Abstract
Lay Abstract
White matter tracts are like the “highways” of the brain, allowing for fast and efficient communication among diverse brain regions. The purpose of this paper is to review the results of autism studies that have used Diffusion Tensor Imaging (DTI), which is a neuroimaging method that allows us to examine the structure and integrity of these white matter tracts. From the 48 studies we reviewed, persons with ASD tended to have decreased white matter integrity spanning across many regions of the brain but most consistently in regions such as the corpus callosum (connecting the left and right hemispheres and associated with motor skill and complex information processing), the cingulum bundles (connecting regions along the middle-line of the brain with important frontal projections and associated with executive function), and white matter tracts that pass through the temporal lobe (connecting temporal lobe regions with other brain regions and associated with social functioning). The pattern of results in these studies suggests that the white matter tracts may be atypical in persons with ASD. Additionally, the review suggests that people with ASD may not exhibit the typical left-greater-than-right-brain asymmetry in white matter integrity compared to people with typical development. White matter alterations in persons with ASD are a target of emerging interventions and may help identify the brain basis of individual differences in this population.
Scientific Abstract
White matter tracts of the brain allow neurons and neuronal networks to communicate and function with high efficiency. The aim of this review is to briefly introduce Diffusion Tensor Imaging (DTI) methods that examine white matter tracts and then to give an overview of the studies that have investigated white matter integrity in the brains of individuals with Autism Spectrum Disorder (ASD). From the 48 studies we reviewed, persons with ASD tended to have decreased fractional anisotropy and increased mean diffusivity in white matter tracts spanning many regions of the brain but most consistently in regions such as the corpus callosum, cingulum, and aspects of the temporal lobe. This decrease in fractional anisotropy was often accompanied by increased radial diffusivity. Additionally, the review suggests possible atypical lateralization in some white matter tracts of the brain and a possible atypical developmental trajectory of white matter microstructure in persons with ASD. Clinical implications and future research directions are discussed.
Keywords: Diffusion Tensor Imaging, Neuroimaging, Autism, White Matter
In the first description of autism, Leo Kanner (1943) highlights a paradoxical cognitive profile that he termed “autistic,” in which many of the individuals in his study exhibited excellent rote memory and reading skills but did not perform as highly as anticipated on formal tests of intelligence. More recent research and clinical descriptions continue to reiterate a possibly uneven cognitive profile in persons with Autism Spectrum Disorder (ASD). For example, individuals with ASD have been shown to perform simple information processing tasks at a level that is similar or superior to age-related peers, but they often demonstrate substantial difficulty when performing higher-order or complex information processing tasks (Minshew, Goldstein, & Siegel, 1997; Minshew, Sweeney, & Luna, 2002; Williams, Goldstein, & Minshew, 2006). In developing neural models of ASD, this particular cognitive profile suggests that it is of the utmost importance to not only examine localized atypicalities of the brain but to also examine distributed neural networks and how efficiently different areas of the brain are able to communicate with each other. Therefore, multiple avenues of research have recently shifted from a localized approach to a more network-based approach in examining possible neural biomarkers of ASD.
Many of these approaches have suggested decreased communication among brain regions in people with ASD. At the molecular level, studies examining the early migration of pyramidal cells and minicolumn structures have found more numerous, smaller, and less compact minicolumns in the postmortem brains of children with ASD (Casanova et al., 2002a, 2002b; Casanova et al., 2006). These results possibly indicate disrupted flow throughout the distributed minicolumnar network, which over the course of development may affect both local and long-distance communication across the brain (for a more detailed explanation of how minicolumnar pathology may relate to connectivity in ASD, see Casanova & Trippe, 2009). Functional connectivity studies also have examined communication among brain regions. These studies use functional magnetic resonance imaging (fMRI) to examine how different brain regions orchestrate their activities. If two or more brain regions consistently activate and deactivate with temporal synchrony, then this may indicate that they are linked through a structurally connected white matter (WM) network. The results of many of these studies suggest decreased connectivity between frontal and posterior areas of the brain across a number of different cognitive tasks and also during a non-task resting state in ASD (for a review see Schipul, Keller, and Just, 2011). Therefore, both molecular and functional connectivity findings suggest that brain connectivity and the underlying WM tracts may be impacted in individuals with ASD. However, these particular methods cannot inform us of the physical characteristics of these WM tracts and their development in living human beings. In parallel, volumetric MRI of white matter can tell us about the macrostructure of these WM tracts but not the microstructure. Fortunately, the development of diffusion tensor imaging (DTI) has enabled researchers to examine both the macro- and microstructure of WM tracts noninvasively in the brain, and the application of DTI to the study of ASD in the past decade has been prolific.
The purpose of the present paper is to provide a brief background on DTI measures and what these measures have indicated about WM integrity in ASD. Because ASD is a developmental disorder, in our review of the literature we strive to take into account developmental trajectories to better characterize WM differences in individuals with ASD compared to individuals with typical development at different points in the life span. Additionally, our review highlights promising areas of future research using DTI methodologies and offers clinical implications.
Diffusion Tensor Imaging (DTI)
DTI (Basser, Mattiello, & LeBihan, 1994) is a noninvasive, exquisitely sensitive method to map and characterize the microstructural properties and macroscopic organization of WM tissues in the brain (Catani & Thiebaut de Schotten, 2008; Jones, Simmons, Williams, & Horsfield, 1999; Mori et al., 2002). DTI measures the random motion or diffusion of water molecules as a function of direction over time. Water diffusion properties are highly sensitive to and modulated by the spacing, orientation, and density of microstructural barriers of brain tissue including cellular membranes, cytoskeleton, and myelin. The fibrous tissue structure of WM bundles (fasciculi) restricts the direction of diffusion perpendicular to the WM tracts more so than in the parallel direction. Therefore, the water diffusion will be more hindered and restricted in the perpendicular directions. This directional dependence of water diffusion is called diffusion anisotropy. WM tracts consist of bundles of axons that allow for efficient communication between brain regions. Changes to the microstructural properties of WM such as myelination, axonal density, and axonal caliber influence diffusion anisotropy and likewise may reflect differences in brain connectivity properties. Conversely, in regions of gray matter and cerebrospinal fluid (CSF), the diffusion properties are more isotropic, e.g., they do not have a strong directional preference.
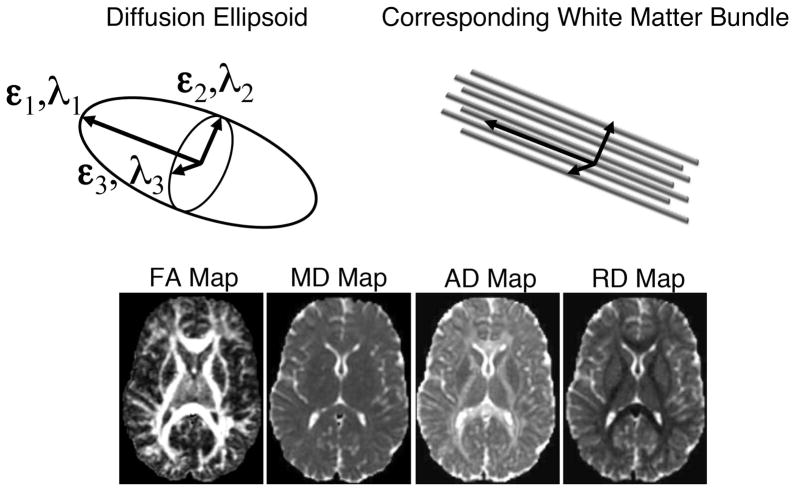
The diffusion tensor models water diffusion as a three-dimensional (3D) Gaussian distribution, which may be represented by a 3D ellipsoid (see Fig 1). The orientations of the ellipsoid directions correspond to the eigenvectors (ε1, ε2, ε3) and the length of each eigenvector is described by the eigenvalues (λ1, λ2, λ3) of the diffusion tensor. In directionally coherent WM, the largest axis of the ellipsoid, defined by the major eigenvector (ε1) and eigenvalue (λ1), is parallel to the direction of the WM fibers (Fig 1). The intermediate and smallest axes of the diffusion tensor ellipsoid, defined by the medium and minor eigenvectors (ε2, ε3) and eigenvalues (λ2, λ3) respectively, are perpendicular to the WM fiber tracts (Fig 1). At each voxel (a 3D pixel) of the brain image, an ellipsoid can indicate the direction and strength of the diffusion.
Figure 1.
Illustration of the diffusion tensor ellipsoid representing anisotropic diffusion in an area of white matter (cartoon region at top). Maps at the bottom are examples of FA, MD, AD and RD.
The most commonly investigated DTI measure is the fractional anisotropy (FA), which is a normalized standard deviation of the diffusion tensor eigenvalues and characterizes the directional variation in the apparent diffusion (Basser & Pierpaoli, 1996).
| (Eq 1) |
where MD is the mean diffusivity defined by the average of the three eigenvalues.
| (Eq 2) |
FA ranges from 0 to 1 with small FA in more isotropic tissues like gray matter and CSF and higher FA (typically > 0.2) in regions of WM (see Fig 1). In other words, a higher value of FA suggests a more elongated and skinnier ellipsoid, with the greatest diffusion parallel to the tract. Conversely, a lower value of FA indicates a more spherical ellipsoid, suggesting more even diffusion among the three directions. FA is highly sensitive to microstructural changes or differences in WM including myelination and axonal density and therefore is often called a measure of WM integrity. However, FA does not provide a complete description of the WM microstructure (Alexander, Hasan, Kindlmann, Parker, & Tsuruda, 2000). Thus, it is strongly encouraged that additional DTI measures be investigated in order to better interpret the underlying changes in tissue microstructure (Alexander, Lee, Lazar, & Field, 2007). For example, MD (Eq. 2) is the average radius of the diffusion tensor ellipsoid and is sensitive to the density of tissue barriers in all directions. The axial diffusivity (AD) is the water diffusivity in the direction parallel to the WM tracts, and is the first eigenvalue of the tensor (AD = λ1). The radial diffusivity (RD), also known as the perpendicular diffusivity, is the mean of the second and third eigenvalues (RD = (λ2+λ3)/2) and has been shown to be modulated by myelin in animal models of dys- and de-myelination (Harsan et al., 2006; Song et al., 2002, 2005; Tyszka, Readhead, Bearer, Pautler, & Jacobs, 2006). However, changes in axonal density, axonal diameter, cytoskeletal properties, swelling from neuroinflammation and white matter complexity (e.g., crossing, curving and branching fibers) are also plausible explanations for changes to RD and other DTI measures (including FA), thus caution must be used to not over-interpret DTI changes (Wheeler-Kingshott & Cercignani, 2009). Through the investigation of multiple measures of the diffusion tensor, one is better equipped to more accurately characterize the WM microstructural differences. The high sensitivity of DTI measures to differences in WM properties has been applied to a broad spectrum of disease processes, injury, disorders, brain development and aging, and response to therapy (Alexander et al., 2007b). More detailed descriptions of diffusion tensor imaging, acquisition methods, measures, and applications are provided in recent review papers (Alexander et al., 2007b; Tournier, Mori, & Leemans, 2011, Basser & Jones, 2002).
The heterogeneity of DTI measures across the brain necessitates the application of brain region specific measurements methods. Techniques for investigating individual and group differences in FA, MD, AD, and RD include voxel-based analysis (VBA), region-of-interest-based analysis (ROI), and tractography-based analysis (TBA) methods. In VBA methods, the brain images are spatially co-registered (normalized) across subjects and statistical testing is performed at each voxel location within the brain (see Lee et al., 2009). The resultant VBA statistical maps often exhibit spatial ‘blobs’ of significance on a normalized brain map. The potential limitations of VBA include poor registration of anatomy between subjects, increased image blurring from the spatial normalization, and decreased statistical power from multiple comparisons (e.g., testing statistical significance at every voxel). Recent improvements to VBA include better spatial normalization methods (e.g., DTI-TK: Zhang et al., 2007) and methods for decreasing the effects of image blurring and multiple comparisons (e.g., T-SPOON: Lee et al., 2009; TBSS: Smith et al., 2006). In ROI analyses, a priori defined regions of interest are defined either manually or by aligning a template to the image, a process called regional segmentation. Manual methods tend to be tedious and are prone to user variability. The main issue with automated regional segmentation template methods is misregistration from inadequate spatial normalization (as in VBA). The main strength of ROI methods is that the voxels-of-interest are combined in a manner that is a priori defined, and thus the number of multiple comparisons is much smaller. Therefore, the statistical testing has more power. Tractography-based methods use the tract reconstructions to define ROIs and can also provide additional measures such as tract density and length (Conturo et al., 1999; Mori, Crain, Chacko, & van Zijl, 1999). To reconstruct the WM tract, tractography uses the principal direction of the tensor using individual streamlines that propagate from voxel-to-voxel following the tensor direction. Knowledge of existing fiber tracts and analyses allow us to combine these streamlines to identify and define WM ROIs (e.g., cingulum, corpus callosum) (Conturo et al., 1999; Mori et al., 1999; Hofer, Merboldt, Tammer, & Frahm, 2008). However, a drawback of tractography is that it is prone to branching and false positive tracts that can lead to variabilities and errors in the ROI definitions, specifically at the ends of the reconstructed tracts (Alexander et al., in press).
DTI is inadequate for describing WM microstructure in regions with crossing WM fibers (Alexander, Hasan, Lazar, Tsuruda, & Parker, 2001; Wedeen et al., 2008). For example, FA is significantly reduced in regions with complex WM fiber crossings. However, methods are being developed for resolving and characterizing crossing tracts (i.e., Weeden et al., 2008; Tournier et al., 2011). Despite this limitation, DTI is still highly sensitive to microstructural differences although it makes the interpretation more challenging.
An Overview of the Reviewed Research
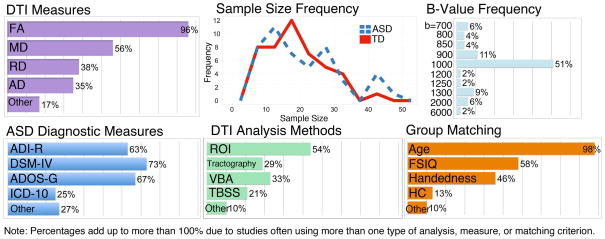
In order to review the body of work that has studied WM in ASD, we searched library databases for all English-language articles that included the term “autism” or “autistic disorder” or “Asperger’s” and “diffusion tensor” or “tractography” or “diffusion spectrum” (for non-tensor based methods) or “FA” or “DTI.” This search method resulted in 48 peer-reviewed research studies of ASD samples. Table 1 of the Supplementary Materials presents an overview of the methods and results of the 48 research studies of ASD. Additionally, Figure 2 illustrates the average sample size and typical diagnostic criteria, matching criteria, DTI measures, and DTI analysis methods for this body of literature. As can be seen in Figure 2, sample sizes for both diagnostic groups tended to be small (ASD: mean = 21.46; Typically Developing: mean = 20.36), with 40% of the studies scanning 15 or fewer participants with ASD. The fact that the majority of these studies had such small ASD sample sizes suggests that caution should be taken when interpreting some of the results. Also, Figure 2 demonstrates that the most common DTI measure was FA, and the most common data analysis method was ROI-based (although many studies used overlapping measures and methods). Figure 2 includes the frequency of the “b-values” used in the studies. The b-value is the strength of the global magnetic diffusion gradient for the scan that is determined by the researchers and sets its diffusion-weighting. Although most studies used a b-value of 1000 s/mm2, there was considerable inter-study variability. The DTI results derived using b-values that are above 2000 are often more difficult to interpret, as they are no longer well modeled by Gaussian distributions (symmetrical bell-shaped curves), which is a primary assumption for diffusion tensor imaging. Only 8% of the reviewed studies used a b-value of 2000 or above. We note these higher b-values when reviewing study results. For an ASD diagnosis, most studies used a combination of diagnostic measures, with the DSM-IV criteria (APA, 2000), Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994), and Autism Diagnostic Observation Scale-General (ADOS-G) (Lord et al., 2000) being the most frequent (58% of studies used both the ADI-R and ADOS-G). Nearly all studies matched the diagnostic groups on age in addition to factors such as IQ and handedness. From Table 1, it can be seen that the majority of the reviewed studies examined participants with ASD during mid-childhood to adolescence, with the average age of the ASD samples being between eight and 16 years in 53% of the studies.
Figure 2.
Key features of the 48 empirical studies reviewed in the present paper, including sample size, how participants were diagnosed with ASD, how participant groups were matched, the types of DTI measures reported in the studies, the DTI methods, and the b-values used for data acquisition.
Table 1.
| Authors | Year | ASD (N) | Control (N) | ASD Age in Years (Mean±SD) | Control Age in Years (Mean±SD) | ASD IQ (Mean±SD) | Control IQ (Mean±SD) | Type of Analyses | DTI Findings | DTI Correlates |
|---|---|---|---|---|---|---|---|---|---|---|
| Barnea-Goraly et al. | 2004 | 7 | 9 | 14.6±3.4 | 13.4±2.8 | 101±12.2 | 107±8.5 | VBA | Reduced FA in ASD near venral prefrontal cortex, ACC, TPJ, STS, occipitotemporal tracts, and corpus callosum | -- |
| Alexander et al. | 2007 | 43 | 34 | 16.2±6.7 | 16.4±6.0 | 107.5±13.0 | 112.8±12.1/ | ROI | Reduced FA in ASD in the corpus callosum (with increased MD and RD) | FA and RD of corpus callosum correlated with age (in both groups) and with PIQ in ASD (not control). Corpus callosum MD and genu FA correlated with processing speed in ASD |
| Ben Bashat et al. | 2007 | 7 | 18 | Range: 1.8–3.3 | Range: 0.3–23.0 | -- | -- | ROI | Increased FA, probability, and displacement in young children with ASD | -- |
| Keller, Kana, & Just | 2007 | 34 | 31 | 18.9±7.3 | 18.9±6.2 | 102.0±14.8 | 109.5±9.0 | VBA | Reduced FA in ASD in 5 regions of white matter, including the corpus callosum, internal capsule, and forceps minor | FA positively correlated with age in ASD group in areas where group differences found |
| Lee et al. | 2007 | 43 | 34 | 16.2±6.7 | 16.4±6.0 | PIQ: 107.5±13.0 | PIQ: 112.8±12.0 | ROI | Reduced FA, increased MD, and increased RD in ASD in STG and temporal stem | Less age-related changes in FA of right STG in ASD group compared to TD group |
| Catani et al. | 2008 | 15 | 16 | 31±9 | 35±11 | 109±17 | 120±21 | ROI, Tractography | Reduced FA in cerebellum of Aspergers but no MD group differences | FA of left superior cerebellar peduncle negatively correlated with ADI-R social domain scores |
| Conturo et al. | 2008 | 17 | 17 | 26.46±2.73 | 26.08±2.69 | 104.41±2.08 | 105.24±2.34 | ROI, Tractography | Reduced RD in ASD in the right hippocampo-fusiform pathway. Increased RD and AD in ASD group in the left hippocampo-fusiform pathway and bilateral amygdalo-fusiform pathways | Decreased across-fiber diffusivity related to poorer Benton face interpretation and PIQ scores |
| Sundaram et al. | 2008 | 50 | 16 | 4.79±2.43 | 6.84±3.53 | -- | -- | ROI, Tractography | Increased MD in ASD in entire frontal lobe, and in long and short-range association fibers. Reduced FA in ASD for short-range but not long-range fibers | No relation between FA and GARS AQ after correcting for multiple comparisons |
| Thakkar et al. | 2008 | 12 | 12 | 30±11 | 27±8 | VIQ: 124±12 PIQ: 120±10 | estimated VIQ: 114±9 | VBA | Reduced FA in ASD in white matter near ACC, as well as DLPFC, VPFC, intraparietal sulcus. Increased FA in the right insula. | Greater ADI repetitive behavior scores related with decreased FA in white matter near the left subgenual rostral ACC and related with greater fMRI activation during correct trials in the right rostral ACC |
| Adluru et al. | 2009 | 41 | 32 | 16.23±6.70 | 16.44±5.97 | -- | -- | VBA, T-SPOON, Classification | ~72% specificity when classifying ASD from controls using the shapes of white matter tracts passing through the splenium of the corpus callosum | -- |
| Brito et al. | 2009 | 8 | 8 | median 9.53±1.83 | median 9.57±1.36 | -- | -- | ROI | Reduced FA in ASD in aspects of the corpus callosum, corticospinal tract, internal capsule, and cerebellum. Increased MD in ASD in aspect of the corpus callosum. Increased FA in aspect of putamen | -- |
| Cheung et al. | 2009 | 13 | 14 | 9.3±2.6 | 9.9±2.5 | 99.5±21.9 | 111.9±19.7 | VBA | Reduced FA in ASD in aspects of prefrontal lobes, ventral and middle temporal lobe, and cerebellum. Increased FA in ASD in superior longitudinal fasciculus and left occipital lobe | ADI-R social and communication scores negatively correlated with FA in fronto-striatal-temporal regions and posterior corpus callosum. ADI-R repetitive behavior scores negatively correlated with FA near basal ganglia, TPJ, splenium of corpus callosum, and cerebellum, but positively correlated with FA in left precentral gyrus |
| Ke et al. | 2009 | 12 | 10 | 8.75±2.26 | 9.40±2.07 | 100.60±18.79 | 99.83±17.93 | VBA, WM density | Reduced FA in ASD in aspects of inferior and middle frontal gyrus, and STG. Increased FA in ASD in aspects of frontal lobe, middle temporal gyrus, and sublobar extra nuclear area | FA of right frontal lobe (sub-gyral) positively correlated with CARS in children with ASD. No significant correlations with ADI-R |
| Knaus et al. | 2009 | 14 | 20 | 16.09 Range: 11–19 | 14.10 Range: 11–19 | VIQ mean: 103.29 PIQ mean: 102.57 | VIQ mean: 119.00 PIQ mean: 113.00 | ROI, Tractography | Atypical functional language laterality more common in ASD. No diagnostic group differences in FA, but those with more typical leftward lateralization had greater FA in the arcuate fasciculus across both ASD and control groups | -- |
| Lee et al. | 2009 | 43 | 34 | 16.23±6.70 | 16.44±5.97 | 107.5±13.0 | 112.8±12.1 | VBA, T-SPOON | Replicated results of Lee et al. (2007) and Alexander et al. (2007) using a T-SPOON voxel-based method. Diffuse MD and RD group differences observed | Found correlations between FA of corpus callosum and processing speed |
| Pardini et al. | 2009 | 10 | 10 | 19.7±2.83 | 19.9±2.64 | 49.20±6.94 | -- | VBA, Tractography | Reduced FA and WM volume in ASD in left OFC. Reduced FA in ASD in white matter near the ACC, the inferior and medial frontal gyri bilaterally, and the right superior frontal gyrus | In ASD group, IQ scores correlated positively with the mean FA values of the left orbitofrontal cortex network. |
| Pugliese et al. | 2009 | 24 | 42 | 23±12 | 25±10 | 104.7±12.05 | 121.2±16.1 | ROI, Tractography | No group differences in FA and MD. Higher number of streamlines in Aspergers in the cingulum and inferior longitudinal fasciculus. Lower number of streamlines in Aspergers in the right uncinate fasciculus | -- |
| Barnea-Goraly, Lotspeich, & Reiss | 2010 | 13 | 11 | 10.5±2.0 | 9.6±2.1 | 85.9±17.4 | 119.9±13.3 | VBA | No group differences in DTI measures between ASD and siblings. Reduced FA and reduced AD in ASD group and sibling group compared to control group in multiple regions across the frontal, temporal and parietal lobes | No significant correlations were found between the ADOS and ADI-R subscale scores and FA values |
| Bloemen et al. | 2010 | 13 | 13 | 39±9.8 | 37±9.6 | 110±15.7 | 115±14.4 | VBA | Reduced FA and increased RD in adults with Asperger syndrome over large areas of the brain. Reduced MD in brainstem in Asperger group | -- |
| Cheng et al. | 2010 | 25 | 25 | 13.71±2.54 | 13.51±2.20 | 101.60±18.91 | 109.04±9.45 | TBSS | Increased and decreased FA in ASD across multiple areas of the brain. Interaction effect of age by group, occurring mostly in the frontal lobe | -- |
| Fletcher et al. | 2010 | 10 | 10 | 14.25±1.92 | 13.36±1.34 | VIQ: 103.7±18.55 | VIQ: 102.7±9.52 | ROI | Increased MD and RD in left arcuate fasciculus in ASD. Lack of typical asymmetry between left and right AF in ASD | Both groups pooled demonstrated RD lateralization and CELF3 correlation |
| Kumar et al. | 2010 | 32 | 16 | 5.0 Range: 2.5–8.9 | 5.5 Range: 2.5–8.6 | -- | ≥85 | TBSS, ROI, WM volume | Reduced FA in ASD and DI groups across multiple areas, but no difference between ASD and DI groups. Longer uncinate and arcuate fibers in right hemisphere in ASD, whereas TD and DI children had longer UF and AF fibers in left hemisphere. | Fiber volume of the UF positively correlated with GARS stereotypic behavior, and fiber length and fiber density of the corpus callosum positively correlated with Vineland communication |
| Lange et al. | 2010 | 30 | 30 | 15.78±5.6 | 15.79±5.5 | 109.57±16.7 | 115.13±12.9 | ROI | Reversed hemispheric asymmetry in diffusion tensor skewness of the STG in ASD. Able to classify ASD from TD using the skew of the STG and temporal stem | FA of STG related to composite Vineland score. |
| Noriuchi et al. | 2010 | 7 | 7 | 13.96±2.68 | 13.36±2.74 | 92.71±6.68 | 116.43±9.50 | VBA | Reduced FA and AD in ASD in the left DLPFC, posterior STS/TPJ, right temporal pole, amygdala, SLF, and occipito-frontal fasciculus | FA of the left DLPFC was negatively correlated with total SRS scores in children with ASD |
| Sahyoun, Belliveau, & Mody | 2010 | 9 | 12 | 12.8±1.5 | 13.3±2.45 | 101.4±12.48 | 106.1±8.56 | TBSS | Reduced FA in ASD in multiple white matter tracts connecting with the frontal lobe. Increased FA in ASD in bilateral UF and right SLF | Group differences in the correlations between FA of different brain areas and task performance |
| Sahyoun, Belliveau, Soulieres, Schwartz, & Mody | 2010 | 9 | 12 | 12.8±1.5 | 13.3±2.45 | 101.4±12.48 | 106.1±8.6 | ROI, Tractography | Reduced FA in bilateral inferior frontal gyrus-fusiform gyrus and right inferior frontal gyrus-middle temporal gyrus tracts in ASD. No group differences in performance on a visuo-spatial task, but fMRI group differences in frontal and temporal activation | -- |
| Shukla, Keehn, Lincoln, & Müller | 2010 | 26 | 24 | 12.7±0.6(SEM) | 13.0±0.6(SEM) | VIQ: 105.6±3.6 (SEM) PIQ: 109.5 ± 3.3(SEM) | VIQ: 108.2 ± 2.6(SEM) PIQ: 110.3 ± 2.5(SEM) | VBA & ROI | Reduced FA, increased MD, and increased RD in ASD for whole-brain white matter and multiple regions across the brain. | Age negatively correlated with MD of the posterior limb of the internal capsule in both groups and with MD of the splenium in ASD. No correlations between DTI and ADOS/ADI-R scores |
| Sivaswamy et al. | 2010 | 27 | 16 | 5.0 Range: 2.6–9.0 | 5.9 Range: 2.6–8.9 | -- | -- | ROI | Increased MD in ASD in bilateral superior cerebellar peduncle, and increased FA in right middle cerebellar peduncle. Increased FA in ASD in left inferior cerebellar peduncle (but decreased FA in right) | -- |
| Thomas, Humphreys, Jung, Minshew, & Behrmann | 2010 | 12 | 18 | 28.5±9.7 | 22.4±4.1 | 106.92±10.47 | 111.6±9.91 | ROI | Decreased number of streamlines and voxels in ASD in the body and forceps minor of the corpus callosum. Leftward increase in volume in ASD in the inferior longitudinal fasciculus, the inferior fronto-occipital fasciculus, and the uncinate fasciculus. No group differences in FA | ADI-R repetitive behavior subscale negatively correlated with number of streamlines and voxels of the forceps minor. |
| Ameis et al. | 2011 | 19 | 16 | 12.4±3.1 | 12.3±3.6 | 98.5±20.4 | 100.7±14.5 | TBSS, post hoc ROI | Increased MD and RD in cortico-cortical and inter-hemispheric white matter tracts in ASD, especially in children and within the frontal lobe | -- |
| Beacher et al. | 2011 | 28 | 30 | Males: 32±10, Females: 32±7 | Males: 28±8, Females: 32±8 | -- | -- | ROI | Sex-by-diagnosis interactions in the corpus callosum, anterior cingulum, and corona radiata, with increased FA in TD males compared to females, but no sex difference in Aspergers | -- |
| Bode et al. | 2011 | 27 | 26 | 14.7±1.6 | 14.5±1.5 | -- | -- | TBSS | Increased FA and reduced RD in ASD in right inferior fronto-occipital fasciculus and right optic radiation | FA not correlated with age |
| Cheon et al. | 2011 | 17 | 17 | 11.0±2.1 | 10.2±2.0 | 112.1±12.0 | 113.8±11.0 | TBSS, ROI | Decreased FA and increased MD in right anterior thalamic radiation, corpus callosum, and left uncinate fasciculus in ASD | FA of right anterior thalamic radiation and right uncinate negatively correlated with SRS scores within ASD group |
| Groen, Buitelaar, van der Gaag, & Zwiers | 2011 | 17 | 25 | 14.4±1.6 | 15.5±1.8 | 98±18 | 105±9 | VBA, WM volume, GM volume | Increased overall MD in ASD. No difference in gray or white matter volume | -- |
| Hong et al. | 2011 | 18 | 16 | 8.7±2.18 | 9.8±1.9 | 105.2±21.1 | 106.1±20.1 | ROI, Tractography | Increased MD and decreased fiber number in ASD in anterior third of corpus callosum | No relations between DTI measures and CARS |
| Ingalhalikar, Parker, Bloy, Roberts, & Verma | 2011 | 45 | 30 | 10.5±2.5 | 10.3±2.5 | -- | -- | Classification, ROI | ~84% specificity in classifying ASD from controls, using MD of the left middle occipital gyrus, right STG, right superior occipital gyrus, right insula, right middle temporal, right internal capsule, right inferior temporal, and left caudate and using FA of the right inferior occipital white matter, the left fornix, the left SLF, the right inferior occipital gyrus, the right external capsule, the left caudate nucleus, the left hippocampus, the left posterior corona radiata, and left cuneus, and the right internal capsule | Classifier scores related to SRS and SCQ scores |
| Jeong, Kumar, Sundaram, Chugani, & Chugani | 2011 | 32 | 14 | 4.88±1.89 | 5.61±1.98 | -- | -- | Tractography, ROI | More curvature, higher bending, reduced FA, and increased RD in ASD in the bilateral arcuate, the bilateral uncinate, and genu of the corpus callosum (especially near the TPJ) | Negative correlations between curvature and FA in both groups in all three white matter ROIs. Positive correlations between curvature and RD in ASD in all three ROIs, but only in genu of the corpus callosum in the TD group |
| Langen et al. | 2011 | 21 | 22 | 25.57±6.08 | 28.45±6.39 | 107.45±15.08 | 109.82±13.71 | ROI | Reduced FA in ASD in left putamen tracts. Increased MD in ASD in right accumbens tract. ASD poorer accuracy on no-go portion of go/no-go task and smaller total brain white matter volume | No relation of FA and repetitive behaviors on ADI-R or ADOS. Possible relation between FA and go/no-go task performance, but not found within either group alone |
| Lo et al. | 2011 | 15 | 15 | 15.2±1.0 | 15.0±0.8 | 108.4±7.3 | 110.6±10.2 | ROI, Tractography | Reduced generalized FA in ASD in the three corpus callosum tracts under investigation. Leftward asymmetry pattern observed in controls but not in the ASD | -- |
| Pardini et al. | 2011 | 22 | -- | Age at post-therapy scan: 21.9±0.5 | -- | PIQ at therapy onset 48.9±1.6 | -- | VBA | Increased FA of the uncinate in those with ASD who highly adhered to the therapy versus those who moderately adhered to the therapy | FA of the uncinate positively correlated with both the difference between pre- and post-therapy autism symptom severity and the length of time in therapy but negatively correlated with the age of therapy onset |
| Poustka et al. | 2011 | 18 | 18 | 9.7±2.1 | 9.7±1.9 | 111.0±14.4 | 112.8±14.9 | VBA, Tractography | Reduced FA in ASD in right SLF and bilateral uncinate fasciculus | FA of left SLF and left uncinate negatively correlated with ADI-R communication & interaction scores. FA of right SLF negatively correlated with ADOS Communication. FA of left fornix negatively correlated with ADOS communication and interaction. FA of right fornix negatively correlated with ADI-R communication |
| Shukla, Keehn, & Müller | 2011 | 26 | 24 | 12.8±.6(SEM) | 13.0±0.6(SEM) | VIQ: 104.3±3.4(SEM) PIQ: 108.8±3.3(SEM) | VIQ: 108.2±2.6(SEM) PIQ: 110.3±2.5(SEM ) | TBSS | Reduced FA and increased MD for ASD in the anterior and posterior limbs of the internal capsule, the corpus callosum, inferior and superior longitudinal fasciculi, inferior fronto-occipital fasciculus, corticospinal tract, cingulum, and anterior thalamic radiation | FA of whole-brain white matter positively correlated with age in TD group but only marginally in ASD. MD and RD of whole-brain white matter negatively correlated with age in TD, but not in ASD |
| Shukla, Keehn, Smylie, & Müller | 2011 | 26 | 24 | 12.6±3.06(SEM ) | 13.0±2.94(SEM ) | VIQ: 106 ± 3.6(SEM) PIQ: 109.1 ±3.3(SEM) | VIQ: 108.2±2.6(SEM) PIQ: 110.3±2.5(SEM) | TBSS | Reduced FA in ASD in the short-distance tracts of the frontal lobes. Increased MD and RD in ASD in the short-distance tracts of the frontal, temporal, and parietal lobes | Positive correlation between age and FA and negative correlation between age and MD and RD for short-distance tracts in each lobe for the TD group. However, for the ASD group, correlations only significant in the frontal lobe |
| Verhoeven et al. | 2011 | 19 | 21 (+13 SLI) | 13.8±1.6 | 10.1±0.4 | -- | -- | ROI, Tractography | FA of SLF similar in ASD-language impairment and controls | FA of SLF not correlated with language scores in ASD |
| Weinstein et al. | 2011 | 21 | 26 | 3.2±1.1 | 3.4±1.3 | -- | -- | TBSS, Tractography | Increased FA and decreased RD in ASD in genu and body of corpus callosum, left SLF, and bilateral cingulum | -- |
| Jou et al. … Pelphrey | 2011 a | 15 | 8 | 10.9±3.8 | 11.5±2.6 | -- | -- | TBSS | Decreased FA in ASD in multiple tracts with the largest group differences occurring in the forceps minor, fronto-occipital fasciculus, and superior longitudinal fasciculus | No relation between FA and SRS after correcting for multiple comparisons |
| Jou et al. … Volkmar | 2011 b | 10 | 10 | 13.06±3.85 | 13.94±4.23 | 91.0±24.79 | 105.0±17.83 | VBA, Tractography | Reduced FA in the arcuate and inferior longitudinal fasciculi, SLF, corpus callosum/cingulum, and inferior fronto-occipital fasciculus | -- |
| Wolff et al. | 2012 | 92 high-risk infants: 28 met ADOS criteria for ASD at 24 month s | 64 did not meet criteria for ASD at 24 months | Time 1: 0.57±0.07 Time 2: 1.06±0.06 Time 3: 2.04±0.05 | Time 1: 0.56±0.07 Time 2: 1.06±0.05 Time 3: 2.06±0.07 | Time 1: 90.4±23.7 Time 2: 89.8±20.3 Time 3: 86.6±22.0 | Time 1: 102.1±15.7 Time 2: 101.3±14.5 Time 3: 99.0±18.0 | Tractography, ROI | Increased FA in ASD-positive high-risk siblings at 6 months of age, similar FA at 12 months, but decreased FA at 24 months compared to ASD-negative high-risk siblings | -- |
Reported asADC, assume equivalent to MD,
Reported as eigenvalues.
Notation changedin table to AD and RD: AD = l1, RD ~ either l2 or l3,
number of b0 not reported
Table 1 (supplementary material) is an overview of the results of each study reviewed. This table, provided in Microsoft Excel format, may be downloaded for personal use. It summarizes the study design, imaging protocol details, measures, and main findings. The following sections summarize the results of all of these studies concisely, describing common themes, trends, and future directions for this body of research. Specifically, we examine indices of general WM integrity in ASD, and then we further review diagnostic group differences in WM integrity in brain regions that have often been implicated in autism, such as the corpus callosum, cingulum, superior temporal gyrus (STG), arcuate fasciculus, and uncinate fasciculus.
Group Differences in Overall White Matter
Although the majority of studies used ROI approaches in examining WM integrity, a handful of studies examined overall WM across the brain (often in addition to an ROI-based approach). These whole-brain analyses have consistently demonstrated reduced FA and increased MD in ASD. For example, in children and adolescents, decreased FA was found in the ASD group, accompanied by significantly increased MD and RD (Shukla, Keehn, Lincoln, & Müller, 2010) (b-value = 2000). Similarly, Groen and colleagues (2011) found increased overall MD in ASD. Other studies using ROI or voxel-based approaches have found extremely widespread areas of WM in which individuals with ASD have decreased FA. For example, Jou et al. (2011a) examined the WM tracts that connect brain areas associated with social functioning (i.e., the fusiform gyrus, amygdala, and STG) in children and adolescents, and found decreased FA in ASD in all of these WM tracts even after controlling for age and IQ. Lee et al. (2009) used a novel voxel-based method that reduced partial volume blurring effects to show that MD was significantly increased for almost all the WM, though FA differences were more localized. Similarly, adults (25–52 years old) with Asperger syndrome were found to have significantly decreased FA in 13 mostly bilateral clusters of the brain with increased RD in 16 clusters, accompanied by four very small clusters (<10 voxels) of higher FA (Bloemen et al., 2010). These studies generally demonstrate that children and adults with ASD are likely to have reduced FA and increased MD and RD compared to individuals with typical development. However, it is important to note that even though the majority of studies have found decreased FA in ASD, a handful of studies have found increased FA (Ben Bashat et al., 2007; Weinstein et al., 2011; Wolff et al., 2012) or a mixture of increased and decreased FA across different WM areas in persons with ASD compared to persons with typical development (Cheng et al., 2010; Cheung et al., 2009; Sivaswamy et al., 2010; Thakkar et al., 2008). The majority of these studies have included very young participants (under the age of four) (i.e., Ben Bashat et al., 2007; Sivaswamy et al., 2010; Weinstein et al., 2011; Wolff et al., 2012).
Group Differences in Corpus Callosum White Matter
The corpus callosum (CC) is a major bundle of WM tracts that connects the left and right hemispheres, and it is possibly the most-studied region in ASD. Characterization of the CC may be a particularly important endeavor in autism and other brain-based research. The CC has been linked to motor skill (Berlucchi, Aglioti, Marzi, & Tassinari, 1995; Eliassen, Baynes, & Gazzaniga, 2000; Schlaug, Jancke, Huang, Staiger, & Steinmetz, 1995; Zahr, Rohlfing, Pfefferbaum, & Sullivan, 2009), complex information processing (Zahr et al., 2009), processing speed (Moseley, Bammer, & Illes, 2002), and working memory (Takeuchi et al., 2010; Zahr et al., 2009). In addition, Anderson and colleagues (2011) found decreased interhemispheric functional connectivity in ASD.
Corpus callosum macrostructure
There are several structural imaging studies that suggest decreased CC volume in ASD (e.g., Alexander et al., 2007a; Hardan, Minshew, & Keshavan, 2000; Keary et al., 2009; Vidal et al., 2006), though Kilian and colleagues (2008) found that this decreased volume may be specific to a subgroup of individuals with ASD who do not have macrocephaly. Decreases in CC volume in ASD have been found in multiple areas such as the forceps major (splenium), forceps minor (genu), and body of the CC (Thomas, Humphreys, Jung, Minshew, & Behrmann, 2011). This decrease in CC volume in ASD has spurred comparisons to another disorder, callosal agenesis, in which a person is born lacking all or most of this structure. Individuals with callosal agenesis have been found to be diagnosed with ASD more often than their peers (Doherty, Tu, Schilmoeller, & Schilmoeller, 2006). Further, individuals with callosal agenesis have similar symptoms to ASD such as impaired social skills (Badaruddin et al., 2007), developmental delays and seizures (Lacey, 1985), and difficulties with complex problem solving and abstract reasoning (Brown & Sainsbury, 2000; David, Wacharasindhu, & Lishman, 1993). The overlap in the clinical profile of agenesis of the CC and ASD also suggests that connectivity of the CC may be one important brain area (of likely many) to study in ASD.
Corpus callosum microstructure
As can be seen in Table 1, 10 ROI studies and 26 VBA studies have made group comparisons of WM microstructure of the CC. As can be seen in Figure 3, a number of these studies have found significantly lower FA in ASD across the entire CC (i.e., genu, body, and splenium). Many of these studies have also measured MD, RD, and AD to better understand the underlying contributions of FA group differences in ASD. Some of these studies found significantly increased RD in ASD in the absence of a group difference in AD (i.e., Alexander et al., 2007a; Jeong, Kumar, Sundaram, Chugani, & Chugani, 2011; Shukla et al., 2010; Shukla, Keehn, Smylie, & Müller, 2011). Similarly, Ameis and colleagues (2011) found increased MD and RD in the forceps minor and forceps major of the corpus callosum in ASD but did not find FA group differences. These findings suggest that persons with ASD may have increased water diffusivity perpendicular to the axon. Recent studies in animal models of myelin abnormalities have found increased RD in animals with less myelination (Harsan et al., 2006; Song et al., 2002, 2005; Tyszka, Readhead, Bearer, Pautler, & Jacobs, 2006), which suggests that the axons of the CC may be less myelinated in ASD. Alternatively, increased RD may be due to less dense and/or thicker axons. Jeong et al. (2011) found that increased curvature of the WM tract appeared correlated with decreased RD.
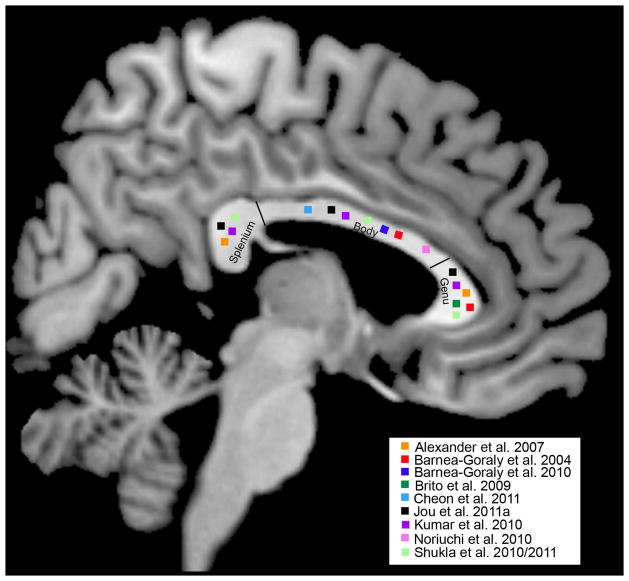
Figure 3.
Studies that have shown decreased fractional anisotropy (FA) in persons with ASD (compared to persons with typical development) in the splenium, body, or genu of the corpus callosum.
Although a good number of studies have found decreased FA in the CC of persons with ASD, there are some notable exceptions. For example, Cheng et al. (2010), Cheung et al. (2009), Hong et al. (2011), and Thomas et al. (2011) did not find a diagnostic group difference in CC FA (although Hong et al. found a significant group difference in MD). Additionally, the two studies with the youngest samples (Ben Bashat et al., 2007; Weinstein et al., 2011) actually demonstrated increased FA of the CC in ASD. One possible explanation is that sample heterogeneity may have contributed to the variation in their findings, as certain characteristics (e.g., intellectual/cognitive ability, language ability, head circumference) may be more indicative of atypical WM microstructure in ASD. Indeed, Alexander and colleagues (2007a) found that approximately 72% of the ASD sample had CC FA at typical levels, but the ASD-Control group difference was driven by a subgroup of 28% of participants with ASD who had low FA of the CC. This subgroup also exhibited decreased performance IQ, increased MD, increased RD, and decreased CC volume compared to their ASD peers, suggesting that within-group differences in WM integrity of the CC may be related to within-group differences in performance IQ. Indeed, DTI measures of the corpus callosum have been associated with performance IQ in non-ASD populations, including adults who were born preterm (Kontis et al., 2009). It is very possible that decreased hemispheric connectivity may be related to the cognitive profile of this subgroup, and further investigations into the behavioral and clinical characteristics of this subgroup are ongoing. However, most studies we reviewed report only ASD-Control group differences, and this subgroup finding underscores the importance of examining and reporting individual variability within the ASD group when examining group differences in white matter integrity, as this may help clarify inconsistent results in the literature and help us better understand the biological basis of within-group heterogeneity in the clinical profiles of ASD. To this end, statistical techniques such as cluster analyses or sophisticated mixed effects models can be used to examine distributions of individuals in these subgroups.
Group Differences in the Cingulum Bundle
The cingulum bundles are the primary intrahemispheric association pathways for the medial cingulate cortex and temporal lobe structures. These tracts run above the CC from the anterior cingulate cortex to the posterior cingulate area and curve around the splenium of the CC and project to the hippocampus (see Fig 4). One limitation with DTI measurements in the cingulum is that the structure is very narrow and surrounded by tissues with much different diffusion properties (CC and cingulate gray matter) that can make the interpretation challenging (Lee et al., 2009). Nevertheless, group differences in the microstructure of the cingulum have been investigated in four ROI studies and 26 VBA studies, and significant differences have been reported consistently. In terms of macrostructure, in an investigation of the limbic tracts, persons with Asperger syndrome were found to have an increased number of streamlines in the bilateral cingulum (Pugliese et al., 2009), suggesting that structural atypicalities may be present in this region. In terms of microstructure, multiple studies have found decreased FA and/or increased MD in the WM in regions of the anterior cingulum in ASD (Barnea-Goraly et al., 2004; Lee et al., 2009; Jou et al., 2011a, b; Kumar et al., 2010; Noriuchi et al., 2010; Pardini et al., 2009; Thakkar et al., 2008) (for an exception, see Cheng et al., 2010). Taken together, these studies offer preliminary findings that suggest decreased FA of the cingulum may be common in persons with ASD.
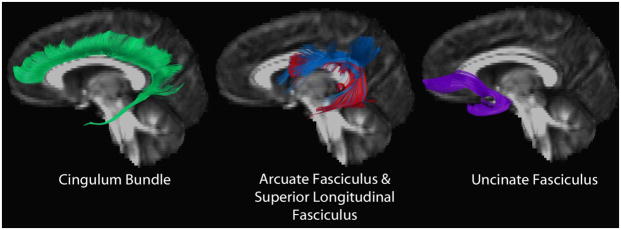
Figure 4.
Tractography of a typically developing cingulum (green), superior longitudinal fasciculus (blue), arcuate fasciculus (red), and uncinate fasciculus (purple).
Group Differences in the Arcuate Fasciculus/Superior Longitudinal Fasciculus
The arcuate fasciculus is a long WM tract known to extend from the inferior frontal gyrus to the temporo-parietal junction (TPJ), and it is thought to be a subsection of the larger superior longitudinal fasciculus, which originates in the superior temporal gray matter and extends to the frontal lobe (See Fig 4). The arcuate fasciculus has been long thought to connect brain areas that are essential for language understanding and production, connecting Broca’s area to Wernicke’s area in the TPJ. Recent research has also demonstrated that the arcuate fasciculus may provide communication between Broca’s area and Wernicke’s area via the inferior parietal lobule (Catani, Jones, & Ffytche 2005). Ten ROI studies and 26VBA studies have investigated the WM microstructure of the arcuate fasciculus/superior longitudinal fasciculus in persons with ASD. However, the results of these studies have been inconsistent, with some results suggesting bilateral decreases in FA (Jeong, Kumar, Sundaram, Chugani, & Chugani, 2011; Noriuchi et al., 2010; Shukla, Keehn, & Müller, 2011), right-only decreases in FA (Barnea-Goraly, Lotspeich, & Reiss, 2010; Poustka et al., 2011; Sahyoun, Belliveau, Soulieres, Schwartz, & Mody, 2010), left-only decreases in FA (Jou et al., 2011a), or right-only increases in FA in persons with ASD (Cheng et al., 2010; Cheung et al., 2009; Sahyoun, Belliveau, & Mody, 2010). Furthermore, a voxel-based study by Lee et al. (2009) did not find any significant group FA differences in the superior longitudinal fasciculus, although MD was significantly increased. The heterogeneity of these results may be caused in part by the length and complexity of fiber geometry and crossing in this region or by participant characteristics such as verbal ability (verbal IQ or ASD communication symptom severity) or age. Using Table 1, we examined if average reported IQ measures or age of the ASD group in these studies accounted for the differences in findings, but these studies used similar ages and IQ groups (with the exception of Jeong et al.’s study who used a younger age group), suggesting that at the group level these studies were similar in these characteristics. However, within one of the studies, Poustka and colleagues (2011) found that the FA of the superior longitudinal fasciculus was related to ADI-R and ADOS-G communication subscales in their sample (discussed in more detail below). Therefore, examining heterogeneity in these regions as a function of characteristics of the individual participants may be a critical avenue for future research.
The arcuate and superior longitudinal fasciculi, however, originate in the TPJ and STG and there is relatively consistent evidence of decreased FA in ASD in this particular aspect of the tract (e.g., Barnea-Goraly et al., 2004; Barnea-Goraly et al., 2010; Lee et al., 2007; Noriuchi et al., 2010); for an exception to this finding see Cheng et al. (2010). The posterior STG is near the TPJ and is implicated in many different aspects of social perception (Allison, Puce, & McCarthy, 2000). Diagnostic group differences in gray matter structure (e.g., Boddaert et al., 2004; Levitt et al., 2003; Scheel et al., 2011), function (e.g., Castelli, Frith, Happe, & Frith, 2002; Koldewyn, Whitney, & Rivera, 2011), and functional connectivity (Kana, Keller, Cherkassky, Minshew, & Just, 2009; Shih et al., 2011) of the posterior STG have been found. The DTI results suggest that although group differences along the entire length of the arcuate and superior longitudinal fasciculi have been inconsistent, there is nevertheless consistent evidence for decreased WM integrity of these tracts near the STG and TPJ, which may be related to aspects of the cognitive and symptom profile of persons with ASD.
Group Differences in the Uncinate Fasciculus
The uncinate fasciculus is a hook-shaped tract that is known to directly connect medial temporal areas, including the amygdala and hippocampus, with frontal cortices, and it is thought to be a part of the limbic system (See Figure 4). Although the exact function of the uncinate fasciculus is still debated, recent research suggests that it may be involved in proper name retrieval (Papagno, 2011; Papagno et al., 2011), emotion recognition (Barbas, 2000), and self-awareness (Levine et al., 1998). Overall, five ROI studies and up to 26 VBA studies examined the microstructure of the uncinate fasciculus in ASD. However, these studies have found inconsistent evidence for group differences. For example, in the bilateral uncinate fasciculus, research has found increased FA (Sahyoun, Belliveau, & Mody, 2010), decreased FA (Cheon et al., 2011; Jeong et al., 201Poustka et al., 2011), increased MD and RD (but no difference in FA) (Ameis et al., 2011; Shukla, Keehn, & Müller, 2011), or no FA or MD group difference (Pugliese et al., 2009).
Atypical Lateralization in Persons with ASD
In persons with typical development, most research has found a leftward asymmetry in DTI measures in the arcuate and uncinate fasciculi (e.g., greater FA and less MD in the left hemisphere) (Catani et al., 2007; Hasan et al., 2009; Kubicki et al. 2002). In persons with ASD, however, multiple studies have found decreased leftward lateralization in the ASD group in the arcuate fasciculus (Fletcher et al., 2010; Lo et al., 2011[b-value between 4000 and 6000]), the uncinate fasciculus (Lo et al., 2011), and in the WM of the STG (Lange et al., 2010), which is a terminal for the arcuate fasciculus. This lack of lateralization appears to be associated with decreased language functioning (CELF-3) (Fletcher et al., 2010). Additionally, Conturo et al. (2008) found decreased lateralization in ASD in WM tracts connecting the hippocampus and amygdala to the fusiform gyrus. Taken together, these results suggest that multiple WM tracts may be atypically lateralized in persons with ASD, which may be associated with the functional hemispheric asymmetry in language tasks that has been observed in individuals with ASD (Bigler et al., 2007; Boddaert et al., 2003; Chiron et al., 1995; Flagg et al., 2005). This atypical lateralization, however, may not be specific to ASD, but ASD may make it more likely for individuals to be less typically lateralized (Knaus et al., 2010). Atypical lateralization in persons with ASD and person with typical development may lead to decreased hemispheric specialization, which may affect highly lateralized tasks such as language ability. Additionally, it is interesting to note that atypical asymmetry of language (as measured by functional activation during language tasks) has been reported in typically developing left-handed individuals (Jorgens et al., 2007; Tzourio et al., 1998) as well as in individuals with reading disorders (Wehner, Ahlfors, & Mody, 2007), developmental stuttering (Blomgren et al., 2003), specific language impairment (Pecini et al., 2005), attention deficit hyperactivity disorder (Hale et al., 2010; Keune et al., 2011), and schizophrenia (Sommer et al., 2001). These disorders may have behavioral symptoms that overlap with ASD, which makes this an intriguing area of study. Examination of the nature of lateralization atypicalities in the WM integrity of persons with ASD and how it may be similar or different to atypical lateralization in other groups is a promising area of future research.
Relation between DTI and behavioral measures
In order to better understand the effect of DTI measures on cognition and symptom severity in ASD, many of the reviewed studies investigated correlations between DTI and ASD symptom measures. We examined relations between DTI measures and ASD symptoms in the literature in order to see which WM tracts may be related to certain aspects of ASD symptoms and to examine if these relations would be able to disambiguate inconsistent results that have been found in the DTI literature. Nevertheless, specific DTI-symptom relations in ASD have not been clear, possibly due to the small sample size of many of the studies or the different methods used to measure these behavioral constructs. For example, two of these studies found significant negative correlations within the frontal lobe, with decreased FA being associated with more severe ASD symptoms, including the ADI-R (Thakkar et al., 2008; ASD n = 12, FA of left subgyral rostral cingulum) and the Social Responsiveness Scale (SRS) (Noriuchi et al., 2010; ASD n=7, FA of DLPFC). However, these findings have implicated quite different regions within the frontal lobe, and another study found decreased FA associated with decreased symptom severity (Ke et al., 2009) (ASD n = 12). Similarly, in the cerebellum, two studies found decreased FA to be related to increased autism symptom severity as measured by the ADI-R (Catani et al., 2008, ASD n = 15; Cheung et al., 2009, ASD n = 13). However, one of these studies found this relation only with the social symptom domain (Cheung et al., 2009), and the other study found it only in the repetitive behavior symptom domain (Catani et al., 2008). The inconsistent findings across the frontal lobe and cerebellum exemplify the inconsistent pattern of DTI-symptom relations across studies.
To further complicate the matter, quite a few studies looked for but did not find significant relations between DTI measures and ASD symptom severity measures. Specifically, Shukla et al. (2010) (ASD sample n = 26), Barnea-Goraly et al. (2010) (ASD sample n = 13), and Langen et al. (2011) (ASD sample n = 21) did not find evidence for significant correlations between their DTI measures and ADOS-G (Autism Diagnostic Observation Scale) or ADI-R scores. Likewise, Hong and colleagues (2011) (ASD sample n = 18) did not find significant correlations between their DTI measures and the Childhood Autism Rating Scale (CARS). Similarly, after correcting for multiple comparisons, Jou and colleagues (2011a) (ASD sample n = 15) did not find significant correlations between FA and SRS scores, and Sundaram and colleagues (2008) did not find significant correlations between FA and Gilliam Autism Rating Scales (GARS). Alexander et al. (2007a) found a significant relation between RD of the CC and the SRS across both groups, but this effect was not found within the groups independently, and may reflect group differences in both RD and SRS scores, rather than a true relation between the two.
Although the correlations between DTI measures and ASD symptoms have not demonstrated a consistent pattern, there is preliminary evidence that performance IQ may be related to FA and RD measures of the CC (Alexander et al., 2007; Lee et al., 2009) and RD of the temporal stem (Lange et al., 2010). This finding may hold promise for improved understanding of the relation between DTI measures and the symptomatic expression of ASD. However, these studies had overlapping samples, and replication with other samples is needed to confirm or deny this effect. Taken together, further work with larger samples is needed to examine if there are indeed true relations between DTI measures and ASD symptoms. In addition, more work is needed to understand exactly where in the brain (and with which measures) these relations occur consistently. This type of investigation will be critical in order to probe the biological basis of individual differences that occur across the Autism Spectrum and to better understand how WM integrity relates to the behavioral expression of ASD at both an individual and group level.
Developmental Trajectory of White Matter Microstructure in Persons with ASD
Because ASD is a developmental disorder, it is important to account for the developmental trajectories of these WM tracts and the age of the participants when interpreting DTI findings. Perhaps taking these developmental trajectories into account may help us better understand why conflicting DTI results arise.
In typically developing individuals, WM develops dynamically and changes across the lifespan, with myelination continuing from childhood into adolescence and generally occurring in a posterior-to-anterior gradient (Yakovlev & LeCours, 1967). Most of the research that has examined WM growth in these individuals has been cross-sectional, but a few recent studies have examined WM growth longitudinally (e.g., Bava et al., 2010; Giorgio et al., 2010; Lebel & Beaulieu, 2011). A consistent theme from this body of research is that individuals with typical development demonstrate increased volume, increased FA, and decreased MD in most WM tracts from childhood into adulthood (Ashtari et al., 2007; Barnea-Goraly et al., 2005; Giorgio et al., 2008; Giorgio et al., 2010; Lebel et al., 2008; Lebel & Beaulieu, 2011; Muetzel et al., 2008). For many of these WM tracts, development was characterized by a relatively steep increase in FA and decrease in MD during childhood, which eventually plateaued (or even dipped) in early adolescence (Lebel & Beaulieu, 2011). However, even within a single fiber bundle, like the CC, there may be slightly different developmental trajectories. Specifically, the genu of the CC appears to reach full development earlier than the splenium (Barnea-Goraly et al., 2005; Giorgio et al., 2008; Giorgio et al., 2010; Lebel et al., 2008; Lebel & Beaulieu, 2011; Paus et al., 1999; Reiss, Abrams, Singer, Ross, & Denckla, 1996).
In persons with ASD, head circumference and structural neuroimaging have suggested differing developmental trajectories of the brains of children with ASD that may affect WM structure. For example, in the first two-to-four years of life, studies have found that children with ASD have atypically rapid brain growth compared to children with typical development (Bloss & Courchesne, 2007; Carper, Moses, Tigue, & Courchesne, 2002; Courchesne et al., 2001; Schumann et al., 2010; Sparks et al., 2002; Nordahl et al., 2011) and more neurons in the prefrontal cortex (Courchesne, et al., 2011; Lainhart & Lange, 2011). Such rapid and early brain growth may be the cause of a larger head circumference of young children with ASD that has been reported previously (Courchesne, Carper, & Akshoomoff, 2003; Dawson et al., 2007; Dementieva et al., 2005; Elder, Dawson, Toth, Fein, & Munson, 2008; Fukumoto et al., 2008; Hazlett et al., 2005; Lainhart et al., 1997, 2006; Nordahl et al., 2011).
It is possible that this rapid early brain development may cause increased FA (and decreased MD) in young children with ASD compared to children with typical development, as FA is known to increase with age and MD is known to decrease with age. Indeed, two studies from the same research group (Ben Bashat et al., 2007; Weinstein et al., 2011) found that young children with ASD (1.5–5.8 years old) demonstrated increased FA compared to a group of individuals with typical development. The authors suggest that this finding may be indicative of an early and rapid increase in FA in ASD that parallels the brain volume findings. Very recent evidence for different rates of FA development in persons with ASD early in life comes from a longitudinal examination of WM in high-risk infant siblings of individuals with ASD (Wolff et al., 2012). These high-risk infants were scanned at six months, 12 months, and 24 months of age, and the results indicated different rates of FA development in those who ended up meeting diagnostic criteria for ASD (Wolff et al., 2012). Specifically, those who met diagnostic criteria at 24 months of age had increased FA at six months of age in regions such as the corpus callosum, fornix, inferior longitudinal fasciculus, uncinate, and posterior limb of the internal capsule (compared to those who did not meet diagnostic criteria). However, by 24 months of age, those who met criteria actually had decreased FA in these regions, suggesting a plateau in FA development compared to the group who did not meet ASD diagnostic criteria. This finding is in partial support of the findings of Ben Bashat et al. and Weinstein et al. who found increased FA in ASD in children younger than four years of age. However, the Wolff et al. results suggest that the increased FA in this group may occur even earlier (around six months of age). Further studies in very young participants will be needed to clarify the developmental trajectory of WM microstructure in ASD.
Age-and-DTI-measure correlations from cross-sectional studies also suggest atypical development of WM integrity in ASD in older subjects. For example, significant age-by-diagnosis interactions have been found across multiple cross-sectional studies, such that age is associated with increased FA and decreased MD or RD in older participants with typical development but not in participants with ASD. This significant age-by-diagnosis interaction has been observed in the FA of the STG WM (Lee et al., 2007) and in the FA of frontal lobe areas such as the bilateral SLF, CC, and right inferior occipito-frontal fasciculus (Cheng et al., 2010). Other studies have found FA (or MD and RD) of only certain regions to be associated with age. For example, positive correlations between age and FA and negative correlations between age and MD and RD were found for tracts in each lobe for the group with typical development but only in the frontal lobe for the ASD group (Shukla Keehn, & Müller, 2011; Shukla, Keehn, Smylie, & Müller, 2011). Similarly, FA in the typically developing group was correlated with age in all subregions of the CC but only in the splenium in the ASD group (with MD negatively correlated with age in both groups). To further complicate the picture, other studies have demonstrated similar age-related changes in FA or MD of both groups (i.e., Bode et al., 2011; Pugliese et al., 2009), or even increased FA with age in the ASD group but not in the typically developing group (Keller, Kana, & Just, 2007). Clearly, future research is needed, and only extended longitudinal designs can directly examine if there is a different developmental trajectory of WM integrity in ASD.
Summary of Findings
From many of the studies under review, it appears that children (>4 years of age) and young adults with ASD tend to have decreased FA in WM tracts spanning across many regions of the brain. This decrease in FA is often accompanied by an increase in both MD and RD. Additionally, this pattern of decreased FA in ASD may be more applicable to some WM tracts than others, with studies finding the most consistent decrease in FA in ASD in regions such as the CC, cingulum, and WM tracts connecting aspects of the temporal lobe. These WM tracts have been associated with diverse functions, including motor skill and complex information processing (the CC), executive functioning (the cingulum), and social functioning (STG and TPJ of the temporal lobe). The results of the present review also suggest possible atypical hemispheric lateralization in persons with ASD occurring in WM tracts such as the uncinate fasciculus and superior longitudinal/arcuate fasciculus. This atypical hemispheric lateralization may relate to decreased hemispheric specialization and language ability in persons with ASD. Nevertheless, we do encourage caution when interpreting what these WM group differences may mean. Although we can speculate as to how these WM group differences may relate to the expression of ASD, the current literature was unclear as to whether or not WM integrity was related to the behavioral or clinical profile of individuals with ASD. The relatively small sample sizes of the studies in this literature review may have made it difficult to detect these DTI-behavioral relations. Future research will benefit from directly exploring how individual differences in WM integrity may relate to behavior, cognition, and symptom severity in larger samples of persons with ASD.
Limitations of the Studies
In contrast to the general pattern of findings to date, there were a number of studies that found no significant group difference or increased FA in ASD (e.g., Ben Bashat et al., 2007; Bode et al., 2011; Cheng et al., 2010; Cheung et al., 2009; Ke et al., 2009; Sivaswamy et al., 2010; Verhoeven et al., 2011; Weinstein et al., 2011). An important issue with comparing studies is that DTI measurements are highly sensitive to a number of non-biological factors (scanner, coils, pulse sequences, parameters, signal-to-noise, spatial resolution, b-value diffusion-weighting) that make it particularly challenging to compare measurement values across sites. This limitation of DTI needs to be addressed before it becomes a clinically useful quantitative modality. In reviewing the protocols for the DTI studies to date, a couple of important considerations for the design of future DTI studies include spatial resolution, diffusion encoding and averaging (see Alexander et al., 2007; Tournier et al., 2011; Basser and Jones, 2002; Alexander et al., in press). Most of the studies had in-plane spatial resolution on the order of 2.5 mm or less, but seven studies used thick slices (5 mm). All imaging methods, including DTI, can only characterize structural features that are on the order of twice the sampling interval or resolution. For example, in studies with slices every 5 mm, we can only reliably characterize structures that are 10 mm or wider. In general, imaging studies with isotropic spatial resolution (i.e., equal dimensions in all 3 directions of the voxel) are preferable to minimize any directional bias effects in the analyses. DTI measures are also highly sensitive to measurement noise, which can lead to increased variance and bias in the measures (Pierpaoli & Basser, 1996). Thus it is important to acquire as many diffusion-weighted and non-diffusion-weighted (b=0; a ratio of ~6:1 is more ideal) images in as many directions a possible. The use of more encoding directions (e.g., ~40 or more) will help to enable the characterization of complex white matter organization including multiple fiber groups within a single voxel.
Furthermore, despite the growing number of DTI studies in ASD populations, most of the studies had samples of 30 subjects or fewer in each group. The considerable heterogeneity of ASD coupled with the relatively small group effect sizes in these studies is likely to lead to instable results. Consequently, there is a strong need for larger samples in future DTI studies to better characterize white matter abnormalities in ASD. These larger sample sizes could be achieved either through increased recruitment from a single site or methodologies that allow for meaningfully combining participant data across sites.
It is still clear that there is much to be learned regarding WM integrity in ASD. Therefore, keeping in mind some of these limitations, we highlight promising future directions, including directly examining WM development longitudinally, examining the role of genetics in WM development, and combining functional connectivity and DTI in the study of network connectivity.
Avenues of Future Research
Longitudinal study of white matter in ASD
Some of the reviewed studies suggest that there may be atypical development of WM microstructure in ASD. With longitudinal studies across a large number of individuals and across a wide age range, we would be able to test this possibility directly. Although any longitudinal study requires substantial time and resources above cross-sectional approaches, such a design would be instrumental in characterizing the ever-changing biomarkers of persons with a developmental disorder such as ASD. Differences in findings between cross-sectional versus longitudinal studies of brain development can be enormous (Brain Development Cooperative Group, 2012). Longitudinal analysis of individual change over time is able to quantify within-person and between-person variation simultaneously, is able to investigate how well subjects “track” along their population growth curves (McMahan, 1981), and is able to greatly reduce previously unexplained residual variation. Such analytic advances enable us to better characterize similarities and differences in developmental trajectories of ASD compared to typical development.
Genetics and white matter in persons with ASD
It will be equally important to investigate genetic contributions to WM development. To date, some research suggests that WM integrity may be closely related to individual genetic composition (e.g., Tan, Doke, Ashburner, Wood, & Frackowiak, 2010). Specific to ASD, Barnea-Goraly et al. (2010) examined WM integrity in children with ASD, their unaffected siblings, and children with typical development with no first-degree relatives with ASD. Their results suggested that both individuals with ASD and their unaffected siblings had decreased FA compared to the children with no family history of ASD in multiple brain areas. These children with ASD and their unaffected siblings did not, however, significantly differ in FA, AD, or hemispheric lateralization. These results offer preliminary evidence that there may be strong genetic contributions to atypicalities in WM microstructure in ASD. Furthermore, these atypicalities may be shared by family members as part of the broader biological autism phenotype (Lainhart & Lange, in press).
DTI and functional connectivity in ASD
Many studies have found decreased functional connectivity between frontal and posterior areas in individuals with ASD (for a review see Schipul, Keller, and Just, 2011). Nevertheless, although structural and functional connectivity relations are plausible, relatively few studies have linked functional connectivity and DTI measures in persons with typical development, let alone in persons with ASD. In persons with typical development, four studies suggest a robust overlap in resting state functional connectivity and DTI measures (Gordon et al., 2011; Greicius, Supeka, Menon, & Dougherty, 2009; Supekar et al., 2010; van den Heuvel, Mandl, Kahn, & Hulshoff Pol, 2009). In ASD, a handful of studies have found relations between functional connectivity and the WM volume of particular tracts (Cherkassky, Kana, Keller, & Just, 2006; Kana, Keller, Cherkassky, Minshew, & Just, 2006; Just, Cherkassky, Keller, Kana, & Minshew, 2007). However, to our knowledge, relations between functional connectivity and DTI measures in ASD have not yet been reported, making this an extremely important avenue for future research.
Clinical Implications
The fact that no consistent correlations between DTI measures and ASD symptom severity have been found to date impedes our understanding of the clinical significance of the reported significant group differences in WM microstructure. As noted previously, however, many studies investigating these correlations had smaller sample sizes and thus were underpowered. There is a pressing need to more fully understand if and how neural markers relate to specific patterns of behavior, cognition, and symptoms in persons with ASD. Future research holds great promise to bridge these current gaps.
Regarding treatment interventions, there is very preliminary evidence suggesting that interventions may be able to affect WM integrity changes in ASD. In the one treatment study we found (Pardini et al., 2011), an intensive communication intervention for 22 individuals with ASD over the course of many years suggested that WM integrity of the uncinate fasciculus was related to the age when treatment began, treatment duration, and decreases in ASD symptom severity over treatment course. Specifically, increased uncinate fasciculus FA was associated with longer treatment duration and decreased symptom severity. Although not a pre- versus post-intervention design, this study provides some hope that particular interventions may affect WM integrity in ASD. There are also promising intervention results outside of ASD research, showing WM integrity changes as a function of motor learning and practice (Bosnell et al., 2011; Taubert et al., 2010), memory training (Engvig et al., 2011), or working memory, episodic memory, and perceptual speed training (Lovden et al., 2010). Another compelling example comes from a study in reading disability, in which poor readers had decreased FA of the left anterior centrum semiovale prior to a reading intervention, but these values increased after a reading intervention (Keller & Just, 2009). These results offer preliminary evidence that WM integrity can be changed through behavioral interventions. However, WM integrity changes as a function of ASD-specific interventions will have to be confirmed or denied by future longitudinal randomized controlled clinical trials.
Finally, there are some initial efforts to use DTI measures to classify and diagnose ASD. These studies have used the shape of splenium fibers (Adluru et al., 2009), tensor coefficients of the bilateral STG and right temporal stem (Lange et al., 2010), and tensor coefficients from many areas across the brain (Ingalhalikar, Parker, Bloy, Roberts, & Verma, 2011) to distinguish between those with ASD and those with typical development. Although there is much research to be done in this domain, these preliminary classification efforts offer hope that DTI methods may some day aid in the diagnosis of ASD.
Conclusions
Since the first DTI study in persons with ASD in 2004, a number of studies have suggested that individuals with ASD may have decreased FA (a measure of fiber coherence) and increased MD (a measure inversely related to tissue density) in a number of WM tracts. These findings have been the most consistent in the corpus callosum, cingulum bundle, and temporal lobe. There is additional evidence of atypical hemispheric lateralization of fiber coherence in ASD.
The specific mechanisms and possible autism neuropathology underlying these reported differences remain unclear. Unfortunately, DTI measures are unspecific indicators of the types of microstructural changes in ASD. Increased RD has been found to be associated with diminished myelination in animal models (Song et al., 2002); however, RD may also be modulated by changes in axonal density and/or width. There are more specific imaging measures of myelination, including magnetization transfer and the myelin water fraction from T2 relaxometry (see review in Alexander et al., in press) that might help disambiguate the specific mechanism.
Overall, there is mounting evidence by these DTI and other studies that is consistent with the general hypothesis that brain connectivity is affected in many people with ASD. The technologies for diffusion imaging of the brain and image analyses are rapidly advancing which will greatly improve the quality of white matter characterization into the future. As the number of DTI and other neuroimaging studies in ASD grow, the understanding of how brain connectivity and white matter are affected in people with ASD will be improved.
Supplementary Material
Acknowledgments
This work was supported by NICHD T32 HD07489 Postdoctoral Training Award (BGT); NICHD P30 HD003352 (ALA); NIMH P50 MH84051 (ALA), NIMH RO1 MH080826 (JEL, ALA, EDB, NL), the Morgridge Institutes for Research (MIR-University of Wisconsin; NA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, NICHD or the NIH. The authors would also like to thank Frances Haeberli and Bimi Pangli for their assistance with selecting and reviewing the articles.
Literature Cited
- Adluru N, Hinrichs C, Chung MK, Lee JE, Singh V, Bigler ED, Alexander AL. Classification in DTI using shapes of white matter tracts. Conference Proceedings: …Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference; 2009; 2009. pp. 2719–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Hasan K, Kindlmann G, Parker DL, Tsuruda JS. A geometric analysis of diffusion tensor measurements of the human brain. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2000;44(2):283–291. doi: 10.1002/1522-2594(200008)44:2<283::aid-mrm16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion-tensor MRI. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2001;45(5):770–780. doi: 10.1002/mrm.1105. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Field AS. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity. doi: 10.1089/brain.2011.0071. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Lainhart JE. Diffusion tensor imaging of the corpus callosum in autism. NeuroImage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Fan J, Rockel C, Voineskos AN, Lobaugh NJ, Soorya L, Anagnostou E. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: A diffusion tensor imaging study. PloS One. 2011;6(11):e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Arlington, VA: American Psychiatric Association; 2000. [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, Lainhart JE. Decreased interhemispheric functional connectivity in autism. Cerebral Cortex (New York, NY: 1991) 2011;21(5):1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Kumra S. White matter development during late adolescence in healthy males: A cross-sectional diffusion tensor imaging study. NeuroImage. 2007;35(2):501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Badaruddin DH, Andrews GL, Bolte S, Schilmoeller KJ, Schilmoeller G, Paul LK, Brown WS. Social and behavioral problems of children with agenesis of the corpus callosum. Child Psychiatry and Human Development. 2007;38(4):287–302. doi: 10.1007/s10578-007-0065-6. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin. 2000;52(5):319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Lotspeich LJ, Reiss AL. Similar white matter aberrations in children with autism and their unaffected siblings: A diffusion tensor imaging study using tract-based spatial statistics. Archives of General Psychiatry. 2010;67(10):1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex (New York, NY: 1991) 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: Theory, experimental design and data analysis - a technical review. NMR in Biomedicine. 2002;15(7–8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher FD, Minati L, Baron-Cohen S, Lombardo MV, Lai MC, Gray MA, Critchley HD. Autism attenuates sex differences in brain structure: A combined voxel-based morphometry and diffusion tensor imaging study. AJNR American Journal of Neuroradiology. 2011 doi: 10.3174/ajnr.A2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Ben Sira L. Accelerated maturation of white matter in young children with autism: A high b value DWI study. NeuroImage. 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S, Marzi CA, Tassinari G. Corpus callosum and simple visuomotor integration. Neuropsychologia. 1995;33(8):923–936. doi: 10.1016/0028-3932(95)00031-w. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lainhart JE. Superior temporal gyrus, language function, and autism. Developmental Neuropsychology. 2007;31(2):217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Bloemen OJ, Deeley Q, Sundram F, Daly EM, Barker GJ, Jones DK, Murphy DG. White matter integrity in asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Research: Official Journal of the International Society for Autism Research. 2010;3(5):203–213. doi: 10.1002/aur.146. [DOI] [PubMed] [Google Scholar]
- Blomgren M, Nagarajan SS, Lee JN, Li T, Alvord L. Preliminary results of a functional MRI study of brain activation patterns in stuttering and nonstuttering speakers during a lexical access task. Journal of Fluency Disorders. 2003;28(4):337–55. doi: 10.1016/j.jfludis.2003.08.002. quiz 355-6. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: A preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(4):515–523. doi: 10.1097/chi.0b013e318030e28b. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Belin P, Chabane N, Poline JB, Barthelemy C, Mouren-Simeoni MC, Zilbovicius M. Perception of complex sounds: Abnormal pattern of cortical activation in autism. The American Journal of Psychiatry. 2003;160(11):2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. NeuroImage. 2004;23(1):364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bode MK, Mattila ML, Kiviniemi V, Rahko J, Moilanen I, Ebeling H, Nikkinen J. White matter in autism spectrum disorders - evidence of impaired fiber formation. Acta Radiologica (Stockholm, Sweden: 1987) 2011;52(10):1169–1174. doi: 10.1258/ar.2011.110197. [DOI] [PubMed] [Google Scholar]
- Bosnell RA, Kincses T, Stagg CJ, Tomassini V, Kischka U, Jbabdi S, Johansen-Berg H. Motor practice promotes increased activity in brain regions structurally disconnected after subcortical stroke. Neurorehabilitation and Neural Repair. 2011;25(7):607–616. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI study of normal brain development. Cerebral Cortex (New York, NY: 1991) 2012;22(1):1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr, Rodrigues Lde S, Gasparetto EL, Calcada CA. Diffusion tensor imaging findings in school-aged autistic children. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 2009;19(4):337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Brown LN, Sainsbury RS. Hemispheric equivalence and age-related differences in judgments of simultaneity to somatosensory stimuli. Journal of Clinical and Experimental Neuropsychology. 2000;22(5):587–598. doi: 10.1076/1380-3395(200010)22:5;1-9;FT587. FT587. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. NeuroImage. 2002;16(4):1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2009;364(1522):1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. Journal of Child Neurology. 2002;17(9):692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Neuronal density and architecture (gray level index) in the brains of autistic patients. Journal of Child Neurology. 2002;17(7):515–521. doi: 10.1177/088307380201700708. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathologica. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, Murphy DG. Altered cerebellar feedback projections in asperger syndrome. NeuroImage. 2008;41(4):1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. NeuroImage. 2010;50(3):873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, Herrington J, Schultz RT. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A diffusion tensor imaging study. Brain Research. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, McAlonan GM. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(9):1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Chiron C, Leboyer M, Leon F, Jambaque I, Nuttin C, Syrota A. SPECT of the brain in childhood autism: Evidence for a lack of normal hemispheric asymmetry. Developmental Medicine and Child Neurology. 1995;37(10):849–860. doi: 10.1111/j.1469-8749.1995.tb11938.x. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(18):10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ. Neuronal fiber pathway abnormalities in autism: An initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. Journal of the International Neuropsychological Society: JINS. 2008;14(6):933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA: The Journal of the American Medical Association. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA: The Journal of the American Medical Association. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- David AS, Wacharasindhu A, Lishman WA. Severe psychiatric disturbance and abnormalities of the corpus callosum: Review and case series. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56(1):85–93. doi: 10.1136/jnnp.56.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Munson J, Webb SJ, Nalty T, Abbott R, Toth K. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biological Psychiatry. 2007;61(4):458–464. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dementieva YA, Vance DD, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, Cuccaro ML. Accelerated head growth in early development of individuals with autism. Pediatric Neurology. 2005;32(2):102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Doherty D, Tu S, Schilmoeller K, Schilmoeller G. Health-related issues in individuals with agenesis of the corpus callosum. Child: Care, Health and Development. 2006;32(3):333–342. doi: 10.1111/j.1365-2214.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Elder LM, Dawson G, Toth K, Fein D, Munson J. Head circumference as an early predictor of autism symptoms in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2008;38(6):1104–1111. doi: 10.1007/s10803-007-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen JC, Baynes K, Gazzaniga MS. Anterior and posterior callosal contributions to simultaneous bimanual movements of the hands and fingers. Brain: A Journal of Neurology. 2000;123(Pt 12):2501–2511. doi: 10.1093/brain/123.12.2501. [DOI] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: A longitudinal diffusion tensor imaging study. Human Brain Mapping. 2011 doi: 10.1002/hbm.21370; 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg EJ, Cardy JE, Roberts W, Roberts TP. Language lateralization development in children with autism: Insights from the late field magnetoencephalogram. Neuroscience Letters. 2005;386(2):82–87. doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Lainhart JE. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. NeuroImage. 2010;51(3):1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto A, Hashimoto T, Ito H, Nishimura M, Tsuda Y, Miyazaki M, Kagami S. Growth of head circumference in autistic infants during the first year of life. Journal of Autism and Developmental Disorders. 2008;38(3):411–418. doi: 10.1007/s10803-007-0405-1. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. NeuroImage. 2010;51(3):943–951. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Johansen-Berg H. Changes in white matter microstructure during adolescence. NeuroImage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Lee PS, Maisog JM, Foss-Feig J, Billington ME, Vanmeter J, Vaidya CJ. Strength of default mode resting-state connectivity relates to white matter integrity in children. Developmental Science. 2011;14(4):738–751. doi: 10.1111/j.1467-7687.2010.01020.x; 10.1111/j.1467-7687.2010.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex (New York, NY: 1991) 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Buitelaar JK, van der Gaag RJ, Zwiers MP. Pervasive microstructural abnormalities in autism: A DTI study. Journal of Psychiatry & Neuroscience: JPN. 2011;36(1):32–40. doi: 10.1503/jpn.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Walshaw PD, Hanada G, Macion J, McCracken JT, Loo SK. Atypical EEG beta asymmetry in adults with ADHD. Neuropsychologia. 2010;48(12):3532–3539. doi: 10.1016/j.neuropsychologia.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55(7):1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, Ghandour MS. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. Journal of Neuroscience Research. 2006;83(3):392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Research. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archives of General Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hofer S, Merboldt KD, Tammer R, Frahm J. Rhesus monkey and human share a similar topography of the corpus callosum as revealed by diffusion tensor MRI in vivo. Cerebral Cortex (New York, NY: 1991) 2008;18(5):1079–1084. doi: 10.1093/cercor/bhm141. [DOI] [PubMed] [Google Scholar]
- Hong S, Ke X, Tang T, Hang Y, Chu K, Huang H, Liu Y. Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Research. 2011;194(3):333–339. doi: 10.1016/j.pscychresns.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Parker D, Bloy L, Roberts TP, Verma R. Diffusion based abnormality markers of pathology: Toward learned diagnostic prediction of ASD. NeuroImage. 2011;57(3):918–927. doi: 10.1016/j.neuroimage.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Kumar AK, Sundaram SK, Chugani HT, Chugani DC. Sharp curvature of frontal lobe white matter pathways in children with autism spectrum disorders: Tract-based morphometry analysis. AJNR American Journal of Neuroradiology. 2011;32(9):1600–1606. doi: 10.3174/ajnr.A2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1999;42(1):37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jorgens S, Kleiser R, Indefrey P, Seitz RJ. Handedness and functional MRI-activation patterns in sentence processing. Neuroreport. 2007;18(13):1339–1343. doi: 10.1097/WNR.0b013e32825a67db. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Jackowski AP, Papademetris X, Rajeevan N, Staib LH, Volkmar FR. Diffusion tensor imaging in autism spectrum disorders: Preliminary evidence of abnormal neural connectivity. The Australian and New Zealand Journal of Psychiatry. 2011;45(2):153–162. doi: 10.3109/00048674.2010.534069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA. Structural neural phenotype of autism: Preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. AJNR American Journal of Neuroradiology. 2011;32(9):1607–1613. doi: 10.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex (New York, NY: 1991) 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain: A Journal of Neurology. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Social Neuroscience. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, Liu Y. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Research. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Keary CJ, Minshew NJ, Bansal R, Goradia D, Fedorov S, Keshavan MS, Hardan AY. Corpus callosum volume and neurocognition in autism. Journal of Autism and Developmental Disorders. 2009;39(6):834–841. doi: 10.1007/s10803-009-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: Remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18(1):23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Keune PM, Schonenberg M, Wyckoff S, Mayer K, Riemann S, Hautzinger M, Strehl U. Frontal alpha-asymmetry in adults with attention deficit hyperactivity disorder: Replication and specification. Biological Psychology. 2011;87(2):306–310. doi: 10.1016/j.biopsycho.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Kilian S, Brown WS, Hallam BJ, McMahon W, Lu J, Johnson M, Lainhart J. Regional callosal morphology in autism and macrocephaly. Developmental Neuropsychology. 2008;33(1):74–99. doi: 10.1080/87565640701729821. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, Tager-Flusberg H. Language laterality in autism spectrum disorder and typical controls: A functional, volumetric, and diffusion tensor MRI study. Brain and Language. 2010;112(2):113–120. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Developmental Science. 2011;14(5):1075–1088. doi: 10.1111/j.1467-7687.2011.01058.x; 10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontis D, Catani M, Cuddy M, Walshe M, Nosarti C, Jones D, Allin M. Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport. 2009;20(4):424–428. doi: 10.1097/WNR.0b013e328325a8f9. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Shenton ME. Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. The American Journal of Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, Chugani DC. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cerebral Cortex (New York, NY: 1991) 2010;20(9):2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Lacey DJ. Agenesis of the corpus callosum. clinical features in 40 children. American Journal of Diseases of Children (1960) 1985;139(9):953–955. doi: 10.1001/archpedi.1985.02140110107042. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Volkmar F. Head circumference and height in autism: A study by the collaborative program of excellence in autism. American Journal of Medical Genetics Part A. 2006;140(21):2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Lange N. Increased neuron number and head size in autism. JAMA: The Journal of the American Medical Association. 2011;306(18):2031–2032. doi: 10.1001/jama.2011.1633. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Lange N. The biological broader autism phenotype. In: Amaral D, Geschwind D, Dawson G, editors. Autism Spectrum Disorders. New York, NY: Oxford University Press; pp. 477–509. (in press) [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(2):282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Lainhart JE. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Research: Official Journal of the International Society for Autism Research. 2010;3(6):350–358. doi: 10.1002/aur.162; 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, Murphy DG. Fronto-striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2011 doi: 10.1016/j.cortex.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Lainhart JE. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424(2):127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lee JE, Chung MK, Lazar M, DuBray MB, Kim J, Bigler ED, Alexander AL. A study of diffusion tensor imaging by tissue-specific, smoothing-compensated voxel-based analysis. NeuroImage. 2009;44(3):870–883. doi: 10.1016/j.neuroimage.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, Mcintosh AR, Toth JP, Stuss DT. Episodic memory and the self in a case of isolated retrograde amnesia. Brain: A Journal of Neurology. 1998;121(Pt 10):1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Smalley S, Thompson PM, Guthrie D, McCracken JT, Toga AW. Cortical sulcal maps in autism. Cerebral Cortex (New York, NY: 1991) 2003;13(7):728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- Lo YC, Soong WT, Gau SS, Wu YY, Lai MC, Yeh FC, Tseng WY. The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: A study using diffusion spectrum imaging tractography. Psychiatry Research. 2011;192(1):60–66. doi: 10.1016/j.pscychresns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lovden M, Bodammer NC, Kuhn S, Kaufmann J, Schutze H, Tempelmann C, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48(13):3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- McMahan CA. An index of tracking. Biometrics. 1981;37:447–455. [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychological Society: JINS. 1997;3(4):303–316. [PubMed] [Google Scholar]
- Minshew NJ, Sweeney J, Luna B. Autism as a selective disorder of complex information processing and underdevelopment of neocortical systems. Molecular Psychiatry. 2002;7(Suppl 2):S14–5. doi: 10.1038/sj.mp.4001166. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, van Zijl PC. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2002;47(2):215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Moseley M, Bammer R, Illes J. Diffusion-tensor imaging of cognitive performance. Brain and Cognition. 2002;50(3):396–413. doi: 10.1016/s0278-2626(02)00524-9. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel MA, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage. 2008;39(4):1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, Amaral DG. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, Kamio Y. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Research. 2010;1362:141–149. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Current Neurology and Neuroscience Reports. 2011;11(6):553–559. doi: 10.1007/s11910-011-0219-6. [DOI] [PubMed] [Google Scholar]
- Papagno C, Miracapillo C, Casarotti A, Romero Lauro LJ, Castellano A, Falini A, Bello L. What is the role of the uncinate fasciculus? surgical removal and proper name retrieval. Brain: A Journal of Neurology. 2011;134(Pt 2):405–414. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- Pardini M, Elia M, Garaci FG, Guida S, Coniglione F, Krueger F, Emberti Gialloreti L. Long-term cognitive and behavioral therapies, combined with augmentative communication, are related to uncinate fasciculus integrity in autism. Journal of Autism and Developmental Disorders. 2011 doi: 10.1007/s10803-011-1281-2. [DOI] [PubMed] [Google Scholar]
- Pardini M, Garaci FG, Bonzano L, Roccatagliata L, Palmieri MG, Pompili E, Emberti Gialloreti L. White matter reduced streamline coherence in young men with autism and mental retardation. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies. 2009;16(11):1185–1190. doi: 10.1111/j.1468-1331.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science (New York, NY) 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Poustka L, Jennen-Steinmetz C, Henze R, Vomstein K, Haffner J, Sieltjes B. Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry. 2011 doi: 10.3109/15622975.2011.591824. [DOI] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell’Acqua F, Thiebaut de Schotten M, Murphy C, Murphy DG. The anatomy of extended limbic pathways in asperger syndrome: A preliminary diffusion tensor imaging tractography study. NeuroImage. 2009;47(2):427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain: A Journal of Neurology. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, Mody M. White matter integrity and pictorial reasoning in high-functioning children with autism. Brain and Cognition. 2010;73(3):180–188. doi: 10.1016/j.bandc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, Soulieres I, Schwartz S, Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, Rotarska-Jagiela A, Schilbach L, Lehnhardt FG, Krug B, Vogeley K, Tepest R. Imaging derived cortical thickness reduction in high-functioning autism: Key regions and temporal slope. NeuroImage. 2011;58(2):391–400. doi: 10.1016/j.neuroimage.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Frontiers in Systems Neuroscience. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33(8):1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(12):4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Muller RA. Functional differentiation of posterior superior temporal sulcus in autism: A functional connectivity magnetic resonance imaging study. Biological Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Lincoln AJ, Muller RA. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: A diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(12):1269–78, 1278.e1–2. doi: 10.1016/j.jaac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(3):286–295. doi: 10.1111/j.1469–7610.2010.02342.x; 10.1111/j.1469–7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Smylie DM, Muller RA. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49(5):1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaswamy L, Kumar A, Rajan D, Behen M, Muzik O, Chugani D, Chugani H. A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. Journal of Child Neurology. 2010;25(10):1223–1231. doi: 10.1177/0883073809358765. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TE. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: Meta-analysis. The British Journal of Psychiatry: The Journal of Mental Science. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cerebral Cortex (New York, NY: 1991) 2008;18(11):2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Kawashima R. Training of working memory impacts structural connectivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(9):3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GC, Doke TF, Ashburner J, Wood NW, Frackowiak RS. Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. NeuroImage. 2010;53(3):1030–1042. doi: 10.1016/j.neuroimage.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: Learning-related changes in cortical areas and associated fiber connections. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(35):11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain: A Journal of Neurology. 2008;131(Pt 9):2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, Jung KJ, Minshew N, Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: A DTI tractography study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2011;47(7):863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2011;65(6):1532–1556. doi: 10.1002/mrm.22924; 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka JM, Readhead C, Bearer EL, Pautler RG, Jacobs RE. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. NeuroImage. 2006;29(4):1058–1065. doi: 10.1016/j.neuroimage.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio N, Crivello F, Mellet E, Nkanga-Ngila B, Mazoyer B. Functional anatomy of dominance for speech comprehension in left handers vs right handers. NeuroImage. 1998;8(1):1–16. doi: 10.1006/nimg.1998.0343. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping. 2009;30(10):3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JS, Rommel N, Prodi E, Leemans A, Zink I, Vandewalle E, Sunaert S. Is there a common neuroanatomical substrate of language deficit between autism spectrum disorder and specific language impairment? Cerebral Cortex (New York, NY: 1991) 2011 doi: 10.1093/cercor/bhr292. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Thompson PM. Mapping corpus callosum deficits in autism: An index of aberrant cortical connectivity. Biological Psychiatry. 2006;60(3):218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wehner DT, Ahlfors SP, Mody M. Effects of phonological contrast on auditory word discrimination in children with and without reading disability: A magnetoencephalography (MEG) study. Neuropsychologia. 2007;45(14):3251–3262. doi: 10.1016/j.neuropsychologia.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, Ben Bashat D. Abnormal white matter integrity in young children with autism. Human Brain Mapping. 2011;32(4):534–543. doi: 10.1002/hbm.21042; 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2006;12(4–5):279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Piven J. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. The American Journal of Psychiatry. 2012 doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford, UK: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. NeuroImage. 2009;44(3):1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Avants BB, Yushkevich PA, Woo JH, Wang S, McCluskey LF, Gee JC. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Transactions on Medical Imaging. 2007;26(11):1585–1597. doi: 10.1109/TMI.2007.906784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.