasis. 1989;19(2):105–111. doi: 10.1159/000215901. [DOI] [PubMed] [Google Scholar]
- 8.Stuart MJ, Setty BN. Hemostatic alterations in sickle cell disease: relationships to disease pathophysiology. Pediatr Pathol Mol Med. 2001 Jan-Feb;20(1):27–46. [PubMed] [Google Scholar]
- 9.Noubouossie DF, Le PQ, Corazza F, Debaugnies F, Rozen L, Ferster A, et al. Thrombin generation reveals high procoagulant potential in the plasma of sickle cell disease children. Am J Hematol. 2011 Sep 30; doi: 10.1002/ajh.22206. [DOI] [PubMed] [Google Scholar]
- 10.Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am Soc Hematol Educ Program. 2007:91–96. doi: 10.1182/asheducation-2007.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Ardoin SP, Shanahan JC, Pisetsky DS. The role of microparticles in inflammation and thrombosis. Scand J Immunol. 2007 Aug-Sep;66(2–3):159–165. doi: 10.1111/j.1365-3083.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 12.Hagger D, Wolff S, Owen J, Samson D. Changes in coagulation and fibrinolysis in patients with sickle cell disease compared with healthy black controls. Blood Coagul Fibrinolysis. 1995 Apr;6(2):93–99. doi: 10.1097/00001721-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Bidot L, Jy W, Bidot C, Jr, Jimenez JJ, Fontana V, Horstman LL, et al. Microparticle-mediated thrombin generation assay: increased activity in patients with recurrent thrombosis. J Thromb Haemost. 2008 Jun;6(6):913–919. doi: 10.1111/j.1538-7836.2008.02963.x. [DOI] [PubMed] [Google Scholar]
- 14.Haidl H, Cimenti C, Leschnik B, Zach D, Muntean W. Age-dependency of thrombin generation measured by means of calibrated automated thrombography (CAT) Thromb Haemost. 2006 May;95(5):772–775. [PubMed] [Google Scholar]
- 15.Bauer KA, Weiss LM, Sparrow D, Vokonas PS, Rosenberg RD. Aging-associated changes in indices of thrombin generation and protein C activation in humans. Normative Aging Study. J Clin Invest. 1987 Dec;80(6):1527–1534. doi: 10.1172/JCI113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 17.Chaari M, Stankovic K, Galea V, Robert F, Khaterchi A, Lionnet F, et al. Steady State Sickle Cell Anemia Is Associated with Increased Formation of Erythrocyte-Derived Microparticles and Acceleration of Thrombin Generation. Blood. 2009 Nov 20;114(22):1537. [Google Scholar]
- 18.Betal SG, Kato GJ, Lawrence MP, Seamon C, Setty Y, Stuart MJ, et al. Thrombin Generation in Sickle Cell Disease: Insights From Computerized Automated Thrombography. Blood. 2009 Nov 20;114(22):1015–1016. [Google Scholar]
- 19.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003 Oct 1;102(7):2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 20.Spronk HM, Dielis AW, Panova-Noeva M, van Oerle R, Govers-Riemslag JW, Hamulyak K, et al. Monitoring thrombin generation: is addition of corn trypsin inhibitor needed? Thromb Haemost. 2009 Jun;101(6):1156–1162. [PubMed] [Google Scholar]
- 21.Dargaud Y, Luddington R, Gray E, Lecompte T, Siegemund T, Baglin T, et al. Standardisation of thrombin generation test--which reference plasma for TGT? An international multicentre study. Thromb Res. 2010 Apr;125(4):353–356. doi: 10.1016/j.thromres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Kluft C, Meijer P. External quality assessment for thrombin generation tests: an exploration. Semin Thromb Hemost. 2010 Oct;36(7):791–796. doi: 10.1055/s-0030-1265296. [DOI] [PubMed] [Google Scholar]
- 23.Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, et al. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998 Jun 1;91(11):4216–4223. [PubMed] [Google Scholar]
- 24.Devine DV, Kinney TR, Thomas PF, Rosse WF, Greenberg CS. Fragment D-dimer levels: an objective marker of vaso-occlusive crisis and other complications of sickle cell disease. Blood. 1986 Jul;68(1):317–319. [PubMed] [Google Scholar]
- 25.Dar J, Mughal I, Hassan H, Al Mekki TE, Chapunduka Z, Hassan IS. Raised D-dimer levels in acute sickle cell crisis and their correlation with chest X-ray abnormalities. Ger Med Sci. 2010;8:Doc25. doi: 10.3205/000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mourad H, Fadel W, El Batch M, Rowisha M. Heamostatic and genetic predisposing factors for stroke in children with sickle cell anemia. Egypt J Immunol. 2008;15(1):25–37. [PubMed] [Google Scholar]